Abstract
Next-generation rationally-designed vaccine adjuvants represent a significant breakthrough to enable development of vaccines against challenging diseases including tuberculosis, HIV, and malaria. New vaccine candidates often require maintenance of a cold-chain process to ensure long-term stability and separate vials to enable bedside mixing of antigen and adjuvant. This presents a significant financial and technological barrier to worldwide implementation of such vaccines. Herein we describe the development and characterization of a tuberculosis vaccine comprised of both antigen and adjuvant components that are stable in a single vial at sustained elevated temperatures. Further this vaccine retains the ability to elicit both antibody and TH1 responses against the vaccine antigen and protect against experimental challenge with Mycobacterium tuberculosis. These results represent a significant breakthrough in the development of vaccine candidates that can be implemented throughout the world without being hampered by the necessity of a continuous cold chain or separate adjuvant and antigen vials.
Keywords: nanoemulsion, lyophilization, vaccine, adjuvant
Introduction
Although lyophilization of protein, live-attenuated or inactivated virus or bacteria-containing vaccines is a routine practice, to date there have been no reports of successful lyophilization and thermostability characterization of an adjuvanted clinical vaccine candidate [1]. Thermostable vaccines can greatly increase effective distribution of vaccines in the developing world and eliminate a prime contributor to high vaccine wastage rates [2, 3]. Development of vaccines that do not require cold-chain maintenance would significantly reduce the cost and technological hurdles of implementation of new vaccines worldwide, especially in low resource settings. Additionally cold-chain maintenance cannot be ensured during natural disasters when power supplies may be compromised. Lyophilization of protein-containing pharmaceuticals such as vaccines is a commonly employed method to prolong shelf-life and increase resistance to thermal stress [4, 5], and multiple marketed vaccines are distributed as lyophilized products [1]. New vaccines under development for induction of cell-mediated immunity against diseases such as malaria or tuberculosis may require adjuvant components in order to enhance and shape immune responses effectively [6]. However, the addition of adjuvant(s) to a vaccine antigen results in a more complex formulation with the potential for multiple interactions among components. Thus, maintaining long-term stability in adjuvanted vaccines can present a significant challenge to vaccine developers; for this reason, some adjuvanted vaccines are administered following bedside-mixing with a separate adjuvant vial [7]. Moreover, none of the existing marketed lyophilized vaccines contain adjuvant in the lyophilized formulation [1]. Indeed, adjuvant formulations already used in approved human vaccines such as aluminum salts or oil-in-water nanoemulsions may be particularly challenging to lyophilize [8, 9]. The complex nature of approved vaccine adjuvants (e.g. alum, oil-in-water nanoemulsions and/or monophosphoryl lipid A (MPLA)) present a substantial hurdle to developing lyophilized adjuvanted vaccines.
Approximately 1.5 million people die of tuberculosis (TB) each year worldwide, with an estimated 2 billion people currently infected, 10% of whom will develop active disease at some point in their lives [10]. The only approved vaccine for TB, Bacillus Calmette-Guérin (BCG), was first used in humans in 1921 and has been effective in reducing the incidence of disseminated TB in children. However, BCG has proven ineffective at preventing pulmonary TB in adolescents and adults [11–13]. Mathematical modeling of the impact of implementing a hypothetical new vaccine against TB with 60% efficacy predicts an 80% drop in incidence by 2050 [14]. Thus there is an urgent need for a new TB vaccine to either boost immunity primed by BCG or replace BCG. Protective immunity against Mycobacterium tuberculosis (Mtb) requires both TNF and IFN-γ production by CD4 T cells [15, 16]. We have developed a recombinant fusion protein antigen consisting of four Mtb proteins, designated ID93, that when paired with an adjuvant such as the synthetic TLR4 agonist glucopyranosyl lipid adjuvant formulated in a squalene-in-water stable nanoemulsion (GLA-SE), induces robust TH1 responses and is protective against Mtb challenge [17–19]. In the absence of the TLR agonist, immunization with ID93 generates a modest TH2 response that is not protective against Mtb challenge [20]. ID93+GLA-SE is currently undergoing Phase I safety testing in human volunteers. An effective, thermostable tuberculosis vaccine formulation could have a dramatic impact on global health, with easier worldwide distribution and reduced vaccine wastage. Herein, we describe the lyophilization, thermostability characterization, and biological efficacy of a nanoemulsion-adjuvanted tuberculosis vaccine candidate, ID93+GLA-SE.
Material and Methods
Sample Preparation and Lyophilization
The construction, expression, and purification of the ID93 tandem fusion protein containing the Mtb genes Rv3619, Rv1813, Rv3620, and Rv2608 have been described previously [17]. Briefly the ID93 fusion protein was expressed in E. coli, purified under denaturing conditions by chromatography on DEAE and Q Sepharose columns, and analyzed by SDS-PAGE on a 4–20% Tris glycine gel (Invitrogen). GLA (also known as PHAD) was purchased from Avanti Polar Lipids Inc. (Alabaster, AL). GLA-SE containing 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) was formulated according to the previously described methods [19, 21]. Briefly, GLA-SE emulsions were produced by mixing a buffered aqueous phase (poloxamer 188 and glycerol in ammonium phosphate buffer pH 5.1) and oil phase (DMPC and GLA dispersed into squalene by sonication at 70ºC) and then microfluidizing the mixture using the Microfluidics M110P (Newton, MA) for 12 passes at 30,000 psi. Component concentrations in the emulsions consisted of 10 % v/v squalene, 1.9 % w/v phosphatidylcholine, 0.1 % w/v poloxamer 188, 2.3 % w/v glycerol, and 25 mM ammonium phosphate buffer. GLA-SE was diluted to the specified concentrations for use.
Liquid and lyophilized samples were prepared with 1.5 mL fill volumes in 3 mL glass vials. Covialed samples containing ID93 (5 μg/mL) + GLA-SE (50 μg/mL, 2% total oil) were prepared in 20 mM tromethamine (Tris) at pH 8.0 [22]. Separately vialed ID93 or GLA-SE were prepared at twice the concentration of covialed samples (10 μg/mL ID93 or 100 μg/mL GLA, 4 % total oil GLA-SE) and mixed 1:1 prior to injection. Samples for SDS-PAGE were prepared at 100 μg/mL ID93 to facilitate analysis. Lyophilized samples also contained 5% (w/v) D-trehalose dehydrate as a stabilizer and were lyophilized using a VirTis (Gardiner, NY) AdVantage 2.0 EL-85 benchtop freeze dryer. The lyophilization recipe utilized a thermal treatment schedule including a 10-hour freezing step from 4 to −40 ºC, and an annealing step at −15 ºC. The primary drying phase (at 100 mTorr) lasted 18 hours from −40 ºC to 25ºC. Finally, a secondary drying phase at 50 mTorr was employed at 25 ºC for 9 hours. All samples were stoppered in atmospheric gas at 500 mTorr, sealed using aluminum caps, and stored at 4 ºC until use. The lyophilization process utilized is a non-optimized developmental process designed to be compatible with a variety of excipient systems. Trehalose was selected as an excipient based on previous observations with other emulsion systems. Heat stressed samples were incubated at 50°C for 30 days and unstressed samples were stored at 4°C prior to injection.
Reducing SDS-PAGE
Reducing SDS-PAGE was performed using Life Technologies (Grand Island, NY) NuPAGE LDS sample buffer, with 1.25% β-mercaptoethanol added, and incubated at 90°C for 15 minutes. Samples were run at 180 V for 65 minutes using 1 μg of ID93 per lane in Life Technologies Novex 4–20% acrylamide tris-glycine precast gel cassettes. Gels were stained overnight using Life Technologies SimplyBlue SafeStain before destaining, drying, and imaging. Band intensities were compared using ImageJ software (NIH) [23].
Particle Analysis
Particle size, polydispersity, and zeta potential measurements were made as described previously[24] using a Malvern (Worcestershire, UK) Nano-ZS after 100 times dilution into ultrapure water filtered througha 20 nm Whatman (Maidstone, Kent, UK) Anotop plus filter. Nanoparticle tracking analysis was performed with a NanoSight LM10 (Amesbury, UK) with a 405 nm laser and a Hamamatsu Orca Flash 2.8 CMOS camera (Hamamatsu, JP). Samples were diluted 1:105 in 20-nm filtered ultrapure water in three steps. Each sample was diluted and analyzed four times, independently, to account for dilution error. Ninety seconds of video were recorded for each sample with optimized shutter and gain settings. The camera histogram gating was adjusted to maximize sensitivity. Data analysis was performed using NanoSight NTA 2.3 software (Wiltshire, UK) in standard mode.
Chemical Integrity of GLA-SE
Concentrations of squalene, DMPC, and GLA were monitored using reversed-phase HPLC (RP-HPLC) as described previously[24]. An Agilent 1200 (Santa Clara, CA) and an ESA Biosciences Corona Charged Aerosol Detector (CAD; Chelmsford, MA) were used with a Waters (Milford, MA) Atlantics C18 5 μm column (4.6 mm × 250 mm). Mobile phase A contained 75:15:10 (v/v/v) methanol, chloroform, and water with 20 mM ammonium acetate and 1 % acetic acid. Mobile phase B contained 50:50 (v/v) methanol and chloroform, 20 mM ammonium acetate, and 1% acetic acid. Samples were prepared by dilution (1:20) into mobile phase B, 9 μL were injected onto a 30°C column, and elution with a gradient of 100% to 10% mobile phase A over 45 minutes was used. Standard curves were fit with a second order polynomial, as recommended by the detector manufacturer, and sample concentrations determined by interpolation.
Animals and immunizations
6–8 week old female C57BL/6 mice were purchased from Charles River maintained in Specific Pathogen Free conditions. After infection animals were maintained in ABSL3 containment according to the regulations and guidelines of the IDRI Institutional Animal Care and Use Committee. Mice were immunized three times three weeks apart by intramuscular injection of 100 μL of the indicated vaccine preparation. For BCG immunization 5×104 CFU (Pasteur strain, Sanofi Pasteur) were injected intradermally once at the time of the first subunit immunization.
Blood cell counts
Peripheral blood was collected from mice (N=5/group) eighteen hours after immunization. Whole blood was stained for CD90.2 (clone 53–2.1) and CD19 (clone 6D5). Sphero AccuCount Rainbow Particles (Spherotech.com) were added according to the manufacturer’s instructions. Cells were washed and resuspended in PBS. Up to 106 events were collected on a four laser LSRFortessa flow cytometer (BD Biosciences). Data were analyzed with FlowJo. Absolute numbers of CD19+ B cells and CD90.2+ T cells per microliter of blood were calculated according to the manufacturer’s instructions.
Antibody responses
Mouse sera (N=5/group) were prepared 21 days after immunization by collection of retro-orbital blood into microtainer serum collection tubes (VWR International, West Chester, PA), followed by centrifugation. Each serum sample was then analyzed by antibody capture ELISA. Briefly, ELISA plates (Nunc, Rochester, NY) were coated with 1 μg/ml recombinant antigen in 0.1 M bicarbonate buffer and blocked with 1% BSA-PBS. Then, in consecutive order and following washes in PBS/Tween20, serially diluted serum samples, anti-mouse IgG, IgG1 or IgG2c-HRP (all Southern Biotech, Birmingham, AL) and ABTS-H2O2 (Kirkegaard and Perry Laboratories, Gaithersburg, MD) were added to the plates. Plates were analyzed at 405nm (ELX808, Bio-Tek Instruments Inc, Winooski, VT).
Intracellular cytokine staining
One month after the final immunization splenocytes were isolated from five animals per group. Red blood cells were lysed using Red Blood Cell Lysis Buffer (eBioscience) and resuspended in RPMI 1640 and 10% FBS. Cells were plated at 2×106 cells/well in 96-well plates and were stimulated for 1 hour with media or ID93 (10 μg/mL) at 37°C. GolgiPlug (BD Biosciences) was added and the cells were incubated for an additional 7 hours at 37°C. Cells were washed and surface stained with fluorochrome labeled antibodies to CD4 (clone GK1.5), CD8 (clone 53–6. 7), and CD44 (clone IM7) (BioLegend and eBioscience) in the presence of anti-CD16/32 (clone 2.4G2) for 20 minutes at 4°C. Cells were washed and permeabilized with Cytofix/Cytoperm (BD Biosciences) for 20 minutes at room temperature. Cells were washed twice with Perm/Wash (BD Biosciences) and stained intracellularly with fluorochrome labeled antibodies to IFN-γ (clone XMG-1.2), IL-2 (JES6-5H4), TNF (MP6-XT22), CD154 (clone MR1), IL-5 (clone TRFK5), and IL-17A (clone TC11-18H10.1) (BioLegend and eBioscience) for 20 minutes at room temperature. Cells were washed and resuspended in PBS. Up to 106 events were collected on a four laser LSRFortessa flow cytometer (BD Biosciences). Data were analyzed with FlowJo. Cells were gated as singlets > lymphocytes > CD4+ CD8− > CD44+ > cytokine positive.
M.tb. aerosol challenge and enumeration
Four weeks after the last immunization, mice (n = 7/group) were aerogenically infected with M. tuberculosis H37Rv (ATCC No. 35718; American Type Culture Collection) using a GlasCol aerosol generator calibrated to deliver 50–100 bacteria into the lungs. To confirm the amount of bacteria delivered an additional three unimmunized animals per infection were euthanized one day later and bacterial burden in the lungs were enumerated. Protection was determined three weeks after challenge by harvesting the lungs from the infected mice, homogenizing the tissue in 0.1% PBS–Tween 80, and plating 5-fold serial dilutions on7H10 agar plates (Molecular Toxicology) for bacterial growth. Bacterial colonies were counted after incubation at 37°C with 5% CO2 for 14–21 days.
Statistical methods
Bacterial burdens were normalized by log10 transformation. Statistical significance of differences in bacterial burdens, cytokine production, blood cell counts, and antibody titers were determined using one-way analysis of variance with the Sidak Multiple Comparisons Test using Prism 5 (GraphPad Software).
Results
Physicochemical Characterization
One approach to improving vaccine thermostability is to lyophilize the antigen component of the vaccine, which is then mixed with the adjuvant at the time of usage. However this requires cold-chain maintenance for the adjuvant and increases the technological burden of vaccination. To surmount this problem we have developed a single vial of both the antigen ID93 and GLA-SE adjuvant (termed “covialed”). We have subsequently developed a lyophilization regimen for this covialed adjuvanted vaccine. Upon lyophilization, a white, partially shrunken cake is formed, and, after reconstitution with water, the emulsion reforms and appears similar to the pre-lyophilized emulsion (Figure 1). The potential to increase stability to heat stress by lyophilization was evaluated by incubating liquid or lyophilized ID93 + GLA-SE at 50°C for 30 days. After heat stress, no visible change in sample quality was observed (Figure 1, bottom row) when compared to unstressed sample (top row). Reconstituted samples maintained the appearance of an emulsion and lyophilized cakes did not show any further signs of collapse or discoloration.
Figure 1. Lyophilization and reconstitution do not affect the appearance of ID93+GLA-SE.
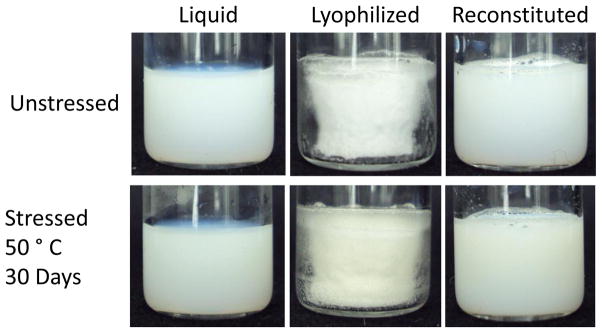
Representative images of liquid (left), lyophilized (center), and reconstituted (right) vials of ID93 + GLA-SE. Vials were unstressed (top row) or stressed (bottom row) at 50°C for 30 days.
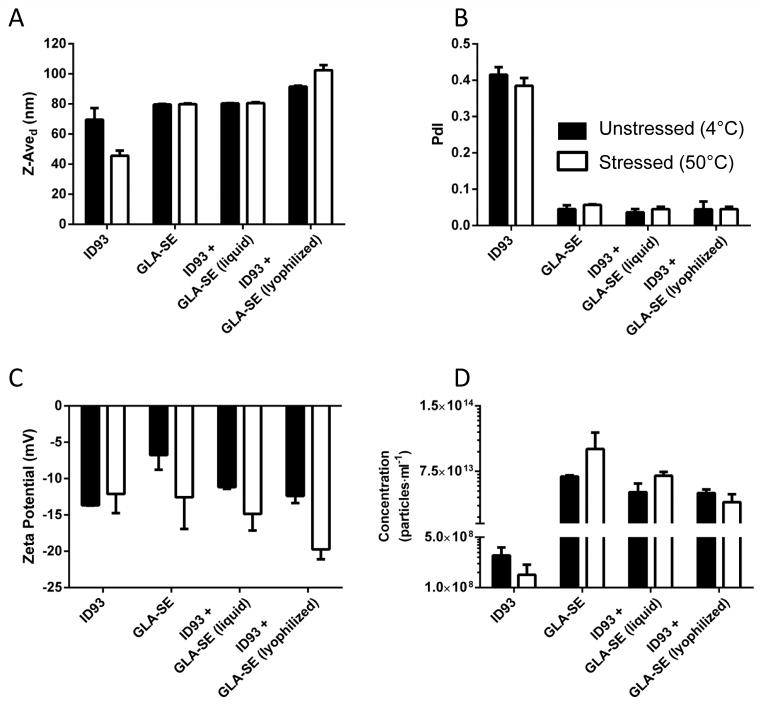
Particle characteristics are critical for effective vaccine development as particle size determines the speed and mechanism of vaccine trafficking in vivo. Maintenance of particle size below 200 nm is desirable in order to allow terminal sterile filtration of the product; in addition, particle sizes <200 nm are able to rapidly access the lymph node [25]. To assess whether covialing or lyophilization and reconstitution of covialed ID93+GLA-SE altered the biophysical properties we examined the particle size, concentration, polydispersity, and overall zeta potential of ID93, GLA-SE, covialed ID93+GLA-SE and lyophilized covial ID93+GLA-SE. Measured particle characteristics after mixing were primarily reflective of the contribution of GLA-SE due to the five orders of magnitude higher particle concentration as compared to ID93. Covialing ID93 and GLA-SE did not affect the particle size relative to GLA-SE alone (80 nm in both cases) (Figure 2A). Lyophilization and subsequent reconstitution of ID93+GLA-SE resulted in a minor increase of approximately 10 nm, within the error of the measurement. ID93 forms polydisperse aggregates with a Z-average diameter of approximately 70 nm. Heat stress of ID93 alone reduced the average particle size observed; however, this was not statistically significant (P>0.05) (Figure 2A). Heat stress of GLA-SE alone or in combination with ID93 did not affect particle size or concentration across any of the platforms tested (Figure 2A and D). GLA-SE is a highly homogenous solution as reflected by the low polydispersity value (Figure 2B). Although the degree of polydispersity observed for ID93 is much higher, the mixture of ID93 and GLA-SE retains the overall low polydispersity characteristic of GLA-SE, reflective of the relative proportions of ID93 and GLA-SE particles. Importantly, lyophilized and reconstituted ID93+GLA-SE retained this uniform particle size. Exposure to heat stress did not affect the polydispersity of ID93, GLA-SE, or covialed ID93+GLA-SE in liquid or lyophilized formats. Both ID93 and GLA-SE have an overall negative zeta potential in the current configuration. Mixing the two resulted in an average zeta potential of -13 mV, which was unaffected by lyophilization (Figure 2C). Upon heat stress a more negative zeta potential was seen for all GLA-SE containing samples; however, this change was only statistically significant for lyophilized ID93 + GLA-SE (P<0.025). Thus overall we find that lyophilization and reconstitution of ID93+GLA-SE results in only minor alterations to the physicochemical characteristics when compared to non-lyophilized ID93+GLA-SE. Similarly sustained exposure to elevated temperatures did not have a pronounced impact on the physicochemical characteristics of the vaccine.
Figure 2. Lyophilization and heat stress do not significantly alter the physicochemical characteristics of ID93+GLA-SE.
Particle characterization of liquid and reconstituted lyophilized samples containing ID93 and/or GLA-SE as indicated in the figure labels. (A) Z-Average diameter and (B) polydispersity index from DLS experiments are shown. (C) Zeta potential measurements and (D) particle concentration measurements from nanoparticle tracking analysis are also shown. Filled bars represent unstressed samples (i.e. stored at 4 °C for 30 days) and open bars represent samples stressed at 50°C for 30 days.
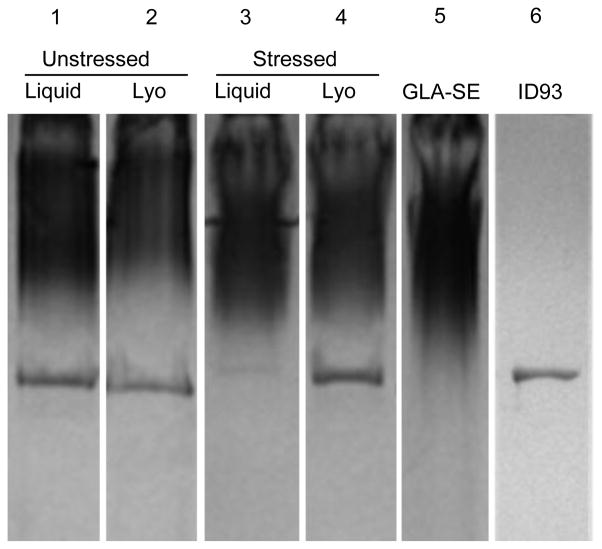
To assess how covialing, lyophilization, and heat stress affect the chemical integrity of ID93+GLA-SE, we evaluated ID93 concentration by SDS PAGE and GLA, squalene and DMPC (the latter two are the major components of the stable nanoemulsion) concentrations by RP-HPLC. Samples containing 100 μg/mL ID93 were evaluated due to the inability to detect ID93 at 5 μg/mL by SDS-PAGE. 100 μg/mL and 5 μg/mL ID93-containing covialed samples behave similarly in terms of particle size, particle concentration, zeta potential, and GLA degradation profiles under liquid, lyophilized, and heat stressed conditions (data not shown). GLA-SE runs as a defuse smear, likely due to the disruption of nanoemulsion particles by SDS, and is visible after staining. Lyophilization and reconstitution of covialed ID93+GLA-SE resulted in a 5–10% decrease in ID93 concentration, expected due to dilution upon reconstitution, indicating that substantial hydrolysis of ID93 has not occurred (Figure 3). Upon exposure to heat stress at 50°C for one month there was a dramatic reduction in the ID93 present in ID93+GLA-SE. Lyophilization of ID93+GLA-SE rendered the protein resistant to this degradation with 6% and 90% of the ID93 band intensity observed after heat stress for the liquid and lyophilized samples, relative to the unstressed samples, respectively (Figure 3). Thus lyophilization of ID93+GLA-SE protected the ID93 protein from heat stress-induced degradation.
Figure 3. Lyophilization of ID93+GLA-SE prevents heat induced loss of ID93.
Reducing SDS-PAGE with SimplyBlue™ staining of covialed ID93 + GLA-SE liquid (lanes 1 and 3) and reconstituted lyophilized samples (lanes 2 and 4). Samples were either unstressed (lanes 1 and 2) or stressed (lanes 3 and 4) at 50°C for 30 days. Unstressed GLA-SE and ID93 are shown for comparison (lanes 5 and 6, respectively).
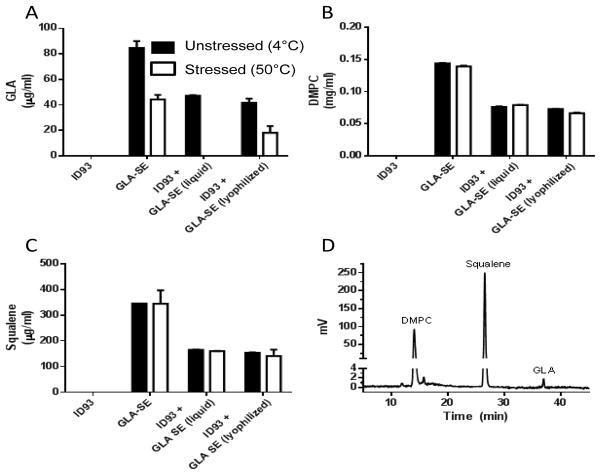
As expected, after mixing GLA-SE 1:1 with ID93, approximately half of the original concentration of GLA, DMPC, and squalene were measured, and no material was lost after lyophilization and reconstitution (Figure 4A). Exposure of liquid GLA-SE to heat stress caused a 50% loss of GLA concentration (P<0.001). This was exacerbated by covialing with ID93 to the point that there was no detectable GLA after heat stress (Figure 4A). This enhanced susceptibility may be due to the more basic pH of the covialed ID93+GLA-SE compared to GLA-SE alone. This loss of GLA was ameliorated by lyophilization of the covialed ID93+GLA-SE, with ~50% of the GLA recovered after reconstitution of the heat stressed lyophilized ID93+GLA-SE (Figure 4D). GLA was the major heat labile component of GLA-SE as neither the DMPC nor the squalene concentration was affected by heat stress (Figure 4B–D). Taken together, these data show that the two active components of ID93+GLA-SE were protected from heat induced degradation by lyophilization.
Figure 4. Lyophilization of ID93+GLA-SE limits heat induced loss of GLA.
Chemical integrity of adjuvant components was determined by reversed-phase HPLC. Liquid and reconstituted lyophilized samples containing ID93 and/or GLA-SE, as indicated in the figure labels, were analyzed. (A) GLA, (B) DMPC, and (C) squalene concentration were determined from standard curves and (D) a representative chromatogram is shown. Filled bars represent unstressed samples and open bars represent samples stressed at 50°C for 30 days.
In summary, lyophilization of ID93+GLA-SE results in a white to off-white cake that retains the chemical and biophysical properties of covialed ID93+GLA-SE upon reconstitution. Exposure of ID93+GLA-SE to heat stress results in a significant loss of both ID93 and GLA. Lyophilization of covialed ID93+GLA-SE largely ameliorated these losses due to heat stress, indicating that this approach may reduce or eliminate the need for cold-chain maintenance of this vaccine candidate.
Vaccine Immunogenicity and Efficacy
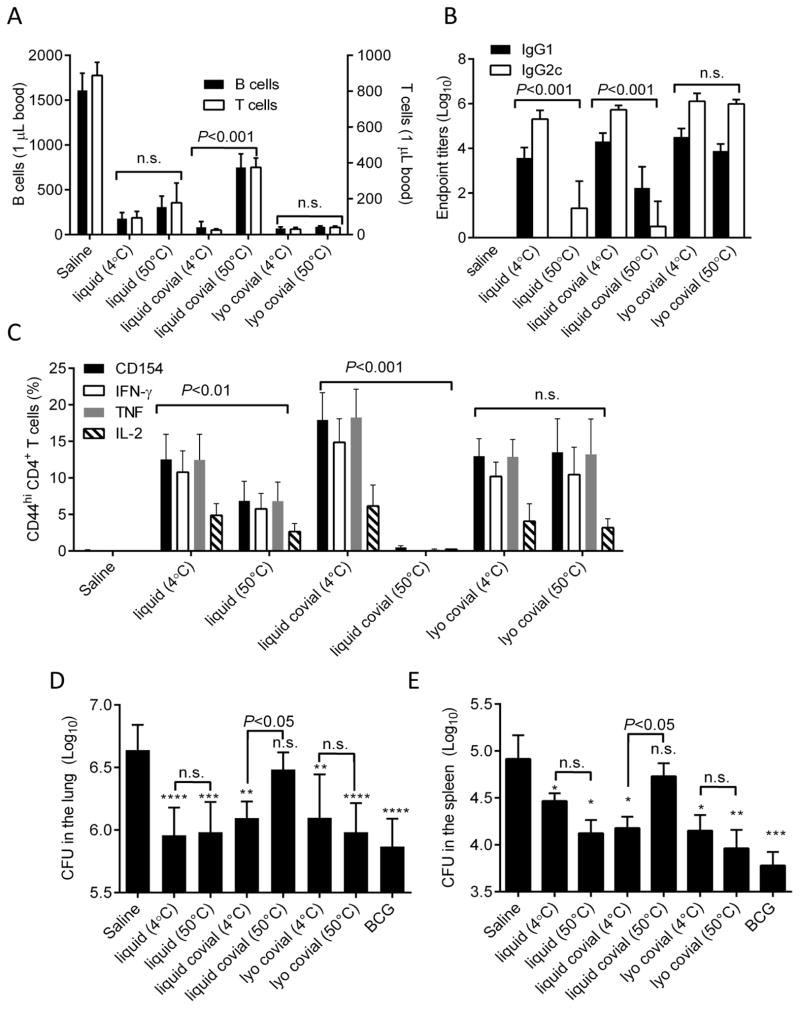
To determine how heat stress affected the biological activity of ID93+GLA-SE and whether lyophilization ameliorated any detrimental effects we immunized mice with saline or ID93+GLA-SE that was stored as separate vials of liquid antigen and adjuvant (liquid), a mixture of antigen and adjuvant (liquid covial) or co-lyophilized antigen and adjuvant (lyo covial). Immunization material was stored at either 4°C (i.e. unstressed) or 50°C (i.e. heat stress) for one month prior to immunization. Following immunization there is a transient loss of circulating B and T cells from the blood as these cells home to the draining lymph node where they encounter antigen [26]. This transient lymphopenia has been termed lymph node shutdown as lymphocytes become transiently trapped in the lymph node. This process is necessary for efficient interactions between antigen presenting cells and cognate lymphocytes. The GLA-SE adjuvant augments this effect which may in part account for its excellent adjuvant activity. Immunization with unstressed liquid ID93+GLA-SE elicited a dramatic loss of both B and T cells from the blood (Figure 5A). Stressing the liquid ID93+GLA-SE at 50°C for a month reduced this effect, suggesting that the activity of GLA was impaired by heat stress. Unstressed liquid covial ID93+GLA-SE induced lymph node shutdown as efficiently as the liquid vaccine, however this liquid covial material was more affected by heat stress as the degree of lymph node shutdown was markedly reduced, likely reflective of the loss of detectable GLA (Figure 5A). Lyophilized covial ID93+GLA-SE elicited transient lymphopenia to a similar degree to the liquid material, however unlike the liquid covial material this effect was not impaired by heat stress of the lyophilized covial vaccine. These data suggest that biological activity of the GLA-SE adjuvant is susceptible to heat stress and this is exacerbated by covialing with the ID93 antigen. Importantly, lyophilization rendered covialed ID93+GLA-SE resistant to the damages of heat stress as read out by this parameter.
Figure 5. Lyophilization of ID93+GLA-SE prevents loss of biological activity due to heat stress.
Mice were immunized with saline or liquid, liquid covial, or lyophilized covial ID93+GLA-SE exposed to 4°C or 50°C for one month. (A) B and T cell blood counts were determined 18 hours after immunization. (B) ID93-specific serum antibody titers were determined three weeks after the first immunization. (D) The frequency of ID93-specific CD4 T cells in the spleen were evaluated one month after the final immunization by analyzing cytokine production following in vitro restimulation with ID93. One month after the final immunization animals were challenged with a low dose of aerosolized M. tuberuculosis. Bacterial burden in the (D) lung and (E) spleen were determined three weeks later. Data are displayed as mean + s.d. of N=5 −7 mice/group. Data are shown from one of two experiments with similar results. *, **, ***, and **** indicate P<0.05, 0.1, 0.001 and 0.0001, respectively, relative to saline. n.s. not significant relative to saline. Statistical comparisons between 4ºC and 50ºC samples are indicated.
To more fully examine the impacts of heat stress and lyophilization on the biological activity of ID93+GLA-SE we evaluated ID93-specific antibody titers following immunization. Immunization with ID93+GLA-SE elicits a mixed IgG1 and IgG2c response that is skewed towards IgG2c production. This is reflective of the IFN-γ dominated CD4 T cell responses produced by ID93+GLA-SE [19]. Exposure to heat stress significantly impaired the ability of liquid ID93+GLA-SE to elicit measurable antibody titers (Figure 5B). This is likely due to degradation of the ID93 protein upon heat stress (Figure 3). Although covialing ID93+GLA-SE did not alter the magnitude of the antibody response when the vaccine was stored at 4°C, this was not sufficient to prevent loss of the antibody-inducing potential caused by heat stress. Conversely the lyophilized covial ID93+GLA-SE elicited robust antibody responses similar in magnitude and IgG1/IgG2c skewing to the liquid unstressed material and this was not impaired by heat stress (Figure 5B).
ID93+GLA-SE protects against M. tuberculosis by inducing ID93-specific CD4 T cells that make IFN-γ, TNF, and IL-2 (i.e. TH1 cells). Exposure to heat stress reduced the frequency of ID93-specific TH1 cells as measured by production of any of these cytokines by almost 50% following the third immunization with liquid ID93+GLA-SE (Figure 5C). That the TH1 response to stressed liquid ID93+GLA-SE is maintained despite degradation of the ID93 protein likely reflects the presence of immunogenic peptides and residual GLA after heat exposure. Covialing of liquid ID93+GLA-SE slightly enhanced the magnitude of the TH1 response when stored at 4°C, however exposure to heat stress completely ablated the ability of liquid covial ID93+GLA-SE to elicit such response. Lyophilized covial ID93+GLA-SE induced TH1 responses similar to that produced with liquid ID93+GLA-SE. Critically, unlike liquid or liquid covialed ID93+GLA-SE, lyophilized covial ID93+GLA-SE fully retained the ability to elicit ID93-specific TH1 cells following heat stress (Figure 5C). We have found previously that ID93-specific CD4 T cells elicited immunization with native ID93+GLA-SE are exclusively TH1 cells, failing to produce IL-5 (TH2) or IL-17 (TH17) upon restimulation [27]. Covialing, lyophilization, and/or exposure to heat stress did not enhance the induction of either TH2 or TH17 cells by ID93+GLA-SE as measured by detectable IL-5 or IL-17 production (data not shown). Production of CD154 following stimulation has been proposed to be a generalized marker of CD4 T cell activation regardless of cytokine production [28]. In all cases CD154 expression levels closely mirrored that of both IFN-γ and TNF, further indicating that there was no deviation from the TH1 programming. Overall the early impairment lymphocyte egress from the blood (Figure 5A) strongly correlated with the subsequent loss of both the antibody (Figure 5B) and CD4 T cell response (Figure 5C) to ID93+GLA-SE vaccination.
To assess how heat stress, covialing, and lyophilization affected the protective efficacy of ID93+GLA-SE we challenged immunized mice with a low dose of aerosolized M. tuberculosis. Three weeks later animals immunized with liquid ID93+GLA-SE were significantly protected against M. tuberculosis relative to the saline immunized animals as measured by reduced bacterial burdens in the lungs and spleen (Figure 5D and E). Heat stressing liquid ID93+GLA-SE separately did not impair this protective efficacy, likely reflective of the residual ID93-specific TH1 response elicited by this immunization (Figure 5C). Covialing of ID93+GLA-SE did not impair protective efficacy when stored at 4°C, but the liquid covialed vaccine lost all protective efficacy when exposed to heat stress. Lyophilization of the covialed ID93+GLA-SE maintained protective efficacy and most importantly lyophilization of ID93+GLA-SE abrogated the loss of protective efficacy due to heat stress (Figure 4D and E).
Discussion
The need for increasing the thermostability of vaccines intended for distribution to the developing world requires special considerations to be taken in the formulation stage of development. Successful lyophilization of multi component systems, including covialed antigen and adjuvant systems, have rarely been described in the literature[29], and present additional challenges that must be addressed. Lyophilization of protective antigen of anthrax in a squalene emulsion has been reported; however the formulation was not reported to contain a bulking agent and therefore would not have produced a cake structure when lyophilized. Importantly neither a description of the thermostability nor a biophysical characterization of the reconstituted system were included [30]. Lyophilization of our system resulted in a product that, when reconstituted, retained the biophysical properties of the liquid sample, and conveyed stability to heat stress under accelerated conditions. Long-term stability studies are underway.
Colyophilization and reconstitution of the antigen and nanoemulsion adjuvant did not significantly alter the physicochemical characteristics of the vaccine. Upon reconstitution the concentrations of both the antigen and the TLR4 agonist GLA, as well as the squalene oil, were not substantially different than that of the starting material. Prolonged exposure to heat stress had little effect on the physical characteristics of either the liquid covialed or colyophilized vaccine, however heat exposure led to chemical degradation of both the antigen and the TLR4 agonist, but not the squalene or phospholipid components of the adjuvant. Colyophilization partially protected against this loss of TLR4 agonist. Further refinements to the lyophilization process including optimization of the lyophilization process and stabilizing excipients will be necessary to enhance thermostability of the colyophilized vaccine.
The loss of GLA due to heat stress was strongly predictive of the impaired immune responses and protective efficacy of the vaccine when administered to experimental animals. Although this relationship was not completely linear, the reduction in GLA in the liquid samples resulted in decreased frequencies of ID93 specific CD4 T cells after immunization. The complete loss of GLA in the heat stressed liquid covialed ID93+GLA-SE matches the loss of CD4 T cell induction. Conversely the degradation of the ID93 protein in the liquid samples had little impact on the magnitude of the CD4 T cell response. This can likely be attributed to retention of the immunodominant peptides necessary to prime the T cell response in the heat stressed samples. On the other hand heat induced degradation of ID93 significantly impaired the magnitude of the antibody response to the vaccine. This is not surprising as many of the ID93-specific antibodies may be conformationally dependent. Futher the residual antibody response was preferentially IgG1 indicating a loss of GLA driven IgG2c skewing. Colyophilization of ID93+GLA-SE largely prevented heat stress induced loss of ID93-specific antibody responses, indicating that the protein structure was protected by this process. This would suggest that protection of antigens against heat stress is a more critical parameter for vaccines that rely on antibody responses for protective efficacy than vaccines such as ID93+GLA-SE that rely on T cells for protective efficacy. Indeed the heat stressed separately vialed liquid vaccine retained a degree of protective efficacy against experimental challenge with aerosolized Mtb. As we have found previously, induction of a TH1 response against ID93 correlates with vaccine efficacy [19]; however, there is not always a direct correlation between TH1 magnitude and magnitude of protective efficacy. This is likely due to limitations of the Mtb infection model in mice. Despite these caveats there is a clear relationship between the ability to retain GLA concentration by lyophilization in the face of thermal stress, the retention of TH1 induction, and maintenance of protective efficacy. Further optimization of the lyophilization process will focus on minimizing the loss of GLA concentration during thermal and other stress events. Additional optimization may be needed to minimize the increased thermal sensitivity of GLA when ID93 and GLA-SE are covialed.
Overall we find that covialing of liquid ID93+GLA-SE does not impair either its immunological activity or the resulting protective efficacy against M.tb. as long as the cold chain is maintained. Covialing has very practical benefits of a decreased burden for shipping and storage resources as a vaccine against M.tb. is implemented globally. Lyophilization of the covialed ID93+GLA-SE rendered the vaccine impervious to the detrimental effects of prolonged exposure to elevated temperatures. By reducing the need for cold chain maintenance we can significantly reduce the practical barriers to delivering a candidate tuberculosis vaccine – and therefore increasing accessibility worldwide. Of note the GLA-SE adjuvant is also included in a number of other vaccine candidates in clinical trials including vaccines for malaria, leishmaniasis, and schistosomiasis. In addition, other formulations of GLA are being considered for vaccines against influenza, HIV, and hookworm. Vaccines against all of these diseases will benefit from the reduced cold-chain requirement achieved by successful lyophilization of these adjuvants.
Acknowledgments
We thank Valerie Reese, David Argilla, Thomas Hudson, Brian Granger, Dean Huang, Ghislain Nana, Susan Lin, Traci Mikasa, and Milllie Fung for excellent technical assistance. The project described was supported by Grant U01AI078054 and Contract HHSN272200800045C from the National Institute of Allergy and Infectious Diseases to RC and grants 42387 and OP1055855 from the Bill and Melinda Gates Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy And Infectious Diseases or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.PATH. Summary of stability data for licensed vaccines. Seattle, WA: 2012. Working in Tandem Ltd. [Google Scholar]
- 2.Chen D, Zehrung D. Desirable attributes of vaccines for deployment in low-resource settings. J Pharm Sci. 2013;102:29–33. doi: 10.1002/jps.23352. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Pritchard E, Hu X, Valentin T, Panilaitis B, Omenetto FG, Kaplan DL. Stabilization of vaccines and antibiotics in silk and eliminating the cold chain. Proc Natl Acad Sci. 2012;109:11981–11986. doi: 10.1073/pnas.1206210109. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Wang W. Lyophilization and development of solid protein pharmaceuticals. Int J Pharm. 2000;203:1–60. doi: 10.1016/s0378-5173(00)00423-3. [DOI] [PubMed] [Google Scholar]
- 5.Kasper JC, Winter G, Friess W. Recent advances and further challenges in lyophilization. Eur J Pharm Biopharm. 2013 doi: 10.1016/j.ejpb.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30:23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration. Vaccines and Related Biological Products Advisory Committee Meeting; 2012. [Google Scholar]
- 8.Clausi AL, Merkley SA, Carpenter JF, Randolph TW. Inhibition of aggregation of aluminum hydroxide adjuvant during freezing and drying. J Pharm Sci. 2008;97:2049–2061. doi: 10.1002/jps.21143. [DOI] [PubMed] [Google Scholar]
- 9.Rossi J, Leroux JC. Principles in the development of intravenous lipid emulsions. In: Wasan KM, editor. Role of Lipid Excipients in Modifying Oral and Parenteral Drug Delivery: Basic Principles and Biological Examples. John Wiley & Sons, Inc; Hoboken, NJ: 2007. pp. 88–123. [Google Scholar]
- 10.WHO global tuberculosis control report 2010. Summary, Cent Eur J Public Health. 2010;18:237. [PubMed] [Google Scholar]
- 11.Checkley AM, McShane H. Tuberculosis vaccines: progress and challenges. Trends Pharmacol Sci. 2011;32:601–606. doi: 10.1016/j.tips.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Rowland R, McShane H. Tuberculosis vaccines in clinical trials. Expert Rev Vaccines. 2011;10:645–658. doi: 10.1586/erv.11.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat Rev Microbiol. 2005;3:656–662. doi: 10.1038/nrmicro1211. [DOI] [PubMed] [Google Scholar]
- 14.Abu-Raddad LJ, Sabatelli L, Achterberg JT, Sugimoto JD, Longini IM, Jr, Dye C, Halloran ME. Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proc Natl Acad Sci U S A. 2009;106:13980–13985. doi: 10.1073/pnas.0901720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 16.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertholet S, Ireton GC, Ordway DJ, Windish HP, Pine SO, Kahn M, Phan T, Orme IM, Vedvick TS, Baldwin SL, Coler RN, Reed SG. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci Transl Med. 2010;2:53ra74. doi: 10.1126/scitranslmed.3001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertholet S, Ireton GC, Kahn M, Guderian J, Mohamath R, Stride N, Laughlin EM, Baldwin SL, Vedvick TS, Coler RN, Reed SG. Identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis. J Immunol. 2008;181:7948–7957. doi: 10.4049/jimmunol.181.11.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orr MT, Fox CB, Baldwin SL, Sivananthan SJ, Lucas E, Lin S, Phan T, Moon JJ, Vedvick TS, Reed SG, Coler RN. Adjuvant formulation structure and composition are critical for the development of an effective vaccine against tuberculosis. J Control Release. 2013;172:190–200. doi: 10.1016/j.jconrel.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ching LK, Mompoint F, Guderian JA, Picone A, Orme IM, Coler RN, Reed SG, Baldwin SL. Transcriptional profiling of TLR-4/7/8-stimulated guinea pig splenocytes and whole blood by bDNA assay. J Immunol Methods. 2011;373:54–62. doi: 10.1016/j.jim.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson RC, Fox CB, Dutill TS, Shaverdian N, Evers TL, Poshusta GR, Chesko J, Coler RN, Friede M, Reed SG, Vedvick TS. Physicochemical characterization and biological activity of synthetic TLR4 agonist formulations. Colloids Surf B Biointerfaces. 2010;75:123–132. doi: 10.1016/j.colsurfb.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 22.Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, Laughlin EM, Duthie MS, Fox CB, Carter D, Friede M, Vedvick TS, Reed SG. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS One. 2011;6:e16333. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox CB, Anderson RC, Dutill TS, Goto Y, Reed SG, Vedvick TS. Monitoring the effects of component structure and source on formulation stability and adjuvant activity of oil-in-water emulsions. Colloids and Surfaces B: Biointerfaces. 2008;65:98–105. doi: 10.1016/j.colsurfb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nature Rev Immunol. 2010;10:787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 26.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 27.Orr MT, Duthie MS, Windish HP, Lucas EA, Guderian JA, Hudson TE, Shaverdian N, O’Donnell J, Desbien AL, Reed SG, Coler RN. MyD88 and TRIF synergistic interaction is required for TH1-cell polarization with a synthetic TLR4 agonist adjuvant. Eur J Immunol. 2013;43:2398–2408. doi: 10.1002/eji.201243124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M, Scheffold A, Thiel A. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med. 2005;11:1118–1124. doi: 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- 29.TKA, Anchordoquy Thomas J, Molina Marion dC, Dean Allison S, Ye Zhang MMP, Lentz Yvonne K, Koe Gary S. Lyophilization of Biopharmaceuticals. American Association of Pharmaceutical Scientists; 2004. [Google Scholar]
- 30.Ivins B, Fellows P, Pitt L, Estep J, Farchaus J, Friedlander A, Gibbs P. Experimental anthrax vaccines: efficacy of adjuvants combined with protective antigen against an aerosol Bacillus anthracis spore challenge in guinea pigs. Vaccine. 1995;13:1779–1784. doi: 10.1016/0264-410x(95)00139-r. [DOI] [PubMed] [Google Scholar]