Abstract
Objective
Primary mediastinal germ-cell tumors are rare, and the effect of newer drugs and treatment strategies in this disease on overall survival is not known. We retrospectively assessed treatment outcomes at a single institution.
Materials and methods
We identified men seen at our institution from 1998 through 2005 for mediastinal germ-cell tumors. Medical records were reviewed for patient characteristics, histology, tumor markers, treatment, and survival outcome.
Results
Thirty-four patients met study criteria, of whom 27 had nonseminomatous germ-cell tumor (NSGCT) and 7 had pure seminoma. Eleven patients (41%) with NSGCT were alive at last contact with a median overall survival time of 33.5 months. Among 13 patients with NSGCT referred to us at initial diagnosis, 7 (54%) were alive and recurrence-free at a median follow-up of 56.5 months. Progression-free survival was associated with absence of risk factors (any histology other than endodermal sinus tumor, β-hCG > 1000 mIU/mL, or disease outside the mediastinum). For the patients whose disease progressed (n = 5) or who had been referred to us for salvage treatment (n = 14), the 3-year overall survival from the date of first progression was 23%. Conversely, patients with seminoma did uniformly well with platinum-based chemotherapy; most did not undergo radiation or surgery.
Conclusion
Chemotherapy given to maximum effect followed by surgical consolidation resulted in long-term progression-free survival for 54% of patients with mediastinal NSGCT. The number of risk factors present at diagnosis may be associated with survival outcome and should be studied in a larger test group.
Keywords: Mediastinal neoplasms, Germ-cell neoplasms, Seminoma, Tumor markers, Resection, Outcome
1. Introduction
Extragonadal germ-cell tumors comprise a rare subgroup of germ-cell neoplasms, most of which arise in the anterior mediastinum [1-3]. Primary mediastinal germ-cell tumors (PMGCTs) account for less than 5% of germ-cell malignancies and are more common in men but also occur in women. PMGCTs in men display a different biological behavior than do primary tumors of the testis or PMGCTs in women.
With standard chemotherapy consisting of 4 courses of bleomycin, etoposide, and cisplatin (BEP), men with nonseminomatous PMGCT have a worse prognosis for survival than do those with mediastinal seminoma or primary tumors of the testis [4]. The reported long-term survival rate for patients treated with chemotherapy in the 1970s and 1980s, not all of whom received cisplatin, was 20% to 27% [5,6]. A report from Indiana University [7] described 31 patients with nonseminomatous PMGCT who received cisplatin, bleomycin, and either vinblastine (20 patients) or etoposide (11 patients) followed by surgical resection; 15 patients (48%) achieved long-term disease-free survival. In a retrospective study of 64 patients with mediastinal nonseminomatous germ-cell tumors (NSGCTs) treated in France from 1983 through 1990, the estimated 2-year overall survival rate was 53% [8]. More recently, a 5-year overall survival rate of 45% was reported in an international analysis of 141 patients with mediastinal NSGCT from 11 cancer centers, treated from 1975 through 1996 in the United States and Europe [9].
The optimal strategy for achieving long-term survival in patients with mediastinal NSGCT remains to be defined. High-dose chemotherapy with autologous peripheral blood stem cell transplantation was studied in the salvage setting [10–12] and as first-line treatment [13] for patients with mediastinal NSGCT and other poor-risk patients, and was not more effective than standard chemotherapy. Other treatment variables that may determine survival outcome include the duration of chemotherapy, use of additional drugs (e.g., taxanes, anthracyclines, or alkylating agents), and timing of surgery. To help identify successful treatment practices and predictors of survival, we performed a retrospective analysis of the recent experience with PMGCTs at The University of Texas M. D. Anderson Cancer Center.
2. Materials and methods
2.1. Patient selection
From a clinical database, we obtained a list of all patients with extragonadal germ-cell tumors who had been seen at M. D. Anderson Cancer Center from 1998 through 2005. After obtaining approval from our institutional review board, we evaluated the records and identified 34 men with PMGCT who had been seen during the study period. Patients were included in the analysis regardless of whether they had been treated or had experienced treatment failure before their referral to us. We retrieved medical records from the referring physicians for patients who had received treatment before their first encounter at M. D. Anderson and for their most recent follow-up. We reviewed the records to determine the patients' characteristics, histologic and tumor marker evaluation results, treatments given, and survival outcomes. Reported patient deaths were also verified with the online Social Security Death Index.
2.2. Treatment
The chemotherapy regimens that had been given and the duration of treatment in each case had been determined by the treating physician. Patients with NSGCT had been offered post-chemotherapy surgical consolidation if they had anatomically resectable disease. Postoperative adjuvant chemotherapy or radiotherapy had been administered at the discretion of the treating physician and with the recommendations of a multidisciplinary team.
2.3. Histopathologic analysis
The pathologic assessment of resected tissue was evaluated for each patient. Residual disease was characterized according to whether elements of viable germ-cell malignancy, non-germ-cell malignancy, or teratoma were present.
2.4. Statistical analysis
Overall survival time was calculated from the date of the initial clinical diagnosis of PMGCT until the patient's death or last follow-up. Progression-free survival (PFS) was calculated as the time from diagnosis to progression, relapse, or death from any cause. Time to progression (TTP) was calculated as the time from diagnosis to progression or relapse. The median survival times were estimated by using the Kaplan-Meier method. For patients who were still alive at the end of our study period, we censored the overall survival data at the latest date when patients were known to be alive.
We developed a risk factor scoring system in which 1 point was given for each of 3 risk factors at diagnosis: extramediastinal disease, histologic characteristics other than those of endodermal sinus tumor (EST), and serum β human chorionic gonadotropin (β-hCG) concentration > 1,000 mIU/mL, for a maximum score of 3 points. Subgroup analysis of TTP was performed using the log-rank test. The significance level was set at 0.05 for all tests.
3. Results
3.1. Patients' characteristics
Among the 34 patients whose records we included, 27 had had NSGCT according to the results of histologic and serum tumor marker evaluations and 7 had had pure seminoma. Eleven of the patients (41%) with NSGCT had had evidence of disease outside the mediastinum at diagnosis; the lungs were the most common site of metastasis (Table 1). Lymph nodes were the only site of metastasis among patients with pure seminoma and were present in 2 patients (29%).
Table 1. Characteristics of 34 patients with primary mediastinal germ cell tumors.
| Characteristic | Nonseminoma n = 27 | Seminoma n = 7 |
|---|---|---|
| At diagnosis | ||
| Median age, years (range) | 30 (20–53) | 32 (20–60) |
| Site of metastasis, no. (%) | ||
| Lung | 7 (26) | 0 |
| Liver | 2 (7) | 0 |
| Bone | 1 (4) | 0 |
| Blood/bone marrow | 1 (4) | 0 |
| Lymph nodes | 0 | 2 (29) |
| None detected | 16 (59) | 5 (71) |
| At time of referral to M. D. Anderson Cancer Center | ||
| Median days from diagnosis to first visit (range) | 145 (0–673) | 26 (0–164) |
| Untreated (%) | 6 (22) | 5 (71) |
| Treated and no progression (%) | 7 (26) | 2 (29) |
| Treated and progressed or relapsed (%) | 14 (52) | 0 |
| Prior treatment modality | ||
| Surgery (%) | 10 (37) | 0 |
| Radiotherapy (%) | 2 (7) | 1 (14) |
| Chemotherapy (%) | 18 (67) | 1 (14) |
| 1–4 courses | 12 | 1 |
| More than 4 courses | 6 | 0 |
3.2. Histologic and tumor marker evaluations
The histologic features of NSGCT were mixed in 15 patients (56%), EST in 6 patients (22%), poorly differentiated carcinoma in 3 patients (11%), choriocarcinoma in 2 patients (7%), and embryonal carcinoma in 1 patient (4%). The serum β-hCG concentration at diagnosis was known for 20 patients with NSGCT; 4 of those patients had concentrations of greater than 1,000 mIU/mL (Table 2).
Table 2. Median TTP in patients with mediastinal nonseminomatous germ cell tumors.
| No. patients | Median TTP, months (range) | Log-rank P | |
|---|---|---|---|
| Newly diagnosed patients, n = 13 | |||
| Extramediastinal metastases: | |||
| Present | 6 | 18.9 (1.4, 52.5+) | |
| Absent | 7 | NE (22.6, 110.3+) | 0.03 |
| Histologic diagnosis: | |||
| Mixed | 8 | 23 (1.4, 52.5+) | |
| Single, EST | 4 | NE (56.6, 110.3+) | 0.09 |
| Single, non-EST | 1 | 14.8 (14.8) | |
| β-hCG concentration: | |||
| ≤1000 mIU/mL | 9 | NE (1.4, 100.0+) | |
| >1000 mIU/mL | 2 | 13.6 (12.4, 14.8) | |
| Unknown | 2 | NE (23.0, 110.3+) | 0.03 |
| Patients referred after relapse, n = 14 | |||
| Extramediastinal metastases: | |||
| Present | 5 | 5.3 (3.9, 9.2) | |
| Absent | 9 | 10 (3.5, 20.2) | 0.04 |
| Histologic diagnosis: | |||
| Mixed | 7 | 5.3 (3.5, 18.1) | |
| Single, EST | 2 | 10.1 (10.0, 10.2) | |
| Single, non-EST | 5 | 8.5 (3.9, 20.2) | 0.76 |
| β-hCG concentration: | |||
| ≤1000 mIU/mL | 7 | 5.3 (3.5, 18.1) | |
| >1000 mIU/mL | 2 | 6.5 (3.9, 9.2) | |
| Unknown | 5 | 10.2 (7.4, 20.2) | 0.27 |
| All patients, n = 27 | |||
| Extramediastinal metastases: | |||
| Present | 11 | 9.2 (1.4, 52.5+) | |
| Absent | 16 | 19.2 (3.5, 110.3+) | 0.14 |
| Histologic diagnosis: | |||
| Mixed | 15 | 12.7 (1.4, 52.5+) | |
| Single, EST | 6 | NE (10.0, 110.3+) | |
| Single, non-EST | 6 | 8.9 (3.9, 20.2) | 0.05 |
| β-hCG concentration: | |||
| ≤1000 mIU/mL | 16 | 20.4 (1.4, 100.0+) | |
| >1000 mIU/mL | 4 | 10.8 (3.9, 14.8) | |
| Unknown | 7 | 12.7 (7.4, 110.3+) | 0.25 |
TTP = time to progression (defined as the time from diagnosis to first recurrence or progression); NE = not estimable; EST = endodermal sinus tumor; β-hCG = β human chorionic gonadotropin.
indicates the observation was censored.
3.3. Chemotherapy regimens
All 27 patients with mediastinal NSGCT had received cisplatin-based chemotherapy, and 17 (63%) had received BEP or etoposide plus cisplatin (EP) as the first treatment regimen (Table 3). Twenty-four men had received at least 1 course of preoperative chemotherapy, whereas the other 3 had undergone surgical resection or debulking initially (before referral to us). Eleven patients (41%) with NSGCT had received more than 4 courses of preoperative chemotherapy. Seven patients with mediastinal seminoma had received BEP (n = 1), EP (n = 5), or radiotherapy (n = 1) as the primary treatment.
Table 3. Initial chemotherapy given to 27 patients with mediastinal NSGCT.
| No. of patients treated | ||||
|---|---|---|---|---|
|
|
||||
| Regimen | First course | Second course | Third course | Fourth course |
| BEP or EP | 17 | 16 | 13 | 13 |
| VIP or VeIP | 1 | 2 | 2 | 2 |
| BOP or OP | 6 | 0 | 0 | 0 |
| CisCA | 0 | 5 | 1 | 0 |
| POMB | 0 | 0 | 4 | 0 |
| ACE | 0 | 1 | 0 | 6 |
| Paclitaxel, ifosfamide, cisplatin | 0 | 0 | 2 | 1 |
| Paclitaxel, bleomycin, vincristine, cisplatin | 1 | 0 | 0 | 0 |
| Vincristine, cisplatin, doxorubicin | 2 | 0 | 0 | 0 |
| Etoposide, ifosfamide, ciplatin, epirubicin | 0 | 1 | 0 | 0 |
| Paclitaxel, cisplatin, doxorubicin | 0 | 0 | 1 | 0 |
| Doxorubicin, ifosfamide | 0 | 0 | 1 | 1 |
| Docetaxel, bleomycin, cisplatin | 0 | 0 | 0 | 1 |
| Total no. | 27 | 25 | 24 | 24 |
NSGCT = nonseminomatous germ cell tumor; BEP = bleomycin, etoposide, cisplatin; EP = etoposide, cisplatin; VIP = etoposide, ifosfamide, cisplatin; VeIP = vinblastine, ifosfamide, cisplatin; BOP = bleomycin, vincristine, cisplatin; OP = vincristine, cisplatin; CisCA, cisplatin, cyclophophamide, doxorubicin; POMB = cisplatin, vincristine, methotrexate, bleomycin; ACE = actinomycin, cyclophosphamide, etoposide.
3.4. Postchemotherapy surgical resection
Nineteen of the 27 patients with NSGCT (70%) and 1 of the 7 patients with seminoma (14%) had undergone postchemotherapy resection of a residual mediastinal mass. Histologic examination had shown only fibrous tissue and necrosis in 2 patients with NSGCT (11% of those who underwent resection) and in the patient with seminoma. Histologic evaluation of residual tumor in the other cases of NSGCT had revealed malignant NSGCT alone in 8 patients (42% of those who underwent resection), malignant germ-cell tumor plus sarcoma in 2 patients (11%), and teratoma alone in 4 patients (21%). The remaining 3 patients' histologic examinations of residual tissue had revealed teratoma plus adenocarcinoma and sarcoma, sarcoma alone (with pretreatment histologic features of embryonal carcinoma), and unknown.
3.5. Hematologic malignancy
None of the men had developed acute leukemia. One who had had an elevated serum α-fetoprotein (AFP) concentration at diagnosis had had abnormal large nucleated cells in the peripheral blood and bone marrow. The abnormal cells had been CD45 negative and had lacked hematopoietic differentiation, being consistent with circulating germ-cell tumor cells rather than leukemia. That patient had died of tumor progression. Another patient had developed pancytopenia after completion of chemotherapy and resection of the mediastinal mass. Bone marrow examination had revealed myelodysplastic syndrome, and he had died of infectious complications without evidence of germ-cell tumor recurrence.
3.6. Survival outcomes
Sixteen patients with nonseminomatous PMGCT were known to have died: 15 had had progressive disease, and the other had died of infectious complications of myelodysplastic syndrome. All of the treatment failures and 14 deaths had occurred within 3 years of diagnosis. Eleven patients with NSGCT (41%) and all 7 with seminoma had been alive at last contact, with a median follow-up of 51.3 months (range, 22–110 months). Figure 1 shows the survival data for the 27 patients with mediastinal NSGCT.
Fig. 1.
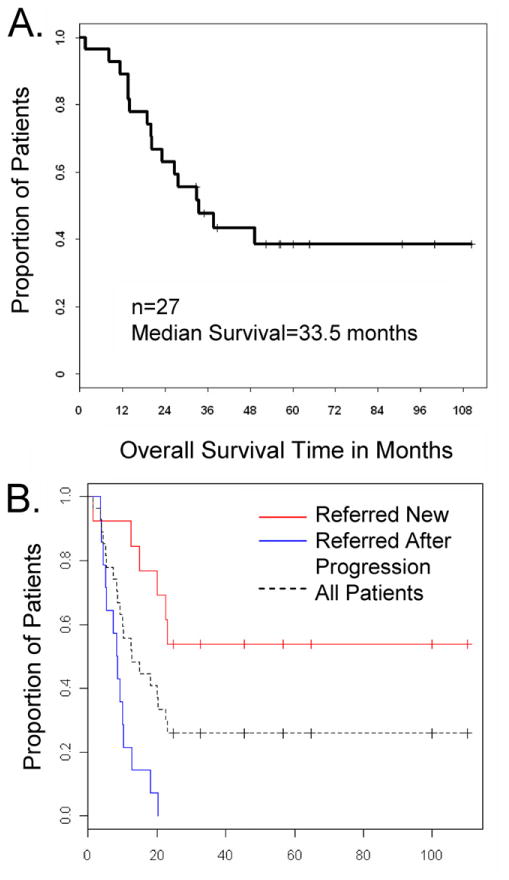
Kaplan-Meier plot of the (A) overall survival and (B) progression-free survival duration for 27 patients with mediastinal nonseminomatous germ-cell tumors. The median progression-free survival from diagnosis was 8.5 months for patients referred in relapse and was not estimable for the patients referred at diagnosis (log rank P < 0.001).
For the subgroup of patients with NSGCT who had been referred to us at or near the time of diagnosis (n = 13), 7 (54%) had been alive and continuously free of recurrence at last contact (Fig. 1B), with a median follow-up of 56.5 months (range, 33–110). This group included patients who had been treated plus those who had been untreated before their referral, as long as there had been no evidence or history of tumor progression after treatment had been started. Treatment was considered to have been any surgery, radiotherapy, or chemotherapy.
Nineteen patients had been considered for second-line or salvage therapy: 5 who had been treated initially at M. D. Anderson and 14 who had been referred to us after progression or relapse. Eighteen of those patients had received salvage chemotherapy, and all 19 had experienced further progression. Ten of those 19 patients (53%) had been alive 1 year from the date of the first progression, and the estimated 3-year overall survival from the date of first progression was 23% (Fig. 2).
Fig. 2.
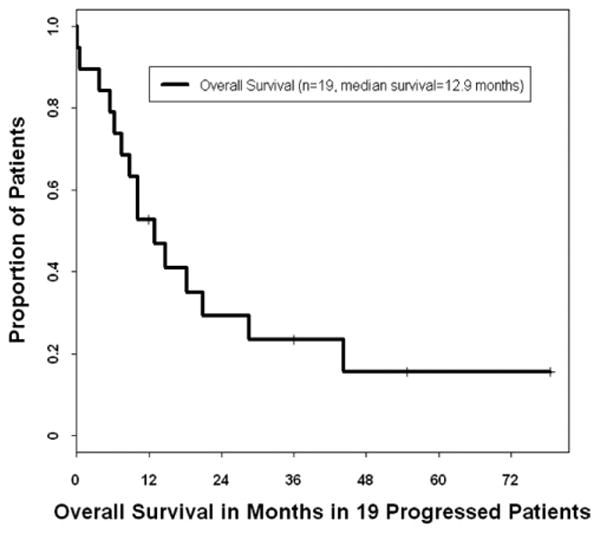
Kaplan-Meier plot of the overall survival duration for 19 patients with relapsed mediastinal nonseminomatous germ-cell tumors. Survival time was calculated from the date of first progression.
3.7. Salvage therapy
Salvage chemotherapy had been administered to 18 patients with mediastinal NSGCT at its first progression or relapse. The responses had been transient, and all 18 patients had eventually experienced further disease progression. Three of the 19 patients with progression or relapse had had surgical resection of all visible disease and were known to be alive and clinically tumor free at 12, 27, and 55 months from the date of the first progression (Fig. 2). One patient had experienced metastatic recurrence of low-grade angiosarcoma, which had also been a component of his primary tumor, and for which he had undergone salvage chemotherapy and multiple surgeries. He was alive with residual lesions but no active disease 6 years after the first progression and was taking low-dose thalidomide.
3.8. Prognostic factors in mediastinal NSGCT
The median TTP of mediastinal NSGCT was calculated according to known prognostic factors, including serum β-hCG concentrations greater than 1,000 mIU/mL at diagnosis [11], EST histology [14], and the presence of extramediastinal tumor at diagnosis [15] (Table 2). Patients with those high β-hCG concentrations had a median TTP of 10.8 months, with no long-term survivors. The median TTP was 9.2 months for patients with extramediastinal disease at diagnosis, and was not estimable (range, 10 to 110+ months) for patients with EST as the only histologic diagnosis. This suggested that EST was a favorable rather than an adverse feature in nonseminomatous PMGCT.
Among the patients with NSGCT who had been referred to M. D. Anderson at diagnosis, 4 (31%) had had a histologic diagnosis of EST with tumor confined to the mediastinum; all had experienced long-term PFS. Two of the patients referred for salvage therapy had had EST initially confined to the mediastinum. One of them had refused to undergo mediastinal resection after chemotherapy, and the other had had mostly necrotic tumor at resection, from which the diagnosis of EST was suspected but never confirmed.
From these data, we identified 3 adverse features as candidate prognostic risk factors: extramediastinal disease at diagnosis, serum β-hCG concentration greater than 1,000 mIU/mL at diagnosis (if known), and any mixed or single histologic diagnosis other than EST. Next, we categorized the 13 patients with NSGCT who had been referred to us at diagnosis into 3 prognostic groups according to their number of risk factors. Table 4 shows the 2-year PFS and TTP analysis for these patients, grouped by adverse features and number of risk factors.
Table 4. Two-year PFS and median TTP analysis by number of adverse features in 13 newly diagnosed patients with mediastinal NSGCTa.
| 2-year PFS | TTP, months | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Patient subgroup | n | No. Patients | % | Median | Log-rank P |
| Adverse features at diagnosis | |||||
| Extramediastinal tumor present | |||||
| Yes | 6 | 1 | 17 | 18.9 | |
| No | 7 | 6 | 86 | NE | 0.03 |
| Histologic diagnosis other than EST | |||||
| Yes | 9 | 3 | 33 | 23 | |
| No | 4 | 4 | 100 | NE | 0.07 |
| Serum β-hCG > 1,000 mIU/mL | |||||
| Yes | 2 | 0 | 0 | 13.6 | |
| Nob | 11 | 7 | 64 | NE | 0.007 |
| Number of adverse features | |||||
| 0 | 4 | 4 | 100 | NE | |
| 1 | 3 | 2 | 67 | NE | |
| 2 or 3 | 6 | 1 | 17 | 18.9 | 0.07 |
| All patients | 13 | 7 | 54 | NE | |
PFS = progression-free survival; TTP = time to progression; NSGCT = nonseminomatous germ cell tumor; NE = not estimable; EST = endodermal sinus tumor; β-hCG = β-human chorionic gonadotropin.
2-Year PFS and TTP were calculated from the date of diagnosis.
Includes patients for whom the serum β-hCG concentration at diagnosis was not known.
4. Discussion
In this contemporary single-institution series of patients with PMGCT, we found a 54% rate of long-term PFS for newly diagnosed mediastinal NSGCT. Historically, the reported survival rates for mediastinal NSGCT were 45% to 48% in the 1990s [7,9] and 20% to 27% in the 1980s [5,6]. Improvements in the prognosis over time are likely due to diverse factors, such as routine use of cisplatin-based chemotherapy, advances in supportive care, improvements in surgery, and newer imaging techniques. Unfortunately, however, the cure rate for mediastinal NSGCT remains significantly below that of intermediate- and good-prognosis testicular cancer and mediastinal seminoma [4]. All 7 of our patients with pure seminoma were alive and free of disease at their last assessment. Six of them had received chemotherapy; most had not undergone radiotherapy, and the results of the only postchemotherapy surgery for seminoma had revealed fibrosis.
Certain patient characteristics at the time of diagnosis were associated with a better prognosis for patients with NSGCT. For patients with newly diagnosed NSGCT, the median TTP had not yet been reached for the subgroups characterized by tumor confined to the mediastinum, serum β-hCG of 1,000 mIU/mL or less at diagnosis, or histologic features of EST (Table 2). A risk factor scoring system based on these 3 parameters is potentially useful in predicting survival, as shown in Table 4. Our results, however, were obtained with a small number of patients and should be statistically validated with a larger test group.
A histologic diagnosis of EST has been described as a poor-risk feature in the setting of testicular NSGCT [16], where it is also more reliably diagnosed on the basis of pathology in the orchiectomy specimen. The diagnosis of mediastinal EST is typically made on clinical grounds, based on a markedly elevated serum AFP concentration, normal or modestly elevated β-hCG concentration, and tumor biopsy results compatible with a diagnosis of EST [14]. EST is relatively insensitive to cisplatin-based chemotherapy yet susceptible to surgical resection. In testicular cancer, the reported survival rate was worse for patients with EST in the setting of advanced metastatic disease (lung metastases >2 cm or extrapulmonary metastases) but not in low-volume disease (lung metastases ≤2 cm) compared with non-EST tumors; moreover, survival did not improve with the use of cisplatin as opposed to non-cisplatin chemotherapy [16]. A similar effect was reported by Kuzur et al. [14] in patients with mediastinal EST in whom advanced or unresectable disease carried a dismal prognosis but occasional cures were achieved surgically.
The association of EST with favorable outcome in our series contrasts with earlier published results, possibly because these patients are now recognized as candidates for aggressive surgery [12,17]. Our data suggest that those patients with EST confined to the mediastinum have a much better prognosis than other subgroups with mediastinal NSGCT, and the proportion of such patients could account in part for some of the observed differences in outcome between studies.
Our series included 19 patients whose tumor had progressed or recurred after primary chemotherapy. Further disease progression was observed in every patient who had undergone salvage chemotherapy, despite the occurrence of transient antitumor responses. Tumor-free status was surgically achieved in 3 of these patients, including 1 man who experienced 47 months of continuous PFS after resection; notably, his tumor had features of EST.
Our data indicate that surgery is the most effective salvage modality for relapsed mediastinal NSGCT, but it is possible that salvage chemotherapy also contributed to positive outcomes by preoperatively reducing the burden of viable tumor. Fizazi et al. [18] reported the results of a retrospective study of patients with mediastinal NSGCT treated between 1976 and 1993, in which 20 patients required salvage chemotherapy and all experienced further disease progression. In 1994, Saxman et al. [10] reported the results of 42 patients who received salvage chemotherapy for recurrent mediastinal NSGCT, of whom only 2 patients (5%) were alive without evidence of disease at their last follow-up; both patients had histologically diagnosed EST. Ganjoo et al. [19] reported the outcome of 17 patients with mediastinal NSGCT who received second- or third-line salvage chemotherapy, of which none were disease-free.
For the treatment of PMGCTs at our institution, we administer chemotherapy before surgery whenever possible [20]. Some patients appear to benefit from more than 4 courses of preoperative chemotherapy when the tumor markers continue to decline, as had been used in 11 patients in our series. There is also potential risk from delaying surgery in patients whose tumors are chemotherapy resistant. The optimal duration of preoperative chemotherapy is difficult to determine without conducting a prospective randomized study.
5. Conclusions
Long-term disease-free survival was achieved in 54% of patients with mediastinal NSGCT, most commonly by using induction chemotherapy followed by consolidation surgery. These results are possible using the standard BEP regimen for patients with mediastinal NSGCT. Cisplatin-based chemotherapy is curative for the vast majority of patients with mediastinal seminoma. Salvage regimens that have been successful in testicular cancer have had, in contrast, little effect on the long-term survival of patients with mediastinal NSGCT. It remains to be determined whether earlier exposure to drugs such as ifosfamide and paclitaxel during the first 4 courses could improve survival for poor-risk patients. Our data suggest that the patients who are at greatest risk are those who have 2 or more risk factors at diagnosis.
Acknowledgments
The authors thank Angela Chinweze for database assistance, Korey Reed for data management, and Robyn Harrell for statistical analysis. The authors also thank Karen Phillips for manuscript editing.
Footnotes
This work was supported by the National Cancer Institute at the National Institutes of Health [core grant CA16672]. This work was presented in part at the 42nd Annual Meeting of the American Society of Clinical Oncology, 2–6 June 2006, Atlanta, GA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moran CA, Suster S. Primary germ-cell tumors of the mediastinum: I Analysis of 322 cases with special emphasis on teratomatous lesions and a proposal for histopathologic classification and clinical staging. Cancer. 1997;80:681–90. [PubMed] [Google Scholar]
- 2.Moran CA, Suster S, Przygodzki RM, et al. Primary germ-cell tumors of the mediastinum: II Mediastinal seminomas—a clinicopathologic and immunohistochemical study of 120 cases. Cancer. 1997;80:691–8. doi: 10.1002/(sici)1097-0142(19970815)80:4<691::aid-cncr7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 3.Moran CA, Suster S, Koss MN. Primary germ-cell tumors of the mediastinum: III Yolk sac tumor, embryonal carcinoma, choriocarcinoma, and combined nonteratomatous germ-cell tumors of the mediastinum—a clinicopathologic and immunohistochemical study of 64 cases. Cancer. 1997;80:699–707. [PubMed] [Google Scholar]
- 4.International Germ-cell Consensus Classification: A prognostic factor-based staging system for metastatic germ-cell cancers International Germ-cell Cancer Collaborative Group. J Clin Oncol. 1997;15:594–603. doi: 10.1200/JCO.1997.15.2.594. [DOI] [PubMed] [Google Scholar]
- 5.Logothetis CJ, Samuels ML, Selig DE, et al. Chemotherapy of extragonadal germ-cell tumors. J Clin Oncol. 1985;3:316–25. doi: 10.1200/JCO.1985.3.3.316. [DOI] [PubMed] [Google Scholar]
- 6.Toner GC, Geller NL, Lin SY, et al. Extragonadal and poor risk nonseminomatous germ-cell tumors Survival and prognostic features. Cancer. 1991;67:2049–57. doi: 10.1002/1097-0142(19910415)67:8<2049::aid-cncr2820670807>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 7.Nichols CR, Saxman S, Williams SD, et al. Primary mediastinal nonseminomatous germ-cell tumors A modern single institution experience. Cancer. 1990;65:1641–6. doi: 10.1002/1097-0142(19900401)65:7<1641::aid-cncr2820650731>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 8.Lemarie E, Assouline PS, Diot P, et al. Primary mediastinal germ-cell tumors Results of a French retrospective study. Chest. 1992;102:1477–83. doi: 10.1378/chest.102.5.1477. [DOI] [PubMed] [Google Scholar]
- 9.Bokemeyer C, Nichols CR, Droz JP, et al. Extragonadal germ-cell tumors of the mediastinum and retroperitoneum: Results from an international analysis. J Clin Oncol. 2002;20:1864–73. doi: 10.1200/JCO.2002.07.062. [DOI] [PubMed] [Google Scholar]
- 10.Saxman SB, Nichols CR, Einhorn LH. Salvage chemotherapy in patients with extragonadal nonseminomatous germ-cell tumors: The Indiana University experience. J Clin Oncol. 1994;12:1390–3. doi: 10.1200/JCO.1994.12.7.1390. [DOI] [PubMed] [Google Scholar]
- 11.Beyer J, Kramar A, Mandanas R, et al. High-dose chemotherapy as salvage treatment in germ-cell tumors: A multivariate analysis of prognostic variables. J Clin Oncol. 1996;14:2638–45. doi: 10.1200/JCO.1996.14.10.2638. [DOI] [PubMed] [Google Scholar]
- 12.Hartmann JT, Einhorn L, Nichols CR, et al. Second-line chemotherapy in patients with relapsed extragonadal nonseminomatous germ-cell tumors: Results of an international multicenter analysis. J Clin Oncol. 2001;19:1641–8. doi: 10.1200/JCO.2001.19.6.1641. [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Nichols CJ, Margolin KA, et al. Phase III randomized trial of conventional-dose chemotherapy with or without high-dose chemotherapy and autologous hematopoietic stem-cell rescue as first-line treatment for patients with poor-prognosis metastatic germ-cell tumors. J Clin Oncol. 2007;25:247–56. doi: 10.1200/JCO.2005.05.4528. [DOI] [PubMed] [Google Scholar]
- 14.Kuzur ME, Cobleigh MA, Greco FA, et al. Endodermal sinus tumor of the mediastinum. Cancer. 1982;50:766–74. doi: 10.1002/1097-0142(19820815)50:4<766::aid-cncr2820500424>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 15.Schneider BP, Kesler KA, Brooks JA, et al. Outcome of patients with residual germ-cell or non-germ-cell malignancy after resection of primary mediastinal nonseminomatous germ-cell cancer. J Clin Oncol. 2004;22:1195–200. doi: 10.1200/JCO.2004.07.102. [DOI] [PubMed] [Google Scholar]
- 16.Logothetis CJ, Samuels ML, Trindade A, et al. The prognostic significance of endodermal sinus tumor histology among patients treated for stage III nonseminomatous germ-cell tumors of the testes. Cancer. 1984;53:122–8. doi: 10.1002/1097-0142(19840101)53:1<122::aid-cncr2820530122>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Radaideh SM, Cook VC, Kesler KA, et al. Outcome following resection for patients with primary mediastinal nonseminomatous germ-cell tumors and rising serum tumor markers post-chemotherapy. Ann Oncol. 2010;21:804–7. doi: 10.1093/annonc/mdp516. [DOI] [PubMed] [Google Scholar]
- 18.Fizazi K, Culine S, Droz JP, et al. Primary mediastinal nonseminomatous germ-cell tumors: Results of modern therapy including cisplatin-based chemotherapy. J Clin Oncol. 1998;16:725–32. doi: 10.1200/JCO.1998.16.2.725. [DOI] [PubMed] [Google Scholar]
- 19.Ganjoo KN, Rieger KM, Kesler KA, et al. Results of modern therapy for patients with mediastinal nonseminomatous germ-cell tumors. Cancer. 2000;88:1051–6. doi: 10.1002/(sici)1097-0142(20000301)88:5<1051::aid-cncr15>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 20.Walsh GL, Taylor GD, Nesbitt JC, et al. Intensive chemotherapy and radical resections for primary nonseminomatous mediastinal germcell tumors. Ann Thorac Surg. 2000;69:337–43. doi: 10.1016/s0003-4975(99)01472-1. [DOI] [PubMed] [Google Scholar]


