Abstract
Objective
Observational evidence supports independent associations between 25-hydroxyvitamin D (25-OHD), parathyroid hormone (PTH) and cardiovascular risk. A plausible hypothesis for these associations is accelerated development of atherosclerosis.
Approach and Results
We evaluated cross-sectional and longitudinal associations of 25-OHD and PTH with carotid intima-media thickness (IMT) and carotid plaques among 3251 participants free of cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. 25-OHD and PTH were measured at baseline by mass spectrometry and immunoassay, respectively. All subjects underwent a carotid ultrasound exam at baseline and 9.4 years later (median, range 8–11.1y). Multivariable linear and logistic regressions were used to test associations of 25-OHD and PTH with the extent and the progression of IMT and the prevalence and incidence of carotid plaque. Mean (SD) 25-OHD and PTH were 25.8ng/ml (10.6) and 44.2pg/ml (20.2). No independent associations were found between 25-OHD or PTH and IMT at baseline [increment of 1.9µm (95%CI −5.1 to 8.9) per 10ng/ml lower 25-OHD; increment of 0.8µm (95%CI −3.2 to 4.8) per 10pg/ml higher PTH] or progression of IMT [increment of 2.6µm (95%CI −2.5 to 7.8) per 10ng/ml lower 25-OHD, increment of 1.6µm (95%CI −1.9 to 5.2) per 10pg/ml higher PTH]. No associations were found with the baseline prevalence of carotid plaque or the incidence of new plaques over the study period. We did not observe any interaction by race or ethnicity (White, Chinese, Black and Hispanic).
Conclusions
The consistent lack of association of vitamin D and PTH with carotid IMT and plaque suggests that these hormones may influence cardiovascular risk through pathways not reflected by carotid atherosclerosis.
Keywords: vitamin D, PTH, mineral metabolism, intima-media thickness, plaque, atherosclerosis, carotid
INTRODUCTION
Lower circulating concentrations of 25-hydroxyvitamin D (25-OHD) and higher circulating concentrations of parathyroid hormone (PTH) have been associated with an increased risk of cardiovascular events in multiple observational cohorts.1, 2,3 There are several plausible explanations for these observations; one hypothesis is that insufficient vitamin D and excessive PTH accelerate atherosclerosis. Low circulating 25-OHD concentrations are associated with obesity, impaired glucose metabolism, hypertension, and dyslipidemia in cross-sectional studies, and with incident hypertension over long-term follow-up.4–7 Inflammatory, immunomodulatory and direct vascular effects of vitamin D have also been implicated.8–10 PTH may affect cardiovascular disease through the development of hypertension,11 left ventricular hypertrophy,12 or endothelial dysfunction.13
Our aim was to test associations of serum 25-OHD and PTH concentrations with carotid intima-media thickness (IMT) and plaque, two non-invasive markers of arterial injury including atherosclerosis that independently predict cardiovascular disease,14 in a large community-based study. We hypothesized that participants with lower 25-OHD or higher PTH would have larger IMT measurements at baseline, more rapid IMT progression over the follow-up period and greater prevalence and incidence of carotid plaques.
MATERIAL AND METHODS
Materials and Methods are available in the online-only Supplement.
RESULTS
Participant characteristics
From 6393 participants with available original IMT measurements, 3251 underwent a second ultrasound for IMT progression and had their baseline IMT re-measured using the images from the baseline ultrasound. Mean age and BMI (SD) of these participants were 60.4y (9.4) and 28.2kg/m2 (5.2) and 46.5% were male. They were racially/ethnically diverse, with 39.6% of White, 13.3% of Chinese, 25.8% of Black and 21.3% of Hispanic subjects. Compared with these, participants who did not have a follow-up carotid ultrasound were older (mean age 64.0y) and had a greater prevalence of treated diabetes (11.5% vs. 8.1%), hypertension (48.3% vs. 40.6%) and current smoking (14.3% vs. 11.5%). Measurements of PTH and 25-OHD were similar in these two groups.
Among the 3251 participants with subsequent carotid ultrasound and new readings of baseline IMT, 1033 (31.8%) had 25-OHD <20ng/ml at baseline (Table 1). Despite being younger, these participants had more cardiovascular risk factors (diabetes, hypertension, smoking, higher BMI, higher CRP), but had higher mean estimated GFR, compared to participants with higher 25-OHD concentrations. Racial/ethnic differences were striking with lower and higher 25-OHD concentrations among Black and White subjects, respectively. The proportion of 25-OHD <20ng/ml was 15.1%, 23.7%, 60.5% and 33.0% among White, Chinese, Black and Hispanic participants, respectively. Three hundred and seventy participants (11.4%) had PTH ≥65pg/ml. We observed a marked increase in the prevalence of hypertension with increasing PTH concentrations and an expected inverse correlation between PTH and GFR. Black and Hispanic participants were more likely to have higher PTH concentrations. The proportion of high PTH concentrations (≥65ng/ml) was higher among Black and Hispanic participants (18.0% and 16.1%) than among White and Chinese participants (6.8% and 3.7%). During follow-up, the prevalence of treatment for traditional cardiovascular risk factors increased. This increase did not differ by 25-OHD or PTH status at baseline. For example, the prevalence of statin use from baseline to exam 5 did not increase more for participants with 25-OHD <20ng/ml (18.9%) than for those with 25-OHD >30ng/ml (23.9%).
Table 1.
Baseline characteristics of 3,251 MESA participants.
| Annualized 25-OH-vitamin D [ng/ml] | PTH [pg/ml] | |||||||
|---|---|---|---|---|---|---|---|---|
| <20 (n=1033) |
20.0–29.9 (n=1155) |
>=30.0 (n=1063) |
<33.0 (n=964) |
33.0–44.2 (n=962) |
44.3–64.9 (n=955) |
>=65 (n=370) |
||
| Age [y] | 58.8 (9.1) | 60.6 (9.4) | 61.6 (9.4) | 58.8 (9.1) | 60.6 (9.4) | 61.6 (9.4) | 58.8 (9.1) | |
| Male, % | 446 (43.3%) | 576 (50%) | 456 (46%) | 446 (43.3%) | 576 (50%) | 456 (46%) | 446 (43.3%) | |
| Race / ethnicity, % | White | 194 (18.8%) | 472 (41%) | 621 (58.5%) | 481 (49.9%) | 400 (41.6%) | 318 (33.3%) | 88 (24%) |
| Chinese | 102 (9.9%) | 195 (16.9%) | 133 (12.5%) | 170 (17.6%) | 149 (15.5%) | 95 (10%) | 16 (4.4%) | |
| Black | 507 (49.2%) | 225 (19.5%) | 106 (10%) | 151 (15.7%) | 221 (23%) | 315 (33%) | 151 (41.3%) | |
| Hispanic | 228 (22.1%) | 260 (22.6%) | 202 (19%) | 162 (16.8%) | 191 (20%) | 226 (23.7%) | 111 (30.3%) | |
| Treated diabetes, % | 106 (10.3%) | 104 (9%) | 54 (5.1%) | 90 (9.4%) | 66 (6.9%) | 72 (7.6%) | 35 (9.8%) | |
| Hypertension, % | 460 (44.6%) | 471 (40.9%) | 310 (36.4%) | 310 (32.2%) | 356 (37%) | 447 (46.9%) | 205 (56%) | |
| Treatment for hypertension, % | 376 (36.5%) | 407 (35.3%) | 268 (31.2%) | 268 (27.8%) | 313 (32.6%) | 366 (38.4%) | 167 (45.6%) | |
| SBP [mmHg] | 126.2 (20.9) | 124.1 (20.1) | 122.4 (19.4) | 119.9 (18.7) | 122.6 (19.6) | 127.4 (20.2) | 131.6 (22) | |
| DBP [mmHg] | 73.1 (10.1) | 71.8 (10) | 70.5 (9.8) | 70.5 (9.5) | 71.5 (10.2) | 72.7 (9.9) | 73.4 (10.8) | |
| BMI [kg/m2] | 29.9 (5.6) | 28 (5) | 26.8 (4.5) | 26.8 (4.6) | 27.9 (5) | 28.9 (5.2) | 30.7 (6.1) | |
| Total cholesterol [mg/dl] | 193.3 (36.5) | 192.8 (34.4) | 195.8 (33.9) | 195.4 (34.6) | 193.5 (35.1) | 194.6 (34.5) | 189.4 (35.9) | |
| LDL [mg/dl] | 118.7 (32.5) | 116.4 (30) | 116 (29.4) | 117.4 (29.4) | 117.1 (31.1) | 117.8 (30.6) | 113.3 (32.5) | |
| HDL [mg/dl] | 49.8 (14.5) | 50 (14.6) | 53.7 (15.7) | 51.3 (14.4) | 50.6 (14.9) | 51.4 (15.6) | 51.6 (15.6) | |
| Treatment with statins, % | 136 (13.2%) | 187 (16.2%) | 129 (16.1%) | 129 (13.4%) | 133 (13.8%) | 158 (16.6%) | 74 (20.2%) | |
| Current smokers, % | 158 (15.4%) | 114 (9.9%) | 101 (9.5%) | 125 (13%) | 116 (12.1%) | 97 (10.2%) | 35 (9.6%) | |
| Former smokers, % | 361 (35.1%) | 415 (36.1%) | 399 (37.6%) | 349 (36.2%) | 343 (35.8%) | 359 (37.6%) | 124 (34%) | |
| GFR [ml/min/1.73m2] | 88.9 (16) | 86 (15.6) | 83 (15.2) | 86.4 (14.8) | 86.4 (15.6) | 86 (15.8) | 83.1 (18.2) | |
| Calcium [mg/dl] | 9.6 (0.4) | 9.6 (0.4) | 9.7 (0.4) | 9.7 (0.4) | 9.7 (0.4) | 9.6 (0.4) | 9.6 (0.5) | |
| Phosphorus [mg/dl] | 3.7 (0.5) | 3.6 (0.5) | 3.7 (0.5) | 3.8 (0.5) | 3.7 (0.5) | 3.6 (0.5) | 3.5 (0.5) | |
| IL-6 [IU/ml] | 38 (13.5) | 40.4 (17.1) | 41.9 (23.1) | 39.5 (14.9) | 39.9 (22.5) | 40.4 (13.1) | 41.5 (25.3) | |
| CRP [mg/l] | 1.7 (1.3) | 1.4 (1) | 1.3 (1) | 1.3 (1.1) | 1.4 (1.1) | 1.5 (1.2) | 1.7 (1.1) | |
Abbreviations:
SBP = systolic blood pressure; DBP = diastolic blood pressure; BMI = body-mass index; LDL = low-density lipoprotein; HDL = high-density lipoprotein; GFR = glomerular filtration rate; IL-6 = interleukin-6; CRP = C-reactive protein.
Carotid IMT and Plaque
At baseline, mean (SD) CCA-IMT and ICA-IMT were 927µm (SD 210µm) and 906µm (SD 399µm), respectively. Median (range) time between ultrasound exams was 9.4 years (y) (8.0–11.1y). Mean (SD) changes in CCA-IMT and ICA-IMT between ultrasound exams were 137µm (SD 140µm) and 164µm (SD 276µm), respectively. At least one carotid plaque was found among 1525/3246 participants at baseline (47.0%). Among participants without plaques at baseline, 698 (40.6%) had developed a carotid plaque at the time of the second ultrasound. Mean plaque scores were 1.08 (SD 1.61) at baseline and progressed by a mean of 1.18 (SD 1.45) over the study period.
25-OHD, IMT, and plaque
At baseline, lower 25-OHD concentrations were associated with modestly greater CCA and ICA IMT in demographic-adjusted analyses (Table 2, left side, model 1). However, in models further adjusted for confounders, we found no independent association of 25-OHD with CCA or ICA IMT or their change over time (Table 2, top and middle rows, model 2). Adjustment for BMI was responsible for most of the attenuation observed from model 1 to model 2. The precision of the null estimates ruled out clinically meaningful associations: the adjusted mean differences in baseline CCA IMT and its change over time, per 10ng/ml lower 25-OHD, were 1.9µm (95%CI −5.1 to 8.9) and 2.6µm (95%CI −2.5 to 7.8), respectively. In addition, 25-OHD concentrations were not associated with the prevalence and incidence of carotid plaque (Table 2, lower rows). No cross-sectional or longitudinal associations with the baseline carotid plaque score or its change over study time were observed: adjusted OR per 10ng/ml lower 25-OHD 1.00 (95%CI 0.93–1.08, p=0.95) and 1.05 (95%CI 0.98–1.13, p=0.17), respectively.
Table 2.
Cross-sectional and longitudinal associations of serum 25-OHD concentration with carotid intima-media thickness and plaque.
| Cross-sectional analyses | Longitudinal analyses | |||||||
|---|---|---|---|---|---|---|---|---|
| 25-OHD | Baseline CCA-IMT | Change in CCA-IMT1 | ||||||
| N | Unadjusted mean IMT [µm (SD)] |
Adjusted difference [µm (95%CI)] |
N | Unadjusted mean difference [µm (SD)] |
Adjusted difference [µm (95%CI)] |
|||
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| ≥30.0 ng/ml | 1047 | 924 (221) | ref. | ref. | 876 | 133 (127) | ref. | ref. |
| 20.0–29.9 ng/ml | 1132 | 923 (207) | 1.3 (−15.1 to 17.7) |
−7.2 (−23.3 to 8.9) |
931 | 136 (134) | 3.2 (−9.1 to 15.6) |
0.1 (−12.3 to 12.5) |
| <20 ng/ml | 1003 | 936 (204) | 23.0 (5.5 to 40.5) |
7.5 (−10.0 to 24.9) |
776 | 144 (162) | 10.0 (−4.9 to 24.8) |
4.6 (−10.6 to 19.8) |
| P value3 | <0.02 | 0.59 | <0.05 | 0.32 | ||||
| Baseline ICA-IMT | Change in ICA-IMT1 | |||||||
| N | Unadjusted mean IMT [µm (SD)] |
Adjusted difference [µm (95%CI)] |
N | Unadjusted mean difference [µm (SD)] |
Adjusted difference [µm (95%CI)] |
|||
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| ≥30.0 ng/ml | 838 | 907 (397) | ref. | ref. | 514 | 168 (285) | ref. | ref. |
| 20.0–29.9 ng/ml | 868 | 917 (429) | 29.7 (−7.8 to 67.3) |
19.9 (−18.9 to 58.6) |
507 | 167 (287) | 7.8 (−27.0 to 42.9) |
1.9 (−34.9 to 38.6) |
| <20 ng/ml | 713 | 890 (362) | 38.3 (−0.7 to 77.3) |
20.2 (−19.8 to 60.3) |
387 | 156 (248) | 7.2 (−31.7 to 38.8) |
−2.4 (−43.7 to 38.8) |
| P value3 | <0.03 | 0.25 | 0.66 | 0.95 | ||||
| Baseline prevalence of carotid plaque | Incidence of a new carotid plaque1,2 | |||||||
| N | Unadjusted prevalence [%] |
Adjusted odds ratio (95%CI) | N | Unadjusted cumulative incidence [%] |
Adjusted odds ratio (95%CI) | |||
| Model 3 | Model 4 | Model 3 | Model 4 | |||||
| ≥30.0 ng/ml | 1048 | 47.00% | 1.0 (ref) | 1.0 (ref) | 556 | 41.20% | 1.0 (ref) | 1.0 (ref) |
| 20.0–29.9 ng/ml | 1140 | 48.10% | 1.19 (0.99–1.42) |
1.17 (0.98–1.41) |
592 | 42.70% | 1.14 (0.89–1.45) |
1.16 (0.90–1.48) |
| <20 ng/ml | 1024 | 44.30% | 1.15 (0.94–1.41) |
1.09 (0.88–1.35) |
570 | 37.90% | 1.04 (0.78–1.37) |
1.04 (0.78–1.39) |
| P value3 | 0.28 | 0.75 | 0.73 | 0.67 | ||||
between the two carotid ultrasounds (9.4y)
among those without carotid plaques at baseline
P-value generated evaluating 25-OHD as a continuous variable.
Linear model 1 adjusted for sex, race, study field center, education, income and time between the 2 ultrasounds.
Linear model 2 further adjusted for physical activity, smoking, BMI, LDL, HDL, use of statins and GFR.
Logistic model 3 adjusted for age, sex, race, site, education, income and time between the 2 ultrasounds.
Logistic model 4 further adjusted for physical activity, smoking, BMI, LDL, HDL, use of statins and GFR.
Abbreviations: CCA-IMT = intima-media thickness of the common carotid artery / ICA-IMT = intima-media thickness of the internal carotid artery
PTH, IMT, and plaque
Serum PTH concentrations were not associated with baseline CCA-IMT or its change over time (Table 3, top rows). The adjusted mean difference per 10pg/ml higher PTH was 0.8µm (−3.2 to 4.8) and 1.6µm (95%CI −1.9 to 5.2), respectively. No association was found between PTH and ICA-IMT at baseline, but participants with higher PTH measurements showed nominally less progression of ICA-IMT between the 2 ultrasounds (−10.6µm, 95%CI −21.3 to 0.1, per 10pg/ml increased PTH). After exclusion of one influential outlier with PTH 14.8pg/m and IMT progression of 3222µm, this association was less pronounced (−7.9µm, 95%CI −17.2 to 1.4, per 10pg/ml increased PTH). PTH was not independently associated with the prevalence or incidence of carotid plaque (Table 3, lower rows) or with the carotid plaque score at baseline or its change over the study period (adjusted OR per 10pg/ml higher PTH: 0.97 (95%CI 0.93–1.01, p=0.18) and 0.99 (95%CI 0.96–1.03, p=0.71), respectively).
Table 3.
Cross-sectional and longitudinal associations of parathyroid hormone concentration with carotid intima-media thickness and plaque
| Cross-sectional analyses | Longitudinal analyses | |||||||
|---|---|---|---|---|---|---|---|---|
| PTH | Baseline CCA-IMT | Change in CCA-IMT1 | ||||||
| N | Unadjusted mean IMT [µm (SD)] |
Adjusted difference [µm (95%CI)] |
N | Unadjusted mean difference [µm (SD)] |
Adjusted difference [µm (95%CI)] |
|||
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| <33.0 pg/mL | 945 | 897 (210) | ref. | ref. | 797 | 130 (125) | ref. | ref. |
| 33.0–44.3 pg/mL | 941 | 925 (207) | 16.8 (−0.3 to 33.8) |
11.8 (−4.9 to 28.4) |
786 | 141 (143) | 11.9 (−1.8 to 25.7) |
10.9 (−2.8 to 24.7) |
| 44.4–64.9 pg/mL | 939 | 950 (216) | 19.5 (1.7 to 37.3) |
10.4 (−7.6 to 28.3) |
735 | 143 (138) | 13.0 (−0.9 to 26.7) |
12.4 (−1.7 to 26.4) |
| ≥65 pg/mL | 357 | 955 (198) | 20.8 (−2.4 to 44.1) |
10.1 (−13.3 to 33.6) |
265 | 131 (154) | 1.2 (−19.7 to 22.0) |
1.0 (−19.6 to 21.5) |
| P value3 | 0.13 | 0.70 | 0.37 | 0.37 | ||||
| Baseline ICA-IMT | Change in ICA-IMT1 | |||||||
| N | Unadjusted mean IMT [µm (SD)] |
Adjusted difference [µm (95%CI)] |
N | Unadjusted mean difference [µm (SD)] |
Adjusted difference [µm (95%CI)] |
|||
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| <33.0 pg/mL | 740 | 881 (361) | ref. | ref. | 465 | 170 (300) | ref. | ref. |
| 33.0–44.3 pg/mL | 737 | 912 (428) | 23.0 (−15.3 to 61.4) |
16.4 (−22.0 to 54.8) |
420 | 165 (256) | −5.3 (−41.5 to 30.9) |
−9.2 (−45.7 to 27.3) |
| 44.4–64.9 pg/mL | 701 | 936 (425) | 29.7 (−10.2 to 69.6) |
15.8 (−25.4 to 57.1) |
393 | 170 (292) | −12.8 (−54.1 to 28.5) |
−16.8 (−60.3 to 26.7) |
| ≥65 pg/mL | 241 | 875 (335) | −23.3 (−73.3 to 26.7) |
−42.3 (−95.1 to 10.5) |
130 | 121 (185) | −65.4 (−112.7 to −18.1) |
−66.2 (−116.6 to −15.8) |
| P value4 | 0.80 | 0.61 | 0.04 | 0.05 | ||||
| Baseline prevalence of carotid plaque | Incidence of a new carotid plaque1,2 | |||||||
| N | Unadjusted prevalence [%]2 |
Adjusted OR (95%CI) | N | Unadjusted cumulative incidence [%]2 |
Adjusted odds ratio (95%CI) | |||
| Model 3 | Model 4 | Model 3 | Model 4 | |||||
| <33.0 pg/mL | 952 | 43.9% | 1.0 (ref) | 1.0 (ref) | 534 | 38.40% | 1.0 (ref) | 1.0 (ref) |
| 33.0–44.3 pg/mL | 952 | 46.9% | 1.11 (0.92–1.34) |
1.12 (0.92–1.36) |
506 | 41.10% | 1.14 (0.88–1.48) |
1.14 (0.88–1.48) |
| 44.4–64.9 pg/mL | 945 | 50.6% | 1.16 (0.95–1.41) |
1.15 (0.94–1.42) |
467 | 43.00% | 1.16 (0.89–1.52) |
1.17 (0.89–1.55) |
| ≥65 pg/mL | 363 | 41.9% | 0.78 (0.60–1.02) |
0.77 (0.58–1.02) |
211 | 39.80% | 1.03 (0.73–1.47) |
1.04 (0.72–1.50) |
| P value4 | 0.27 | 0.33 | 0.98 | 0.99 | ||||
between the two carotid ultrasounds
among those without carotid plaques at baseline
P value for continuous 25-OHD
Linear model 1 adjusted for sex, race, study field center, education, income and time between the 2 ultrasounds.
Linear model 2 further adjusted for physical activity, smoking, BMI, LDL, HDL, use of statins, GFR and 25-OHD.
Logistic model 3 adjusted for age, sex, race, site, education, income and time between the 2 ultrasounds.
Logistic model 4 further adjusted for physical activity, smoking, BMI, LDL, HDL, use of statins, GFR and 25-OHD.
Abbreviations: CCA-IMT = intima-media thickness of the common carotid artery / ICA-IMT = intima-media thickness of the internal carotid artery
Additional analyses
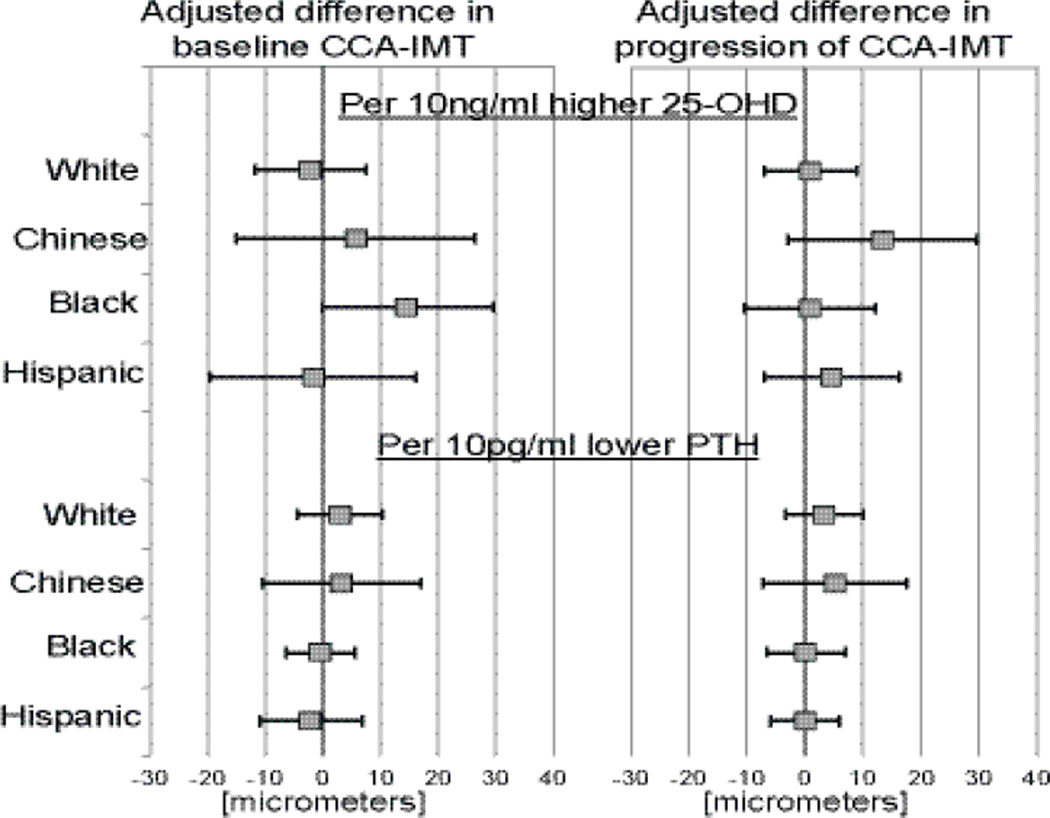
There was no heterogeneity in the associations of 25-OHD and PTH with IMT or its change over time by race/ethnicity (all p-interaction >0.05, Figure 1).
Figure 1.
Associations between 25-OHD / PTH and baseline maximum CCA-IMT or its progression in subgroups of race / ethnicity (differences in IMT (in micrometers) per 10ng/ml decrement in 25-OHD or 10pg/ml increment in PTH).
number of participants for baseline CCA-IMT - White (1261), Chinese (425), Black (823), Hispanic (673) / number of participants for progression of CCA-IMT – White (1048), Chinese (360), Black (623), Hispanic (549)
To confirm that our findings were not influenced by selection bias, and in particular survivorship bias, we repeated cross-sectional analyses on the 6,393 MESA participants who had baseline measurements of 25-OHD, PTH, and IMT, regardless of the presence of a second carotid ultrasound. Null results were similar, including the absence of effect modification by race/ethnicity.
DISCUSSION
In this large cohort study of racially and ethnically diverse adults without clinical cardiovascular disease at baseline, we observed no independent associations of serum 25-OHD or PTH concentration with CCA-IMT, ICA-IMT, or carotid plaque. Moreover, we observed consistent null results for both cross-sectional associations and longitudinal associations evaluating change in IMT and incident plaque over 10 years of follow-up, which have not been reported previously. Estimated magnitudes of association were close to zero, excluded clinically relevant relationships, and did not vary by race/ethnicity. These robustly null results suggest that 25-OHD and PTH do not influence the development of carotid IMT and atherosclerosis in generally healthy adults.
Our null cross-sectional results are in agreement with most previous studies. Five smaller studies have reported a lack of association of 25-OHD with carotid IMT in diverse populations: postmenopausal women recruited from a specialty clinic in Korea,9 adults from an Amish population,15 a Dutch population-based study of 600 adults,16 a community-based study of 900 older Korean adults17, and a clinical trial of type 1 diabetes.18
In contrast, two clinic-based studies reported positive cross-sectional associations between lower vitamin D and larger IMT. These examined selected populations (type II diabetes, HIV) and did not exclude participants with known cardiovascular disease, resulting in potential for confounding or bias.19, 20 Our results highlight the important role that confounding can play in analyses of 25-OHD: lower 25-OHD was associated with greater IMT and greater progression of CCA-IMT in models adjusted for demographic variables, as hypothesized, but not with further adjustment for confounding variables. Also, Reis et al. studied 654 subjects from a community-based cohort in California with a mean age of 76 years and a high average 25-OHD (41.5ng/ml). This study reported an independent association between vitamin D status and ICA-IMT, but not CCA-IMT,21 which conflicts with our results without a clear explanation. In the same study, PTH was not associated with either ICA- or CCA-IMT.
Several explanations could be advanced for the lack of associations between mineral metabolism markers and carotid injury in our study. First, one measure of 25-OHD and PTH may not adequately represent the true average individual status of these hormones due to variability over time. PTH has a substantial within-subject variability,22, 23 but the validity of one measure of 25-OHD is very high, with a correlation of 0.85 between 2 measurements taken 8 months in White and Black American subjects.24 Second, carotid IMT measurement error (which would bias estimates towards the null) cannot be excluded, even with the very good intra- and inter-reader reproducibility measurements used in this study. Third, CCA-IMT may be more closely related to aging and hypertensive medial hypertrophy than atherosclerotic processes.25 However, ICA-IMT and carotid plaque, which yielded similar results in our analysis, are thought to represent early phenotypes of atherosclerosis. Fourth, more aggressive treatment for cardiovascular risk factors among participants with low 25-OHD or high PTH during follow-up could have attenuated the true associations, but we found that the increase in cardiovascular treatment did not differ by baseline 25-OHD or PTH status. Finally, and most likely in our opinion, our results may suggest that mineral metabolism disturbances affect cardiovascular risk through pathways distinct from carotid atherosclerosis.
Previous experimental and epidemiological evidence support effects of PTH and 25-OHD on cardiovascular risk that do not involve carotid atherosclerosis. PTH is an independent predictor of cardiovascular mortality in the general population,3 but its association with incident heart failure appears much stronger than with myocardial infarction.1 The detrimental effects of PTH on the myocardium (left ventricular hypertrophy, fibrosis, calcifications) or endothelial function may be more important than the effects on arterial wall injury, at least in the carotid arteries.11–13 Vitamin D may act on cardiovascular risk through several different pathways, such as through an immuno- or inflammatory modulation or a direct effect on the endothelial or smooth-muscle vascular cell.8, 26, 27 Of cardiovascular outcomes, lower circulating concentrations of 25-OHD have been most consistently and strongly associated with increased risk of coronary artery disease. 1, 28–30 The extent of coronary artery calcium and carotid IMT are only moderately correlated,31 suggesting that their pathogeneses may differ. Whether 25-OHD influences the development of coronary atherosclerosis, suggested by previous work,32 needs to be further explored.
Our study design and population bring important strengths to our results. Precise estimates of associations, of utmost importance given the null findings, were possible due to the large sample size and strict quality of the outcome measures. The possibility of residual confounding was reduced by the well-measured confounding variables and the lack of clinical cardiovascular disease at baseline. Survivorship bias was minimized by showing similar results for cross-sectional associations at baseline between the entire cohort and the subcohort with both ultrasound examinations. Finally, the participants’ diversity in race/ethnicity, age range and gender broaden the generalizability of the results. Study limitations included its observational design, possible measurement error in mineral metabolism and carotid biomarkers, especially for longitudinal IMT and plaque measurements, the use of surrogate markers of carotid atherosclerosis as well as lack of data on vitamin D supplementation.
In conclusion, data from this large, diverse cohort do not support clinically meaningful relationships of circulating 25-OHD or PTH concentrations with carotid IMT or plaque. If previously-observed relationships of these biomarkers with cardiovascular events are causal, pathways other than carotid atherosclerosis are likely responsible.
Supplementary Material
Significance.
Lower circulating concentrations of 25-hydroxyvitamin D (25-OHD) and higher circulating concentrations of parathyroid hormone (PTH) are associated with increased risk of cardiovascular events, but potential disease pathways are poorly defined. In this study, we measured 25-OHD and PTH in 3251 participants without cardiovascular disease who underwent two carotid ultrasounds a mean of 9.4 years apart. 25-OHD and PTH were associated neither with the severity or progression of intima-media thickness nor with the prevalence or incidence of carotid plaques. These null results were observed among all races and ethnicities. The absence of associations suggests that the pathways mediating the increased cardiovascular risk of vitamin D and PTH may be independent of carotid atherosclerotic processes.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Sources of funding
This research was supported by grant R01-HL096875 and contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute, grant ES015915 from the National Institute of Environmental Health Sciences, grant R831697 from the US Environmental Protection Agency, and grants UL1-RR-024156 and UL1-RR-025005 from NCRR.
M Blondon is supported by a grant for prospective researchers from the Swiss National Science Foundation.
IH de Boer has received research funding from Abbott Laboratories.
Abbreviations
- 25-OHD
25-hydroxyvitamin D
- PTH
parathyroid hormone
- IMT
intima-media thickness
- MESA
Multi-Ethnic Study of Atherosclerosis
- CV
coefficient of variation
- ICA
internal carotid artery
- CCA
common carotid artery
- BMI
body-mass index
- GFR
glomerular filtration rate
- LDL
low-density lipoprotein
- HDL
high-density lipoprotein
- CRP
C-reactive protein
- µm
micrometer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
All other authors report no conflicts of interests.
REFERENCES
- 1.Kestenbaum B, Katz R, de Boer I, Hoofnagle A, Sarnak MJ, Shlipak MG, Jenny NS, Siscovick DS. Vitamin d, parathyroid hormone, and cardiovascular events among older adults. Journal of the American College of Cardiology. 2011;58:1433–1441. doi: 10.1016/j.jacc.2011.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L, Song Y, Manson JE, Pilz S, Marz W, Michaelsson K, Lundqvist A, Jassal SK, Barrett-Connor E, Zhang C, Eaton CB, May HT, Anderson JL, Sesso HD. Circulating 25-hydroxy-vitamin d and risk of cardiovascular disease: A meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5:819–829. doi: 10.1161/CIRCOUTCOMES.112.967604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hagstrom E, Hellman P, Larsson TE, Ingelsson E, Berglund L, Sundstrom J, Melhus H, Held C, Lind L, Michaelsson K, Arnlov J. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation. 2009;119:2765–2771. doi: 10.1161/CIRCULATIONAHA.108.808733. [DOI] [PubMed] [Google Scholar]
- 4.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin d, diabetes, and ethnicity in the third national health and nutrition examination survey. Diabetes Care. 2004;27:2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 5.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis d is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin d and the metabolic syndrome among u.S. Adults. Diabetes Care. 2005;28:1228–1230. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- 7.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. Plasma 25-hydroxyvitamin d levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 8.Dusso AS, Brown AJ, Slatopolsky E. Vitamin d. American journal of physiology. Renal physiology. 2005;289:F8–F28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 9.Choi HS, Kim SH, Rhee Y, Cho MA, Lee EJ, Lim SK. Serum parathyroid hormone is associated with carotid intima-media thickness in postmenopausal women. International journal of clinical practice. 2008;62:1352–1357. doi: 10.1111/j.1742-1241.2008.01801.x. [DOI] [PubMed] [Google Scholar]
- 10.Shoben AB, Rudser KD, de Boer IH, Young B, Kestenbaum B. Association of oral calcitriol with improved survival in nondialyzed ckd. J Am Soc Nephrol. 2008;19:1613–1619. doi: 10.1681/ASN.2007111164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor EN, Curhan GC, Forman JP. Parathyroid hormone and the risk of incident hypertension. Journal of hypertension. 2008;26:1390–1394. doi: 10.1097/HJH.0b013e3282ffb43b. [DOI] [PubMed] [Google Scholar]
- 12.Saleh FN, Schirmer H, Sundsfjord J, Jorde R. Parathyroid hormone and left ventricular hypertrophy. Eur Heart J. 2003;24:2054–2060. doi: 10.1016/j.ehj.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Bosworth C, Sachs MC, Duprez D, Hoofnagle AN, Ix JH, Jacobs DR, Jr., Peralta CA, Siscovick DS, Kestenbaum B, de Boer IH. Parathyroid hormone and arterial dysfunction in the multi-ethnic study of atherosclerosis. Clin Endocrinol (Oxf) 2013 doi: 10.1111/cen.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 15.Michos ED, Streeten EA, Ryan KA, Rampersaud E, Peyser PA, Bielak LF, Shuldiner AR, Mitchell BD, Post W. Serum 25-hydroxyvitamin d levels are not associated with subclinical vascular disease or c-reactive protein in the old order amish. Calcified tissue international. 2009;84:195–202. doi: 10.1007/s00223-008-9209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pilz S, Henry RM, Snijder MB, van Dam RM, Nijpels G, Stehouwer CD, Tomaschitz A, Pieber TR, Dekker JM. 25-hydroxyvitamin d is not associated with carotid intima-media thickness in older men and women. Calcified tissue international. 2009;84:423–424. doi: 10.1007/s00223-009-9238-6. [DOI] [PubMed] [Google Scholar]
- 17.Lim S, Shin H, Kim MJ, Ahn HY, Kang SM, Yoon JW, Choi SH, Kim KW, Song JH, Choi SI, Chun EJ, Shin CS, Park KS, Jang HC. Vitamin d inadequacy is associated with significant coronary artery stenosis in a community-based elderly cohort: The korean longitudinal study on health and aging. J Clin Endocrinol Metab. 2012;97:169–178. doi: 10.1210/jc.2011-1580. [DOI] [PubMed] [Google Scholar]
- 18.Sachs MC, Brunzell JD, Cleary PA, Hoofnagle AN, Lachin JM, Molitch ME, Steffes MW, Zinman B, de Boer IH. Circulating vitamin d metabolites and subclinical atherosclerosis in type 1 diabetes. Diabetes Care. 2013 doi: 10.2337/dc12-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Targher G, Bertolini L, Padovani R, Zenari L, Scala L, Cigolini M, Arcaro G. Serum 25-hydroxyvitamin d3 concentrations and carotid artery intima-media thickness among type 2 diabetic patients. Clin Endocrinol (Oxf) 2006;65:593–597. doi: 10.1111/j.1365-2265.2006.02633.x. [DOI] [PubMed] [Google Scholar]
- 20.Choi AI, Lo JC, Mulligan K, Schnell A, Kalapus SC, Li Y, Hunt PW, Martin JN, Deeks SG, Hsue PY. Association of vitamin d insufficiency with carotid intima-media thickness in hiv-infected persons. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;52:941–944. doi: 10.1093/cid/ciq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reis JP, von Muhlen D, Michos ED, Miller ER, 3rd, Appel LJ, Araneta MR, Barrett-Connor E. Serum vitamin d, parathyroid hormone levels, and carotid atherosclerosis. Atherosclerosis. 2009;207:585–590. doi: 10.1016/j.atherosclerosis.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viljoen A, Singh DK, Twomey PJ, Farrington K. Analytical quality goals for parathyroid hormone based on biological variation. Clin Chem Lab Med. 2008;46:1438–1442. doi: 10.1515/CCLM.2008.275. [DOI] [PubMed] [Google Scholar]
- 23.Ankrah-Tetteh T, Wijeratne S, Swaminathan R. Intraindividual variation in serum thyroid hormones, parathyroid hormone and insulin-like growth factor-1. Ann Clin Biochem. 2008;45:167–169. doi: 10.1258/acb.2007.007103. [DOI] [PubMed] [Google Scholar]
- 24.Major JM, Graubard BI, Dodd KW, Iwan A, Alexander BH, Linet MS, Freedman DM. Variability and reproducibility of circulating vitamin d in a nationwide u.S. Population. J Clin Endocrinol Metab. 2013;98:97–104. doi: 10.1210/jc.2012-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: A meta-analysis. Atherosclerosis. 2012;220:128–133. doi: 10.1016/j.atherosclerosis.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 26.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-dihydroxyvitamin d(3) is a negative endocrine regulator of the renin-angiotensin system. The Journal of clinical investigation. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Somjen D, Weisman Y, Kohen F, Gayer B, Limor R, Sharon O, Jaccard N, Knoll E, Stern N. 25-hydroxyvitamin d3-1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation. 2005;111:1666–1671. doi: 10.1161/01.CIR.0000160353.27927.70. [DOI] [PubMed] [Google Scholar]
- 28.de Boer IH, Levin G, Robinson-Cohen C, Biggs ML, Hoofnagle AN, Siscovick DS, Kestenbaum B. Serum 25-hydroxyvitamin d concentration and risk for major clinical disease events in a community-based population of older adults: A cohort study. Ann Intern Med. 2012;156:627–634. doi: 10.1059/0003-4819-156-9-201205010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin d and risk of myocardial infarction in men: A prospective study. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 31.Folsom AR, Kronmal RA, Detrano RC, O'Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, Tracy RP, Watson KE, Burke GL. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: The multi-ethnic study of atherosclerosis (mesa) Arch Intern Med. 2008;168:1333–1339. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS. 25-hydroxyvitamin d levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol. 2009;20:1805–1812. doi: 10.1681/ASN.2008111157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.