Abstract
Protein-transduction technology has been attempted to deliver macromolecular materials, including protein, nucleic acids, and polymeric drugs, for either diagnosis or therapeutic purposes. Herein, fusion protein composed of an arginine-rich cell-penetrating peptide, termed low-molecular-weight protamine (LMWP), and a transcriptional coactivator with a PDZ-binding motif (TAZ) protein was prepared and applied in combination with biomaterials to increase bone-forming capacity. TAZ has been recently identified as a specific osteogenic stimulating transcriptional coactivator in human mesenchymal stem cell (hMSC) differentiation, while simultaneously blocking adipogenic differentiation. However, TAZ by itself cannot penetrate the cells, and thus needs a transfection tool for translocalization. The LMWP-TAZ fusion proteins were efficiently translocalized into the cytosol of hMSCs. The hMSCs treated with cell-penetrating LMWP-TAZ exhibited increased expression of osteoblastic genes and protein, producing significantly higher quantities of mineralized matrix compared to free TAZ. In contrast, adipogenic differentiation of the hMSCs was blocked by treatment of LMWP-TAZ fusion protein, as reflected by reduced marker-protein expression, adipocyte fatty acid-binding protein 2, and peroxisome proliferator-activated receptor-γ messenger ribonucleic acid levels. LMWP-TAZ was applied in alginate gel for the purpose of localization and controlled release. The LMWP-TAZ fusion protein-loaded alginate gel matrix significantly increased bone formation in rabbit calvarial defects compared with alginate gel matrix mixed with free TAZ protein. The protein transduction of TAZ fused with cell-penetrating LMWP peptide was able selectively to stimulate osteogenesis in vitro and in vivo. Taken together, this fusion protein-transduction technology for osteogenic protein can thus be applied in combination with biomaterials for tissue regeneration and controlled release for tissue-engineering purposes.
Keywords: protein transduction, low-molecular-weight protamine (LMWP), transcriptional coactivator with PDZ-binding motif (TAZ), selective osteogenesis, bone-tissue engineering
Introduction
Protein transduction is a potential tool for the delivery of proteins into mammalian cells, and has drawn significant interest from the medical and pharmaceutical communities.1–3 Basic and exceptionally potent cell-penetrating peptides (CPPs) derived from the protein-transduction domains of virus-related proteins (including peptides from human immunodeficiency virus, the Tat protein, and herpes simplex virus type I (VP22) have been applied in this protein-transduction technology.4,5 Dozens of diverse proteins, including transcription-factor enzymes, other therapeutic proteins, and even solid nanoparticles have been linked to CPPs, thereby demonstrating translocation into the cells in different organs, including the brain.6–9
Herein, we focus on the intracellular delivery of transcriptional factor/cofactor protein to enhance selective osteogenesis in vitro and in vivo using protein-transduction technology. Current therapies to expedite bone formation, either for repairing bone defects or for the treatment of osteoporosis, include recombinant growth factors, hormones, gene therapy, and tissue engineering in combination with bone-graft materials.10–12 Growth factors, including bone morphogenetic protein (BMP)-2, have been widely applied in enhanced bone formation and gained some success in bone-forming results.13–15 However, clinical application of these growth factors is beset by side effects related to dose, due to their nonselective cellular proliferation and differentiation, followed by unpredictable physiologic consequence, such as tumor cell promotion and uncontrolled mineral creation due to the lack of a possible localization delivery carrier.16,17 Unlike growth factors and hormones, cytoplasmic transcription factors/cofactors would be the obvious molecular target of choice in attempting to direct specific osteogenic differentiation. In this regard, the delivery strategies of selective, specific factors for enhancing bone formation while avoiding unwanted divergent differentiation seem promising.
A transcriptional coactivator with a PDZ-binding motif (TAZ) was initially identified through its ability to interact with 14-3-3 proteins, and is also called WW domain-containing transcription regulator 1. Recent studies have reported that BMP-2 stimulates expression of TAZ, which induces subsequent Smad signaling and expression of the osteocalcin (OCN) gene by Runt-related transcription factor 2 (Runx2), followed by increased osteoblast differentiation.18 The two transcriptional factors core-binding factor alpha (Cbfa)1/Runx2 and peroxisome proliferator-activated receptor (PPAR)γ drive mesenchymal stem cells (MSCs) to differentiate into either osteoblasts or adipocytes, respectively, and the differentiation of each lineage appears to be transcriptionally controlled by the presence of TAZ.19–23 One of the functions of TAZ is as a regulator of MSC differentiation by promoting osteoblastic differentiation through activating Runx2, while simultaneously impairing adipocyte differentiation by suppressing PPARγ expression.20–22 It would be advantageous not only for bone-defect healing but also for correcting imbalance between osteogenesis and adipogenesis in osteoporotic disorders. Therefore, it is anticipated that delivery of TAZ can specifically induce osteogenesis by osteogenic/adipogenic balance recovery or bone regeneration by TAZ transduction into cell membrane. However, application of TAZ protein itself is limited due to the fact that it is not able to enter the cells. So far, most studies investigating the transcription factor have mainly focused on gene delivery.24 Viral and nonviral vectors have been used to achieve gene delivery into cells; however, viral vectors raise serious concerns with regard to safety, and nonviral vectors, such as Lipofectamine, have low efficiency for gene delivery. As indicated earlier, a transducible protein can be directly applied to target cells due to fusion with a small-peptide tag that promotes translocation of the protein into the cells. The authors previously identified the nontoxic CPP low-molecular-weight protamine (LMWP; VSRRRRRRGGRRRR) and its conjugates with therapeutic anticancer protein and even with solid nanoparticulates for stem cell labeling/trafficking purposes.9,25
Herein, we propose a fusion protein of the LMWP peptide and TAZ protein for specific bone formation in vitro and in vivo. TAZ was fused with a gene fragment encoding the amino acids of LMWP peptide in a bacterial expression vector to produce a genetic in-frame LMWP-TAZ fusion protein. We expect that the cell-penetrating LMWP-TAZ fusion protein can enhance the osteogenic differentiation of human MSCs (hMSCs), while blocking adipogenic differentiation.
Materials and methods
Plasmid construction
For generation of the LMWP-TAZ fusion protein, the oligonucleotides 5′-AATTCGTGTCCCGACGTCGT CGCAGGAGAGGAGGCCGCAGGAGGCGTTA-3′ and 5′-AGCTTTAACGCCTCCTGCGGCCTCCTCTC-CTGCGACGACGTCGGGACACCC-3′ were annealed and inserted into the EcoRI-Hind III sites of pET-21b+ vector (Merck Millipore, Billerica, MA, USA) in frame with the six-histidine open-reading frame to generate the expressions of plasmid LMWP (pLMWP). Full-length TAZ complementary deoxyribonucleic acid (cDNA) was amplified by polymerase chain reaction (PCR) with the primers 5′-AGCTTATGAATCCGTCCTCGGTG-3′and 5′-TCGAGTTAGAAAGGGCTCGCTTTTG-3′ and inserted into the Hind III-Xhol sites of pET21b+ and pLMWP. The program for PCR consisted of 30 cycles of denaturation at 94°C for 40 seconds, annealing at 55°C for 1 minute, extension at 72°C for 3 minutes, and the final extension at 72°C for 10 minutes. The TAZ cDNA was subcloned into pET-21b+ and pLMWP expression vectors that had been digested with the same restriction enzymes (Figure 1). The nucleotide sequences of all PCR products cloned into the plasmid were confirmed by sequencing.
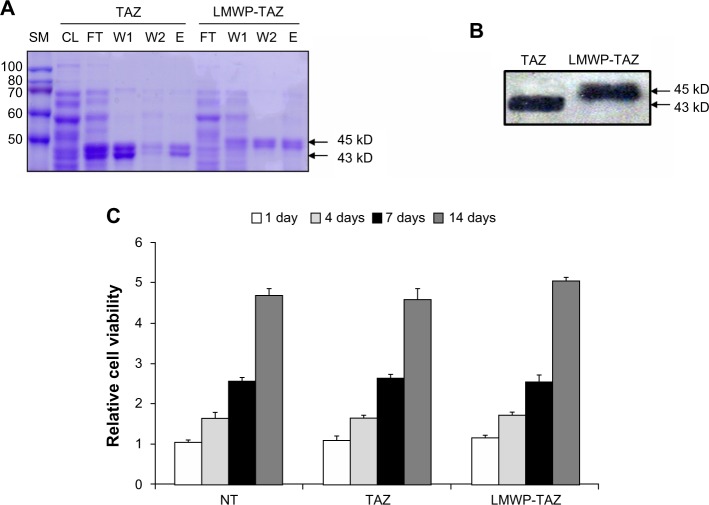
Figure 1.
(A–C) Identification of LMWP-TAZ fusion protein.
Notes: (A) Purification of LMWP-TAZ employing Ni(II)-affinity chromatography was detected by Coomassie staining. (B) Western blot analysis of purified fusion proteins with anti-TAZ antibody. (C) Effect of the fusion proteins on cell viability. Changes in cell viability according to the TAZ and the LMWP-TAZ protein were examined using the MTT assay. The hMSCs were treated with TAZ (100 nM) and LMWP-TAZ (100 nM) for 1, 4, 7, and 14 days in complete medium.
Abbreviations: LMWP, low-molecular-weight protamine; TAZ, PDZ-binding motif; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; hMSCs, human mesenchymal stem cells; SM, size marker; CL, cleared lysate; FT, flow-through; W, wash; E, elution. NT, no treatment.
Expression and purification of recombinant proteins
The pET-21b+ plasmids were used to transform Escherichia coli strain BL21(DE3) and induced using Isopropyl β-D-1-thiogalactopyranoside (IPTG). The recombinant proteins tagged a six histidine (6× His) were isolated by affinity chromatography with Ni-nitrilotriacetic acid (NTA) agarose (Qiagen, Venlo, the Netherlands), eluted with 100 mM NaH2PO4/10 mM Tris/8 M urea, pH 4.5, and desalted on a PD-10 column (GE Healthcare Bio-Sciences Corp, Piscataway, NJ, USA). The purity of the eluted proteins was analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie Brilliant Blue R staining. The obtained protein was frozen and stored at −20°C until further use. The fusion proteins LMWP-TAZ and TAZ were labeled with fluorescent dye (fluorescein isothiocyanate [FITC]). In brief, the protein solution (pH 9.3, carbonate buffer) was reacted in a 1:2 molar ratio with the FITC solution dissolved in dimethylformamide overnight in the dark at room temperature. Reaction was monitored by high-pressure liquid chromatography (HPLC) of the absorbance change at 280 nm of the protein peak. The labeled protein was purified by HPLC (purity >95%), lyophilized, and then stored at −20°C in the dark until further use.
Cell-viability assay
Bone marrow-derived hMSCs were ordered from Lonza, Walkersville, MD, USA. Cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay. Before the experiments, the hMSCs were plated in 24-well plates and maintained in complete medium. At the indicated time, the cells in the 24-well plates were incubated with 0.5 mg/mL MTT for 2 hours at 37°C. The intensity of the MTT product was measured at 550 nm using a microplate reader (BioTek, Winooski, VT, USA).
Confocal microscopic observation
The cells were plated on four-well (Lab-Tek™; Thermo Fisher Scientific, Waltham, MA, USA) chambered cover glasses at a density of 1×104 cells/cover glass and incubated at 37°C in humidified 5% CO2. After complete adhesion, the FITC-labeled LMWP-TAZ protein or TAZ was then added to the cells at a final protein concentration of 100 nm. Following 20 minutes’ incubation at 37°C in humidified 5% CO2, cells were washed three times with phosphate-buffered saline (PBS) and fixed with 3.7% formaldehyde in PBS. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; 20 minutes at 300 ng/mL), and the cells were visualized and photographed using an FV-300 laser scanning microscope (Olympus, Tokyo, Japan).
Flow-cytometric analyses of LMWP-TAZ protein internalization
The cells were seeded at a density of 1×106 cells per well in six-well plates. After 24 hours, the cells were washed and incubated with samples including FITC-labeled LMWP-TAZ or TAZ protein (100 nm) for 30 minutes at 37°C in humidified 5% CO2. After incubation, cells were washed with PBS, extensively treated with trypsin-ethylenediaminetetraacetic acid (EDTA) to remove surface-bound FITC-labeled samples, and washed again, thereby avoiding any artifacts. Analysis was conducted on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA) equipped with a 488 nm air-cooled argon laser. The filter settings for emission were 530/30 nm band pass (FL1) for FITC. The fluorescence of 10,000 vital cells was acquired, and data were visualized in logarithmic mode.
SDS-PAGE and Western blot analysis
hMSCs were incubated with LMWP-TAZ fusion protein in Dulbecco’s Modified Eagle’s Medium (DMEM) for the indicated times. Cells were lysed at 4°C in a lysis buffer containing 20 mM Tris at pH 7.5, 150 mM NaCl, 2 mM EDTA, 2 mM ethylene glycol tetraacetic acid, 1 mM sodium orthovanadate, 10 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride, and 0.5% Triton X-100 and incubated on ice for 30 minutes. Protein concentration was measured with a Bradford protein-assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Equal aliquots of protein (40 μg) were boiled for 5 minutes in 5× sample buffer (250 mM Tris-HCl [pH 6.8], 50% glycerol, 10% SDS, 500 mM dithiothreitol, 0.5% bromophenol blue) and separated on 10% SDS-PAGE gels. Samples were transferred to nitrocellulose membranes (GE Healthcare Bio-Sciences), and probed with anti-TAZ (Imgenex Corporation, San Diego, CA, USA). Anti-TAZ was incubated 1:5,000 in 1× Tris-buffered saline with Tween 20 (TBS-Tween 20) containing 5% dry milk, and anti-6× His tag antibody (Abcam, Cambridge, MA, USA) was used 1:2,000 in 1× TBS-Tween 20 containing 3% bovine serum albumin. Blocking was carried out in corresponding buffers without antibody for 1 hour at room temperature. After three washes, the membranes were incubated with secondary antibody (horseradish peroxidase-conjugated goat antirabbit immunoglobulin G 1:2000 in TBS-Tween 20) for 60 minutes, followed by another three washes. Protein bands were visualized with chemiluminescence reagents (WEST-ZOL®; iNtRON Biotechnology, INC., Seoul, South Korea).
Osteogenic and adipogenic differentiation assay
Mineralization of the extracellular matrix was determined by alizarin red S stain (Junsei Chemical Co., Tokyo, Japan), using previously described methods.26 Briefly, the hMSCs (1×106 cells) were treated with either LMWP-TAZ or TAZ protein at 100 nm concentration and incubated for 14 days in mineralization medium containing 20% fetal bovine serum, 50 µg/mL ascorbic acid, 10 mM β-glycerophosphate, and 10−7 M dexamethasone. For the alizarin red S staining, the cells were washed three times with PBS and fixed in ice-cold 95% ethanol for 15 minutes at 4°C. After additional washing in water, they were incubated in 2% alizarin red S in water for 10 minutes at room temperature, followed by three washes with water and finally incubated with 70% ethanol followed by absolute ethanol and air-dried. To observe effects of the fusion proteins on osteogenesis, total ribonucleic acid (RNA) was isolated at 1, 3, and 7 days post-transduction of cells using a Trizol RNA isolation kit (Life Technologies, Carlsbad, CA, USA), and quantified by ultraviolet spectroscopy. PCR was performed with primers specific for alkaline phosphatase (ALP), type I collagen, osteopontin (OPN), Runx2, PPARγ, adipocyte fatty acid-binding protein (aP)-2, and glyceraldehyde 3-phosphate dehydrogenase.
To induce adipogenesis on hMSCs, the cells were exposed to fresh DMEM containing TAZ or LMWP-TAZ fusion protein for 2 hours at 100 nm. The medium was discarded, and fresh DMEM with 5% fetal bovine serum was added. The control group was treated with the same amount of vehicle. After 48 hours, cells were washed with PBS, and human adipogenic maintenance medium (50 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, and 100 μM indomethacin) was added to the cells for 48 hours, which was followed by culturing in adipogenic induction medium containing 50 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, 10 μg/mL insulin, and 100 μM indomethacin for 72 hours. Cells were stained with Oil Red O (Sigma-Aldrich, St Louis, MO, USA).
Loading LMWP-TAZ protein in alginate gel and in vitro protein release
The purified recombinant TAZ or LMWP-TAZ protein solution (2 mg in 100 μL PBS, pH 8.5) was added to 1 mL 3% sodium alginate solution. Then 200 μL of 0.2 M calcium chloride was added to the alginate solution and mixed homogeneously to create a gel. A release experiment was conducted under 0.1 M sodium bicarbonate buffer (pH 8.5) for 7 days. In brief, 2 mg LMWP-TAZ-loaded alginate gel was placed in 1 mL sodium bicarbonate buffers in the glass vials and shaken at a speed of 80 rpm at 37°C. At specific time intervals, the releasing medium was replenished with fresh bicarbonate buffer solution. Then, 0.25 mL 0.01% 2,4,6-trinitrobenzene sulfonic acid was added to 0.5 mL of each sample solution and incubated at 37°C for 2 hours. SDS solution (10%) 0.25 mL and 0.125 mL 1 M HCl were added to each sample to terminate the reaction. Protein concentration was measured at 335 nm using a microplate reader (BioTek) using an established calibration curve. A calibration curve was constructed in the concentration range of 1–1,000 μg using bovine serum albumin (1 mg/mL in 0.1 M sodium bicarbonate) solution. In addition, the activity of the released TAZ was confirmed by Western blot assay using an anti-TAZ antibody (1:500).
In vivo bone-formation study
New Zealand White male rabbits weighing 2–2.5kg (eight animals per test group) were used to assess in vivo bone formation by 2 mg TAZ- or LMWP-TAZ-loaded alginate gel per site. The rabbits were anesthetized with an intramuscular injection of xylazine (3.5 mg/kg body weight). After the surgical site had been wiped with Betadine (Povidone-iodine; Alcon Laboratories, TX, USA), local anesthesia was provided with a 2% lidocaine solution. A skin incision and a periosteal flap exposed the cranium. A craniotomy defect (8 mm in diameter) was then performed using a trephine needle in a dental handpiece whilst being supplemented with physiological saline. After dissection of the calvarial disc, the samples including LMWP-TAZ fusion protein or TAZ protein with alginate gel were placed into the defect, and the soft tissues and skin were closed using 5-0 chromic gut and 4-0 silk (Ethicon; Johnson and Johnson, New Brunswick, NJ, USA). The alginate gel without LMWP-TAZ fusion protein or TAZ protein and untreated defect served as controls. The animals were killed 4 weeks after implantation.
The retrieved specimens were fixed in a formalin solution and embedded in superlow-viscosity embedding media (Polysciences, Inc., Pittsburgh, PA, USA) without prior decalcification. The coronal sections (20 μm in thickness) were sliced and stained with a hematoxylin and eosin staining-solution kit (Sigma-Aldrich). Microscopic examination was conducted using a BH-2 optical microscope (Olympus). Histomorphometric measurements of newly formed bone were conducted using an automated image-analysis system equipped with a charge-coupled-device camera on a light microscope. Stained sections were measured semiquantitatively using Bioquant Image Analysis (Nashville, TN, USA) software. Furthermore, the specimens were evaluated by micro-computed tomography (microCT) scan. The microCT scans were obtained with a SkyScan 1076 (SkyScan, Aartselaar, Belgium). Scanning was performed with a resolution of 35 μm and a scanning width of 68 μm in the direction parallel to the coronal aspect of the calvarial bone. Digital microradiographic images were acquired at 120 kV and 100 mA. The images were then reconstructed in 3-D using Cone-Beam CT-reconstruction A Sasov software (SkyScan). Also, a cylindrical region of interest (ROI, 8×1 mm) was positioned over the defect site. The total volume of newly formed bone within the ROI was measured by assigning a threshold for total bone content and subtracting bone volume of the control group. The total volume of bone in four defects is reported (mm3).
Statistical analysis
All values are presented as means ± standard error for all controls and experiments (n= the total number of independent cultures). Data were analyzed by one-way analysis of variance followed by Fisher’s protected least significant difference post hoc test (StatView; SAS Institute, Cary, NC, USA). P-values <0.05 were considered significant.
Results and discussion
Generation of LMWP-TAZ fusion proteins
Regulating the switch between proliferation and differentiation of MSCs is critical for the normal tissue healing while presenting with a tumor. In particular, under such pathologic conditions as bone fracture, selective osteogenesis is needed without affecting overproliferation or other unwanted differentiation. Other than bone fracture, osteoporosis presents the imbalance of MSC differentiation into adipocytes rather than osteoblasts, ie, arrested bone formation accompanied by an increase in bone marrow adipogenesis occurs with aging and is further related to osteoporosis. Growth factor-related signal transduction has been used to recover bone-forming capacity with some success, though with accompanying unnecessary cell proliferation. In this regard, selective direction of bone formation by transcriptional factors seems promising. However, as described earlier, TAZ itself is not transducible into the cells, and thus requires a potent intracellular delivery carrier.
We designed the 3.5 kb pLMWP-TAZ construct containing the eleven-amino acid-long LMWP sequence fused to the 5′-end of the TAZ tag. Six histidine residues to allow for affinity purification were followed by the TAZ sequence. The plasmids pLMWP-TAZ and control pTAZ were then sequenced to confirm that the coding region was in frame. E. coli BL21(DE3) cells were transduced with either construct and induced by IPTG. Purification of the supernatant through an Ni-NTA-Sepharose affinity column yielded mature fusion proteins of either LMWP-TAZ or TAZ. Each step of purification was confirmed by Coomassie staining (Figure 1A). The yield of the purified fusion proteins was approximately 20 mg/L culture. The eluted fraction of each culture verified their molecular weight using SDS-PAGE with size marker. The presence of active TAZ fusion proteins was further verified by Western blot analysis using anti-TAZ polyclonal antibody and displayed the expected size of 45 kDa (Figure 1B), indicating that the TAZ protein was linked to LMWP by a 1:1 molar ratio. The difference in molecular weight between LMWP-TAZ and TAZ was almost identical to the molecular weight of LMWP.
We investigated the cytotoxicity of LMWP-TAZ compared with TAZ in hMSCs using MTT. Human MSCs were proliferated for 14 days, and there was no significant effect on cell viability by LMWP-TAZ and TAZ (Figure 1C). This result suggested that LMWP-TAZ can be utilized as an intracellular delivery tool without affecting cell viability. The LMWP peptide possesses several biological advantages as a CPP over TAT47–57 in that it has significantly reduced antigenicity, mutagenicity, and complement-activating activity, due to being originally derived from a naturally existing protein – a protamine. Moreover, LMWP showed fewer hemodynamic/hematologic toxic effects than the parent protamine, which has been confirmed by extensive and conclusive animal studies.25,27,28 A previous report of ours also showed that polyethyleneimine, a transfecting agent currently in use, reduced cell viability much more than LMWP.25 These findings point to the potential of LMWP as a delivery carrier in protein therapy.
Internalization of LMWP-TAZ fusion protein into hMSCs
We assessed the cell permeability of the LMWP-TAZ and TAZ fusion proteins. To monitor the localization of the proteins during cellular uptake in living cells, FITC-labeled proteins were used. As shown by the FACS result (Figure 2A), labeled TAZ was detected after 20 minutes of incubation of these proteins with hMSCs at a final concentration of 100 nm. To avoid possible fluorescence detection by surface-bound protein, extracellular-bound protein was removed using trypsin-EDTA, thereby allowing detection of internalized protein. LMWP-TAZ-FITC protein rapidly internalized into ~100% of cells, achieving maximum intracellular concentration in 20 minutes, which was in contrast to negligible detection by TAZ-FITC protein. We investigated intracellular translocation of the LMWP-TAZ by live cell-imaging instead of fixation/membrane permeation. Nucleus staining (blue) was accompanied by live cell observation. FITC-labeled LMWP-TAZ penetrated into the cytoplasm as well as the nucleus. On the other hand, LMWP unfused TAZ was not detected in cytosol after 40 minutes incubation and was followed by washing with PBS (Figure 2B).
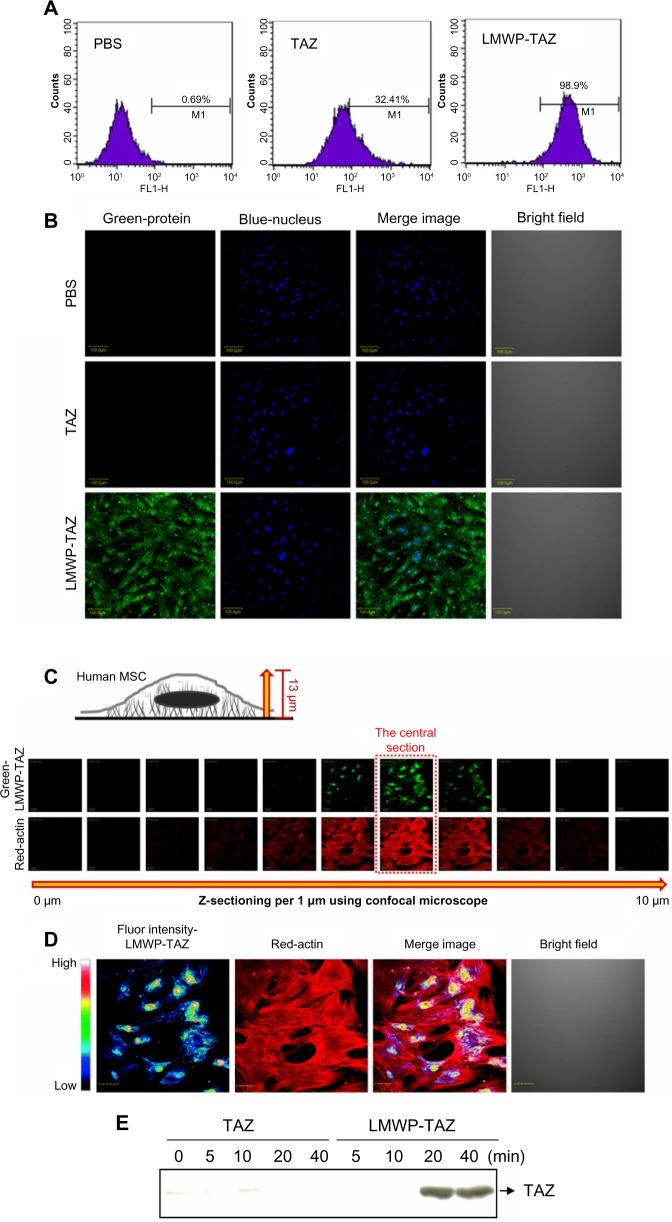
Figure 2.
(A–E) Quantification of LMWP-TAZ fusion protein transduction in hMSCs.
Notes: (A) Flow-cytometric analysis of hMSCs treated with TAZ and LWMP-TAZ fusion proteins at a concentration of 100 nm for 20 minutes. FL1-H indicates fluorescence intensity of FITC tagged protein in the cells. (B) Confocal microscopy of LMWP-TAZ fusion protein into the hMSCs. Cells were incubated in medium containing 100 nM TAZ and LMWP-TAZ fusion protein for 20 minutes. Nuclei were counterstained with DAPI. Scale bar 200 μm. (C) Z-sectioned images of the LMWP-TAZ-treated cells from the bottom to the highest surface of cells by 1 μm. (D) A pseudocolor confocal image showing penetration, distribution, and intensity profile in the central sections of the cells incubated with LMWP-TAZ at 37°C for 40 minutes. Magnification 400×. (E) Time dependence of LMWP-TAZ internalization efficiency was determined by Western blot analysis of transduced hMSCs. The cells were treated with 100 nm of TAZ and LMWP-TAZ fusion proteins for 0, 5, 10, 20, and 40 minutes.
Abbreviations: PBS, phosphate-buffered saline; LMWP, low-molecular-weight protamine; TAZ, PDZ-binding motif; DAPI, 4′,6-diamidino-2-phenylindole; hMSCs, human mesenchymal stem cells; min, minutes; Fluor, fluorescence; FITC, fluorescein isothiocyanate.
Z-section per micrometer was performed from the cell surface to the slide surface using confocal laser scanning microscopy (Figure 2C). With this method, it is easy to observe cytosolic internalization and distribution of protein without cell loss. The central section (at 6 μm from the slide bottom) was magnified 400× and presented as a pseudocolor depending on the intensity of the green fluorescence of penetrated LMWP-TAZ (Figure 2D). The green fluorescence of LMWP-TAZ was not detected at either the highest or lowest section. High fluorescence intensity of LMWP-TAZ was observed in the nucleus as a red color. Therefore, it is expected that nucleus-penetrating LMWP-TAZ could function as a transcriptional factor or cofactor by binding DNA.
The internalized protein was further identified as a fusion protein of TAZ. Western blot analysis was conducted against protein extracts from cell lysate using anti-TAZ. LMWP-TAZ proteins were added to the culture media of hMSCs at 1 μM concentration for various periods. The intracellular concentration of transduced LMWP-TAZ fusion protein into cultured cells was detected at 20 minutes and remained up to 2 hours in hMSCs, while TAZ protein itself was not detected (Figure 2E). This fast translocation of LMWP-TAZ into cells suggests that the LMWP-TAZ fusion protein has the ability to penetrate efficiently the cell membrane in a short time.
LMWP, which consists of two clusters of arginine sequences similar to those of TAT47–57, showed comparable translocation capacity.25 Polyarginine peptides show cell-translocation activity and possesses an internalization pathway similar to those of TAT47–57.29,30 Naturally derived LMWP, developed by the authors, possess high arginine content and carries significant sequence similarity to TAT, by far the most potent protein-transduction domain peptide. In our previous study, LMWPs demonstrated their capability of transducing a nonpenetrating protein toxin and small interfering RNA (siRNA; vascular endothelial growth-factor siRNA) into the tumor cells by chemical conjugation as efficiently as TAT, and determined cytotoxicity of transduced protein toxin or siRNA against cancer cell lines and a tumor-bearing mouse.25,31 In this study, the LMWP-TAZ fusion protein was produced using bacterial expression, a frequently used method for the production of recombinant proteins.
Osteogenic differentiation by LMWP peptide-TAZ fusion protein
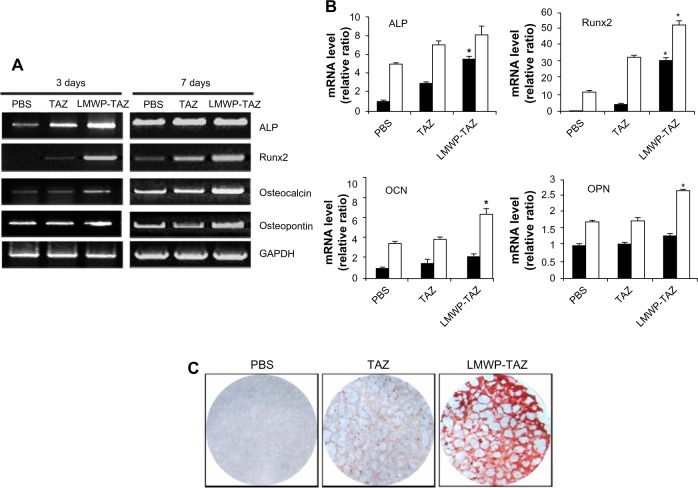
Expression of osteogenic gene markers was examined at 3 and 7 days after protein transduction by conducting reverse-transcription (RT)-PCR. As expected, ALP and Runx2 expression was significantly higher in the LMWP-TAZ treated cells than either TAZ or untreated cells at 3 days. In addition, cells treated with LMWP-TAZ fusion protein significantly increased OCN and OPN levels at 7 days after transduction, which lies in the late stage of osteoblastic differentiation, compared to TAZ protein (Figure 3A and B). Osteogenic marker proteins have different expression times, such as with early or late-stage markers. ALP and Runx2 are early markers, while OCN and OPN are late markers. The role of LMWP-TAZ was evident at each stage of differentiation. At day 3, it increased ALP and Runx2 expression, resulting in further stimulation of the late markers OCN and OPN. The primary biological function of ALP in bone formation is associated with calcification of the skeleton. This function is exerted either by its ability to catalyze the hydrolysis of organic phosphatase esters, thereby providing inorganic phosphate, or by removing the inorganic pyrophosphate that inhibits calcification at the site of active mineralization. OPN and OCN are secreted proteins, which are late stage markers of osteoblastic differentiation. TAZ is known to trigger the signal of Runx2 expression, thereby stimulating OCN expression.32 Our RT-PCR result is consistent with the previous report that followed similar osteogenic stimulation flow from Runx2 through OCN expression;26 however, stimulation was obtained only when the LMWP was conjugated with TAZ to translocate into the cells.
Figure 3.
(A–C) Effect of osteogenic differentiation by LMWP-TAZ fusion protein.
Notes: The hMSCs were treated with fusion proteins for indicated times in osteogenic media containing 20% fetal bovine serum, 50 µg/mL ascorbic acid, 10 mM β-glycerophosphate, and 10−7 M dexamethasone for detection of osteogenic differentiation. (A) The expression levels of specific osteogenic genes were detected by reverse-transcription polymerase chain reaction, and (B) quantified at 3 days (black column) and 7 days (white column). (C) Detection of matrix mineralization by alizarin red S. Three independent experiments were performed in duplicate, and significant differences are denoted by asterisks (P<0.05), indicating significantly different to time-matched TAZ group.
Abbreviations: mRNA, messenger ribonucleic acid; ALP, alkaline phosphatase; OCN, osteocalcin; OPN, osteopontin; PBS, phosphate-buffered saline; LMWP, low-molecular-weight protamine; Runx2, runt-related transcription factor 2; TAZ, PDZ-binding motif; hMSCs, human mesenchymal stem cells; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Next, alizarin red S staining for calcium deposits was used to detect matrix mineralization of hMSCs at 14 days of post-transduction. As expected, treatment by LMWP-TAZ fusion protein displayed strong positive staining for mineralization compared to TAZ treatment (Figure 3C). In contrast, cells treated with TAZ did not create calcium deposits until day 14, the same as those without treatment, simply because TAZ on its own could not internalize the cells. Coadministration of LMWP with TAZ did not yield any mineralization either, indicating that TAZ itself was still not able to cross the cell membrane without conjugation with LMWP (data not shown). On the contrary, the LMWP-TAZ fusion protein was found to increase mineralization, indicating internalization into the cells. These results suggest that the exogenous LMWP-TAZ fusion protein, when transduced into the cell membrane, can be a transcriptional modifier of MSC differentiation into osteoblastic cells.
Inhibition of adipogenic differentiation by cell-penetrating LMWP-TAZ fusion protein
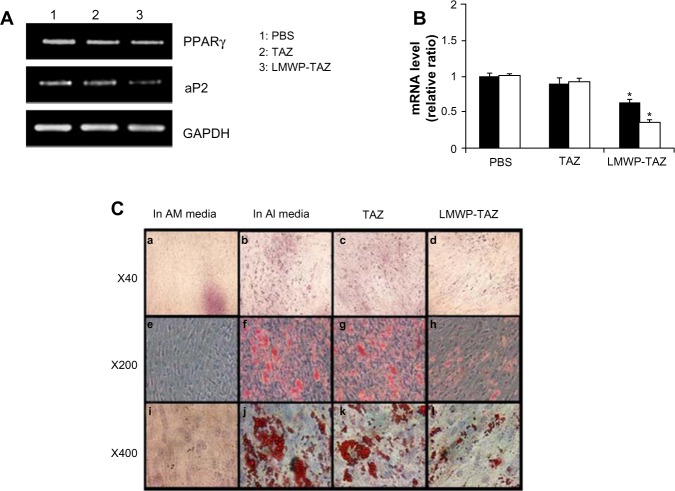
To examine the role of the LMWP-TAZ protein on adipogenic differentiation, the expression of representative adipogenic marker proteins, such as PPARγ and aP2, was examined 3 days after protein transduction. PPARγ, a fat-specific transcription factor, plays a role in preadipocyte commitment while aP2 gene is known to be upregulated during adipogenesis. Expression levels of PPARγ and aP2 mRNA were significantly decreased in the hMSCs treated with LMWP-TAZ, compared to cells treated with TAZ protein or cells without treatment (Figure 4A and B). To confirm further the inhibition of adipogenesis by LMWP-TAZ, an Oil Red O staining assay was conducted on the cells treated with each protein. After 14 days of protein transduction, marked red-stained lipid droplets were observed in cells without treatment as well as cells treated with TAZ protein (Figure 4C). In sharp contrast, negligible numbers of lipid droplets were seen in cells treated with LMWP-TAZ fusion protein, due to the internalized fusion protein inhibiting the expression of adipogenic protein, as seen from the inhibited adipogenic markers.
Figure 4.
(A–C) Effect of adipogenic differentiation by LMWP-TAZ fusion protein.
Notes: Cells were grown in adipogenic-inducing (AI) media and adipogenic-maintaining (AM) media with fusion proteins, as described in the Materials and methods section. (A) Downregulation of adipogenic gene markers detected, and (B) quantified PPARγ (black column) and aP2 (white column). Three independent experiments were performed in duplicate, and significant differences are denoted by symbols. Significant differences are denoted by asterisks (P<0.05), indicating significantly different to time-matched TAZ group. (C) After 14 days, cells were fixed, and cells containing lipid droplets were stained with Oil red O and photographed. Cells cultured in AM media (a, e and i), cells cultured in AI media (b, f and j), TAZ fusion protein-treated cells (c, g and k), LMWP-TAZ fusion protein-treated cells (d, h and l).
Abbreviations: mRNA, messenger ribonucleic acid; LMWP, low-molecular-weight protamine; TAZ, PDZ-binding motif; PBS, phosphate-buffered saline; PPAR, peroxisome proliferator-activated receptor; aP, adipocyte fatty acid-binding protein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
BMPs are potent local factors that increase osteoblast differentiation and thereby osteogenesis. The addition of recombinant BMP-2 and/or BMP-7 to a pluripotent mouse mesenchymal progenitor cell line (C3H10T1/2) induces the cells to differentiate into three cell types: osteoblasts, chondroblasts, and adipocytes.33–35 In a previous report, BMP-2 induced the differentiation of a mesenchymal progenitor cell line into not only mature osteoblasts but also adipocytes, implying nonselective differentiation.36 It should be pointed out that LMWP-TAZ induces differentiation primarily into osteoblasts compared to BMP-2, and thus this selectivity might be applied to the targeted osteogenesis and/or the treatment of age-related osteoporosis with abnormal adipocyte increase in bone marrow. Previous human and animal studies have shown that osteoporosis presents an inverse relationship between the amount of trabecular bone and adipose tissue in the bone marrow.37,38 Therefore, the enhancement of osteogenesis by the LMWP-TAZ fusion protein with inhibition of adipogenesis could provide a therapeutic modality of selective osteogenesis as well as osteoporosis treatment.
In vitro release of LMWP-TAZ from alginate gel
Most proteins, including LMWP-TAZ, are soluble and easily washed out with the bloodstream or physiologic fluid. Therefore, combination with biomaterials is inevitable when protein is applied in vivo to secure effective local maintenance. The LMWP-TAZ protein was loaded in the alginate solution, and gel was created by the addition of calcium chloride. Alginate gel with LMWP-TAZ was able to retard LMWP-TAZ release from the surface while preventing rapid washout at the surface (Figure 5). The release of protein from alginate gel showed an initial burst effect and was maintained up to 7 days. Half the amount of the loaded LMWP-TAZ fusion protein in alginate gel was released by 3 days, and the release amount was maintained up to 7 days. Most of the protein released from biomaterials shows an initial burst, except for the chemical immobilized proteins on the surface of the biomaterials. The release condition in vitro is not precisely correlated with that in vivo environment, in that fluid volume in an in vivo bone defect or implanted site is smaller than in in vitro release media. In this regard, the initial high concentration of fusion protein confers rapid uptake and osteogenesis by surrounding preosteoblastic cells, while the slow release of protein secures the maintenance of an effective protein concentration for a longer period. Alginate is a linear polysaccharide, and has several favorable properties as as a biomaterial: nontoxicity, biodegradability, and ease of processing into the desired shape. The combination of alginate gel with growth factor has been attempted to enhance healing capacity in tissue regeneration.39 As a carrier material for fusion proteins, alginate gel was applied with the proteins. Therefore, the in vivo osteogenic potential of LMWP-TAZ was anticipated, and further in vivo evaluation was conducted in rabbit calvarial defect.
Figure 5.
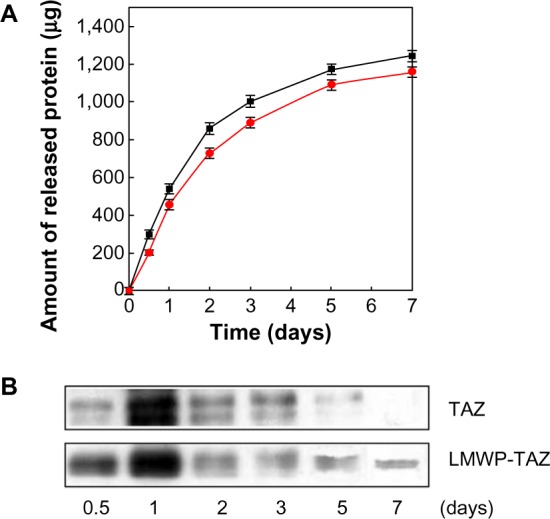
(A and B) Recombinant protein release from alginate gel.
Notes: (A) The amounts of released TAZ protein (■) and LMWP-TAZ fusion protein (•) from alginate gel were measured by 2,4,6-trinitrobenzene sulfonic acid assay. (B) The release amounts of TAZ and LMWP-TAZ fusion proteins on bone minerals were confirmed by Western blot analysis using TAZ antibody.
Abbreviations: LMWP, low-molecular-weight protamine; TAZ, PDZ-binding motif.
In vivo bone formation by LMWP-TAZ fusion protein
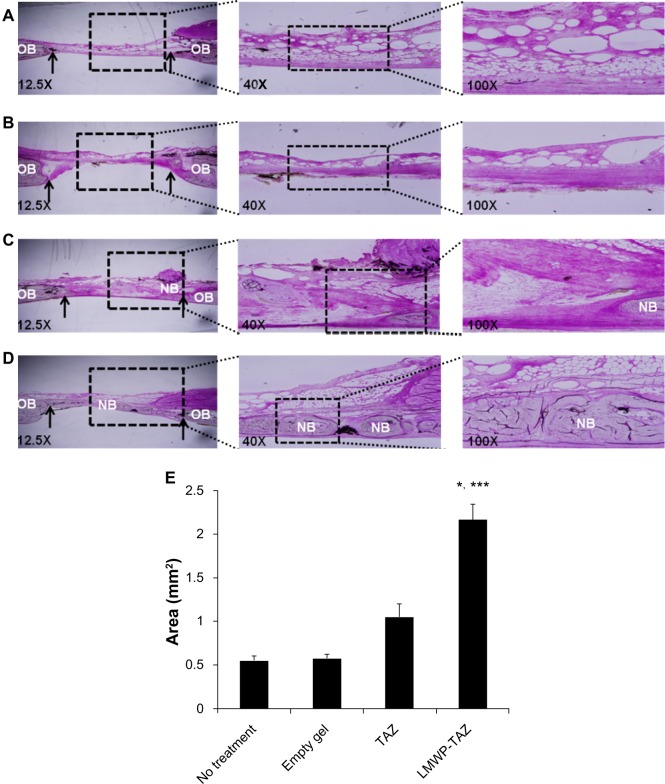
We examined bone regeneration by mixing the fusion protein with alginate gel as a scaffold in the rabbit calvarial defect model. Figure 6 shows histological sections of the rabbit calvarial defects with no treatment, gel-only matrix as a scaffold, TAZ protein-, or LMWP-TAZ fusion protein-loaded alginate gel matrix at 4 weeks after surgery. In the group without treatment, dense and fibrous connective tissue at the defect site was observed. No severe inflammatory reaction was observed along the side of dura mater in untreated groups. The group treated with alginate gel showed little bone formation from the defect, similar to the group without treatment. Connective tissue rather than new osteoid was evident in the specimens treated with TAZ protein-loaded alginate gel. However, in the group treated with LMWP-TAZ protein-loaded alginate gel, the new bone formation was marked, bone growth starting from the bony borders toward the center of the defect. In addition, prominent bony bridges consolidating the central region of the calvarial defect were observed. This result was consistent with histomorphometric analysis, in that LMWP-TAZ fusion protein was revealed to induce faster bone formation than TAZ-treated defects.
Figure 6.
(A–E) Histological evaluation of LMWP-TAZ fusion protein assembled alginate gel matrix in rabbit calvarial defects after 4 weeks’ implantation with hematoxylin and eosin staining.
Notes: (A) No-treatment group (original magnification 12.5×, 40×, 100×), (B) empty alginate gel-treated group (original magnification 12.5×, 40×, 100×), (C) TAZ protein-loaded alginate gel-treated group (original magnification 12.5×, 40×, 100×), (D) LMWP-TAZ fusion protein-loaded alginate gel-treated group (original magnification 12.5×, 40×, 100×). Bone margin is indicated by an arrow. (E) Histomorphometric result at 4 weeks. Total new bone is expressed as area (mm2). *P<0.05 compared to time-matched empty defect; ***P<0.05 compared to time-matched TAZ-treated group.
Abbreviations: OB, old bone; NB, new bone; LMWP, low-molecular-weight protamine; TAZ, PDZ-binding motif.
3-D microCT images were taken to examine the microarchitecture and distribution of mineralization within the protein-loaded alginate gel (Figure 7A, dotted circles superimposed on the images indicate the cranial defect edges). The empty-gel group exhibited minimal mineralized regions, and the mineralized regions were confined mostly to the defect edge. TAZ-loaded alginate gel displayed small patches of mineralization. In contrast, LMWP-TAZ-loaded alginate gel demonstrated multiple layers and islets of dispersed irregularly shaped mineralized regions within the implant region. In addition, the scaffolds incorporating LMWP-TAZ appeared to have had more ingrowth of mineralized areas from the defect edge, relative to the empty group and TAZ-loaded gel (Figure 7B). Bone formation within LMWP-TAZ-loaded gel was qualitatively and quantitatively superior to that obtained with scaffolds containing TAZ or empty defects. In previous studies, stimulating methodology, such as bone grafting, growth-factor treatment, and incorporating condensed DNA has been used to improve bone regeneration.40–42 In particular, growth factors like recombinant human BMPs (rhBMPs) incorporating bone minerals have been applied in dental clinical research many times.43 However, concerns regarding commercially available rhBMPs include carcinogenicity, unwanted bone formation, short-term effects, and immune reaction.43,44 On the contrary, the TAZ protein is a transcriptional factor that determines the lineage between osteoblasts and adipocytes via regulating transcription factors, such as Cbfa1/Runx2 and PPARγ, respectively.20 TAZ could be applied as a substitution material for rhBMPs in regenerative medicine, because it is safer and more selective than rhBMPs for bone regeneration.
Figure 7.
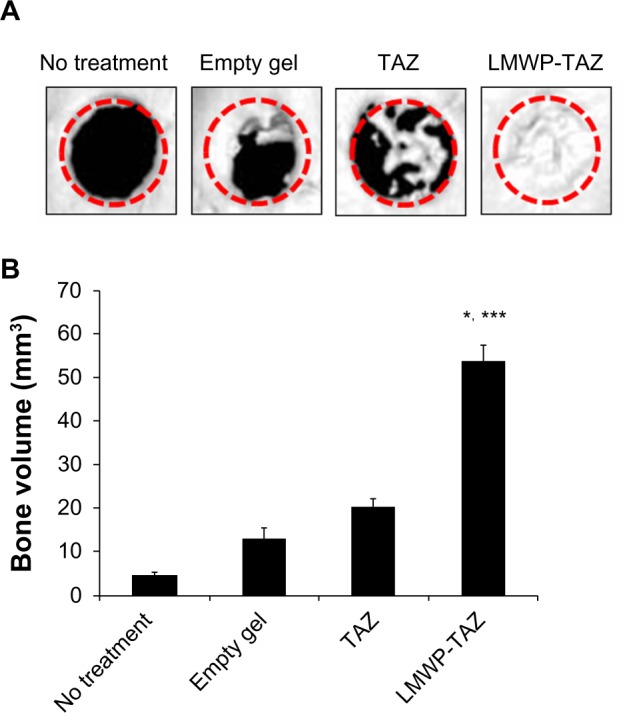
(A and B) Micro-computed tomography (CT) images reconstructing each group in 3-D at 4 weeks.
Notes: (A) 3-D micro-CT images of four groups. Image reconstructions were performed at a resolution of 35 μm. The dotted circles indicate the cranial defect edges. (B) Bone volume (mm3) from the newly calcified area was calculated by micro-CT image analysis according to the lower threshold level. *P<0.05 compared to time-matched empty defect; ***P<0.05 compared to time-matched TAZ treated group.
Abbreviations: LMWP, low-molecular-weight protamine; TAZ, PDZ-binding motif.
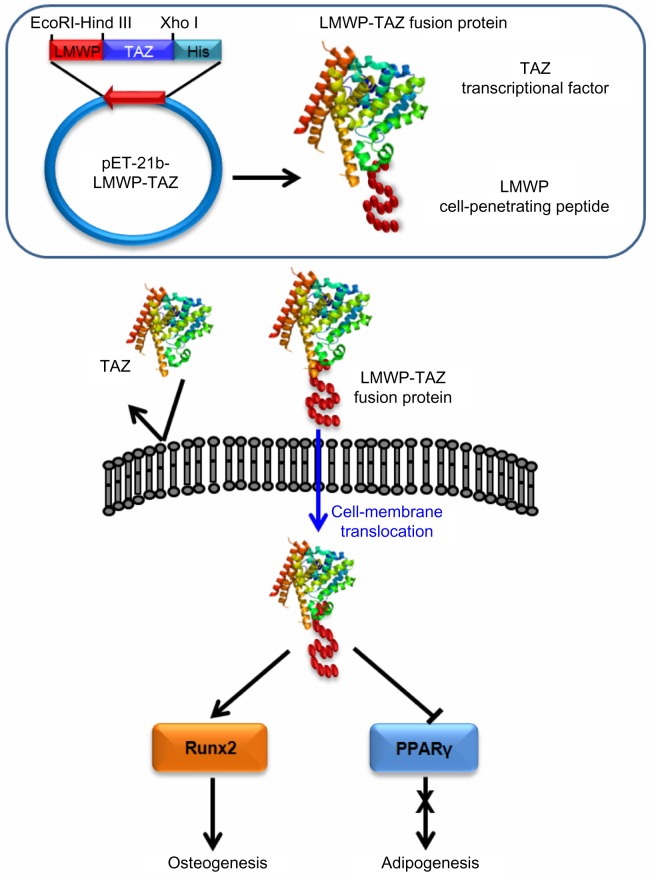
All things considered, the LMWP-TAZ fusion protein was able to induce significant osteogenesis in combination with alginate gel in vivo. It is noteworthy that this is, to our knowledge, one of few reports on in vivo bone-formation efficacy involving the cell-penetrating peptide-transcriptional cofactor fusion protein for clinical application. Our current in vivo study used the concept of intracellular protein delivery using nontoxic cell-penetrating peptides and local release from such biomaterials as alginate gel. In addition, considering that hMSCs have been attempted as vehicles for tissue engineering, the protein transduction of TAZ for precise tuning of the balance between osteoblast and adipocyte differentiation of hMSCs might be also important in medical therapeutics (Figure 8).
Figure 8.
Schematic figure of overall concept of selective osteogenic differentiation of mesenchymal stem cells by cell-penetrating LMWP-TAZ.
Abbreviations: LMWP, low-molecular-weight protamine; Runx2, runt-related transcription factor 2; TAZ, PDZ-binding motif; PPAR, peroxisome proliferator-activated receptor; His, 6× Histidine tag.
Conclusion
The protein fusion of TAZ with cell-penetrating LMWP not only showed an ability to penetrate through the cell membrane but also enabled selective osteogenic differentiation of MSCs in vitro and in vivo. The present experimental results demonstrate that the LMWP-TAZ fusion protein might be useful for directing specific osteogenesis while correcting the imbalance with adipogenesis. In conclusion, protein transduction of a newly identified therapeutic protein in combination with biomaterial and controlled release can be a potential modality for selective tissue regeneration and can further be applied in protein- or cell-based tissue engineering.
Acknowledgments
This work was supported in part by the Bio and Medical Technology Development Program of the National Research Foundation (NRF), funded by the Ministry of Science, ICT and Future Planning (2012049727) and in part by a National Research Foundation of Korea Grant, through the Oromaxillofacial Dysfunction Research Center for the Elderly (2012000912) at Seoul National University in Korea.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Crombez L, Morris MC, Deshayes S, Heitz F, Divita G. Peptide-based nanoparticle for ex vivo and in vivo drug delivery. Curr Pharm Des. 2008;14:3656–3665. doi: 10.2174/138161208786898842. [DOI] [PubMed] [Google Scholar]
- 2.Jones SW, Christison R, Bundell K, et al. Characterisation of cell-penetrating peptide-mediated peptide delivery. Br J Pharmacol. 2005;145:1093–1102. doi: 10.1038/sj.bjp.0706279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang K, Fang H, Chen Z, Taylor JS, Wooley KL. Shape effects of nanoparticles conjugated with cell-penetrating peptides (HIV Tat PTD) on CHO cell uptake. Bioconjug Chem. 2008;19:1880–1887. doi: 10.1021/bc800160b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drin G, Cottin S, Blanc E, Rees AR, Temsamani J. Studies on the internalization mechanism of cationic cell-penetrating peptides. J Biol Chem. 2003;278:31192–31201. doi: 10.1074/jbc.M303938200. [DOI] [PubMed] [Google Scholar]
- 5.Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 6.Giorello L, Clerico L, Pescarolo MP, et al. Inhibition of cancer cell growth and c-Myc transcriptional activity by a c-Myc helix 1-type peptide fused to an internalization sequence. Cancer Res. 1998;58:3654–3659. [PubMed] [Google Scholar]
- 7.Fawell S, Seery J, Daikh Y, et al. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci U S A. 1994;91:664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jo D, Nashabi A, Doxsee C, et al. Epigenetic regulation of gene structure and function with a cell-permeable Cre recombinase. Nat Biotechnol. 2001;19:929–933. doi: 10.1038/nbt1001-929. [DOI] [PubMed] [Google Scholar]
- 9.Suh JS, Lee JY, Choi YS, et al. Efficient labeling of mesenchymal stem cells using cell permeable magnetic nanoparticles. Biochem Biophys Res Commun. 2009;379:669–675. doi: 10.1016/j.bbrc.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 10.Fan H, Liu H, Wong EJ, Toh SL, Goh JC. In vivo study of anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold. Biomaterials. 2008;29:3324–3337. doi: 10.1016/j.biomaterials.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Tai K, Pelled G, Sheyn D, et al. Nanobiomechanics of repair bone regenerated by genetically modified mesenchymal stem cells. Tissue Eng Part A. 2008;14:1709–1720. doi: 10.1089/ten.tea.2007.0241. [DOI] [PubMed] [Google Scholar]
- 12.Zhou XZ, Leung VY, Dong QR, Cheung KM, Chan D, Lu WW. Mesenchymal stem cell-based repair of articular cartilage with polyglycolic acid-hydroxyapatite biphasic scaffold. Int J Artif Organs. 2008;31:480–489. doi: 10.1177/039139880803100603. [DOI] [PubMed] [Google Scholar]
- 13.Cowan CM, Aalami OO, Shi YY, et al. Bone morphogenetic protein 2 and retinoic acid accelerate in vivo bone formation, osteoclast recruitment, and bone turnover. Tissue Eng. 2005;11:645–658. doi: 10.1089/ten.2005.11.645. [DOI] [PubMed] [Google Scholar]
- 14.Pang EK, Im SU, Kim CS, et al. Effect of recombinant human bone morphogenetic protein-4 dose on bone formation in a rat calvarial defect model. J Periodontol. 2004;75:1364–1370. doi: 10.1902/jop.2004.75.10.1364. [DOI] [PubMed] [Google Scholar]
- 15.Sekiya I, Larson BL, Vuoristo JT, Reger RL, Prockop DJ. Comparison of effect of BMP-2, -4, and -6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell Tissue Res. 2005;320:269–276. doi: 10.1007/s00441-004-1075-3. [DOI] [PubMed] [Google Scholar]
- 16.Langenfeld EM, Kong Y, Langenfeld J. Bone morphogenetic protein 2 stimulation of tumor growth involves the activation of Smad-1/5. Oncogene. 2006;25:685–692. doi: 10.1038/sj.onc.1209110. [DOI] [PubMed] [Google Scholar]
- 17.Langenfeld EM, Langenfeld J. Bone morphogenetic protein-2 stimulates angiogenesis in developing tumors. Mol Cancer Res. 2004;2:141–149. [PubMed] [Google Scholar]
- 18.Hong JH, Yaffe MB. TAZ: a beta-catenin-like molecule that regulates mesenchymal stem cell differentiation. Cell Cycle. 2006;5:176–179. doi: 10.4161/cc.5.2.2362. [DOI] [PubMed] [Google Scholar]
- 19.Cui CB, Cooper LF, Yang X, Karsenty G, Aukhil I. Transcriptional coactivation of bone-specific transcription factor Cbfa1 by TAZ. Mol Cell Biol. 2003;23:1004–1013. doi: 10.1128/MCB.23.3.1004-1013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong JH, Hwang ES, McManus MT, et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074–1078. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- 21.Byun MR, Jeong H, Bae SJ, et al. TAZ is required for the osteogenic and anti-adipogenic activities of kaempferol. Bone. 2012;50:364–372. doi: 10.1016/j.bone.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 22.Kanai F, Marignani PA, Sarbassova D, et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000;19:6778–6791. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murakami M, Nakagawa M, Olson EN, Nakagawa O. A WW domain protein TAZ is a critical coactivator for TBX5, a transcription factor implicated in Holt-Oram syndrome. Proc Natl Acad Sci U S A. 2005;102:18034–18039. doi: 10.1073/pnas.0509109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohba S, Ikeda T, Kugimiya F, et al. Identification of a potent combination of osteogenic genes for bone regeneration using embryonic stem (ES) cell-based sensor. FASEB J. 2007;21:1777–1787. doi: 10.1096/fj.06-7571com. [DOI] [PubMed] [Google Scholar]
- 25.Park YJ, Chang LC, Liang JF, Moon C, Chung CP, Yang VC. Nontoxic membrane translocation peptide from protamine, low molecular weight protamine (LMWP), for enhanced intracellular protein delivery: in vitro and in vivo study. FASEB J. 2005;19:1555–1557. doi: 10.1096/fj.04-2322fje. [DOI] [PubMed] [Google Scholar]
- 26.Suh JS, Kim KS, Lee JY, Choi YJ, Chung CP, Park YJ. A cell-permeable fusion protein for the mineralization of human dental pulp stem cells. J Dent Res. 2012;91:90–96. doi: 10.1177/0022034511424746. [DOI] [PubMed] [Google Scholar]
- 27.Lee LM, Chang LC, Wrobleski S, Wakefield TW, Yang VC. Low molecular weight protamine as nontoxic heparin/low molecular weight heparin antidote (III): preliminary in vivo evaluation of efficacy and toxicity using a canine model. AAPS PharmSci. 2001;3:E19. doi: 10.1208/ps030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsui B, Singh VK, Liang JF, Yang VC. Reduced reactivity towards anti-protamine antibodies of a low molecular weight protamine analogue. Thromb Res. 2001;101:417–420. doi: 10.1016/s0049-3848(00)00427-8. [DOI] [PubMed] [Google Scholar]
- 29.Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 30.Tung CH, Mueller S, Weissleder R. Novel branching membrane translocational peptide as gene delivery vector. Bioorg Med Chem. 2002;10:3609–3614. doi: 10.1016/s0968-0896(02)00248-1. [DOI] [PubMed] [Google Scholar]
- 31.Choi YS, Lee JY, Suh JS, et al. The systemic delivery of siRNAs by a cell penetrating peptide, low molecular weight protamine. Biomaterials. 2010;31:1429–1443. doi: 10.1016/j.biomaterials.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301–316. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang EA, Israel DI, Kelly S, Luxenberg DP. Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors. 1993;9:57–71. doi: 10.3109/08977199308991582. [DOI] [PubMed] [Google Scholar]
- 34.Asahina I, Sampath TK, Hauschka PV. Human osteogenic protein-1 induces chondroblastic, osteoblastic, and/or adipocytic differentiation of clonal murine target cells. Exp Cell Res. 1996;222:38–47. doi: 10.1006/excr.1996.0005. [DOI] [PubMed] [Google Scholar]
- 35.Katagiri T, Yamaguchi A, Ikeda T, et al. The nonosteogenic mouse pluripotent cell line, C3h10t1/2, is induced to differentiate into osteoblastic cells by recombinant human bone morphogenetic protein-2. Biochem Biophys Res Commun. 1990;172:295–299. doi: 10.1016/s0006-291x(05)80208-6. [DOI] [PubMed] [Google Scholar]
- 36.Kato S, Kawabata N, Suzuki N, Ohmura M, Takagi M. Bone morphogenetic protein-2 induces the differentiation of a mesenchymal progenitor cell line, ROB-C26, into mature osteoblasts and adipocytes. Life Sci. 2009;84:302–310. doi: 10.1016/j.lfs.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Tornvig L, Mosekilde LI, Justesen J, Falk E, Kassem M. Troglitazone treatment increases bone marrow adipose tissue volume but does not affect trabecular bone volume in mice. Calcif Tissue Int. 2001;69:46–50. doi: 10.1007/s002230020018. [DOI] [PubMed] [Google Scholar]
- 38.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–154. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 39.Choi YJ, Lee JY, Park JH, et al. The identification of a heparin binding domain peptide from bone morphogenetic protein-4 and its role on osteogenesis. Biomaterials. 2010;31:7226–7238. doi: 10.1016/j.biomaterials.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 40.Minear S, Leucht P, Jiang J, et al. Wnt proteins promote bone regeneration. Sci Transl Med. 2010;2:29ra30. doi: 10.1126/scitranslmed.3000231. [DOI] [PubMed] [Google Scholar]
- 41.Huang YC, Simmons C, Kaigler D, Rice KG, Mooney DJ. Bone regeneration in a rat cranial defect with delivery of PEI-condensed plasmid DNA encoding for bone morphogenetic protein-4 (BMP-4) Gene Ther. 2005;12:418–426. doi: 10.1038/sj.gt.3302439. [DOI] [PubMed] [Google Scholar]
- 42.Kim YS, Kim SH, Kim KH, et al. Rabbit maxillary sinus augmentation model with simultaneous implant placement: differential responses to the graft materials. J Periodontal Implant Sci. 2012;42:204–211. doi: 10.5051/jpis.2012.42.6.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benglis D, Wang MY, Levi AD. A comprehensive review of the safety profile of bone morphogenetic protein in spine surgery. Neurosurgery. 2008;62:ONS423–ONS431. doi: 10.1227/01.neu.0000326030.24220.d8. discussion ONS431. [DOI] [PubMed] [Google Scholar]
- 44.Vaidya R, Sethi A, Bartol S, Jacobson M, Coe C, Craig JG. Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech. 2008;21:557–562. doi: 10.1097/BSD.0b013e31815ea897. [DOI] [PubMed] [Google Scholar]