Abstract
Objective
Resting-state functional magnetic resonance imaging (fMRI) uncovers correlated activity between spatially distinct functionally related brain regions and offers clues about the integrity of functional brain circuits in people with chronic subjective tinnitus. We chose to investigate auditory network connectivity, adopting and extending previously used analyses methods to provide an independent evaluation of replicability.
Design
Independent components analysis (ICA) was used to identify coherent patterns arising from spontaneous brain signals within the resting-state data. The auditory network component was extracted and evaluated. Bivariate and partial correlation analyses were performed on pre-defined regions of bilateral auditory cortex to assess functional connectivity.
Study sample
Our design carefully matched participant groups for possible confounds, such as hearing status. Twelve patients (seven male, five female; mean age 66 years) all with chronic constant tinnitus and eleven controls (eight male, three female; mean age 68 years) took part.
Results
No significant differences were found in auditory network connectivity between groups after correcting for multiple statistical comparisons in the analysis. This contradicts previous findings reporting reduced auditory network connectivity; albeit at a less stringent statistical threshold.
Conclusions
Auditory network connectivity does not appear to be reliably altered by the experience of chronic subjective tinnitus.
Keywords: Tinnitus, fMRI, functional connectivity, resting-state, auditory network
Abbreviations
- BAI
Beck anxiety inventory
- BDI
Beck depression inventory
- BOLD
Blood oxygen level dependent
- EEG
Electroencephalography
- fMRI
Functional magnetic resonance imaging
- FWE
Family-wise error
- GIFT
Group ICA for fMRI toolbox
- HQ
Hyperacusis questionnaire
- ICA
Independent component analysis
- MEG
Magnetoencephalography
- NAC
Nonprimary auditory cortex
- NIHR
National Institute for Health Research
- PAC
Primary auditory cortex
- PET
Positron emission tomography
- ROI
Region of interest
- RSN
Resting-state network
- TCHQ
Tinnitus case history questionnaire
- THQ
Tinnitus handicap questionnaire
Tinnitus can be described as the involuntary perception of sound in the absence of any corresponding external auditory source. Despite its high prevalence across the world (Baguley et al, 2013), relatively little is known about its specific causes and underlying perpetuating neural mechanisms. Neuroimaging approaches are providing new ways of investigating such mechanisms. These have been applied to electroencephalography (EEG), magnetoencephalography (MEG), positron emission tomography (PET), and functional magnetic resonance imaging (fMRI) measures of people with tinnitus (see Adjamian et al, 2009; Lanting et al, 2009 for reviews).
So far, many neuroimaging tinnitus studies have used sound-evoked paradigms (Golm et al, 2013; Husain et al, 2011; Gu et al, 2010; Melcher et al, 2009; Adjamian et al, 2009) or focused on anatomical differences in brain structure (e.g. Melcher et al, 2012). However, in the case of chronic health conditions such as depression and schizophrenia, there is a growing interest in investigating the patterns of brain activity during rest (Veer et al, 2010; Garrity et al, 2007). Being able to understand the dynamic interactions between different neural networks in healthy and diseased states may help inform future treatment strategies or be used as biomarkers when measuring the efficacy of new treatments (Narayanan, 2010). Moreover, in the context of tinnitus research, the use of resting-state neuroimaging may be better suited as an experimental paradigm to record activity relating to the ‘typical’ on-going experience of the tinnitus percept.
Human brain function is not localized but engages spatially distributed, functionally linked anatomical regions which are in constant exchange of information. This inter-relationship can be referred to as ‘connectivity’ and can be quantified in humans using neuroimaging methods such as fMRI, PET, EEG and MEG. The present study investigates connectivity using resting-state fMRI which is sensitive to low frequency (< 0.1 Hz) spontaneous fluctuations in the blood oxygenation level dependent (BOLD) signal (Ogawa et al, 1990; Fox & Raichle, 2007) and offers a high-degree of spatial resolution.
Biswal et al (1995) were the first group to use fMRI to identify coherent patterns of spatially independent, temporally correlated BOLD signal fluctuations during a resting-state. That is, when no explicit task is being performed by the participant. These patterns have since been termed ‘resting-state networks’ (RSNs) (Greicius et al, 2003) and are thought to reflect functional systems supporting core perceptual and cognitive processes (Cole et al, 2010). Several RSNs have been identified including visual, attention, auditory, and default mode (Beckman et al, 2005). Resting-state networks are generally reported to show reliable and consistent patterns of functional connectivity (Zhang et al, 2008). Rogers et al (2007) define the term ‘functional connectivity’ as the quantification of the operational interactions of multiple spatially-distinct brain regions that are simultaneously engaged. See van den Heuvel & Pol (2010) for a review of resting-state fMRI functional connectivity.
Recently, the application of resting-state fMRI has been used to investigate functional connectivity differences in those with tinnitus (Burton et al, 2012; Kim et al, 2012; Lee et al, 2012; Maudoux et al, 2012a, b; Wineland et al, 2012). In a preliminary study of four people with tinnitus and six controls, Kim et al (2012) used resting-state fMRI to investigate underlying brain activity within the auditory cortex of people with tinnitus. Results from an independent component analysis (ICA) followed by bivariate correlation between regions of interest (ROI) indicated a reduced functional connectivity between left and right auditory cortices in the tinnitus group. The authors interpret this finding as indicative of a loss of coherence in intrinsic oscillatory activity, potentially indicating disequilibrium between neural excitation and inhibition across the hemispheres. Two further studies (Maudoux et al, 2012a, b) also adopted ICA with a customized automated component selection approach. Both studies describe results for the same cohort of 13 people with chronic tinnitus and 16 age-matched controls. A large number of connectivity differences were observed between the two groups in auditory and distributed non-auditory regions. Tinnitus individuals showed increased connectivity in the brainstem, basal ganglia, cerebellum, parahippocampal, right prefrontal, parietal, and sensorimotor areas and decreased connectivity in the right primary auditory cortex, left prefrontal, left fusiform gyrus, and bilateral occipital regions. Overall, Maudoux and colleagues concluded that the presence of tinnitus was able to modify functional connectivity in networks which encompass memory, attention, and emotion. A pair of studies targeted larger samples of people with bothersome (n = 17) and non-bothersome tinnitus (n = 18), adopting the same methodology (Burton et al, 2012; Wineland et al, 2012). Their research investigated potential correlations between the auditory network and other brain networks using an exploratory seed correlation approach. The main finding was a negative connectivity correlation between auditory and visual networks only in those with bothersome tinnitus. The authors thought these findings may reflect neuroplastic adaptations to reduce phantom noise salience and conflict between non-auditory tasks.
Although all of these studies found that the presence of tinnitus modifies brain connectivity, the results differ markedly between studies. Many fMRI tinnitus studies have identified some potential confounds which might explain difficulties in replication of findings across studies. These include factors such as age and gender (Lanting et al, 2009), laterality of the tinnitus percept (Melcher et al, 2000; Smits et al, 2007), severity of symptoms (Burton et al, 2012; Wineland et al, 2012), hearing loss (Husain et al, 2011), and hyperacusis (Gu et al, 2010). The present study therefore sought to address some of these design limitations by using a larger cohort of age, sex, and hearing-matched participants. Differences in analysis methodology could certainly be sufficient to explain much of the variability in findings. In the present study, we made an a priori decision to follow the same analysis steps described by Kim et al, (2012), as they used widely available proprietary software, naturally lending itself to replication. By doing this, we could ensure that this aspect of the analysis methodology was comparable between studies. We also employed additional analyses to more fully explore the data: (1) we defined regions that separated primary and secondary auditory cortex, and (2) we included a partial correlation approach, which allowed us to assess functional connectivity relationships between two auditory regions both within and between hemispheres, whilst controlling for the effects of the remaining ROIs specified in the model (e.g. Smith et al, 2011).
Methods
Participants
All participants were recruited through Nottingham Audiology Services or the Ear, Nose and Throat (ENT) department, Queen's Medical Centre, Nottingham. Twelve participants (seven male, five female; mean age 65.8 years) all with chronic (two years minimum duration), constant subjective tinnitus participated in the study. Two of the twelve participants had lateralized tinnitus, the remaining ten had bilateral (n = 7) or central (perceived in the centre of the head) (n = 3) tinnitus. We also recruited eleven age- and hearing-matched controls (eight male, three female; mean age 68.5 years). All participants were aged 49–75 years without a history of neurological disorder. The study was approved by the National Research Ethics Committee (REC: 09/H0407/8). All participants gave written informed consent prior to taking part. See Table 1 for participant demographics and tinnitus characteristics.
Table 1.
Group demographics, questionnaire scores, and tinnitus characteristics. M = male, F = female, L = left, R = right, In head = central tinnitus.
|
No tinnitus group
|
Tinnitus group
|
Tinnitus characteristics
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age | HQ | BAI | BDI | Sex | Age | HQ | BAI | BDI | Laterality | Duration (years) | THQ | TCHQ % annoy | |
| F | 68 | 6 | 2 | 0 | M | 72 | 24 | 10 | 4 | L | 15 | 25.1 | 28 | |
| F | 71 | 9 | 8 | 3 | M | 64 | 14 | 2 | 2 | L&R | 2 | 35.5 | 10 | |
| M | 58 | 13 | 2 | 0 | M | 72 | 14 | 2 | 2 | L&R | 2 | 60.4 | 70 | |
| M | 68 | 19 | 0 | 3 | F | 67 | 8 | 6 | 0 | L&R | 4 | 61.3 | 50 | |
| M | 75 | 8 | 3 | 0 | F | 73 | 11 | 4 | 2 | In head | 70 | 21.1 | 5 | |
| M | 68 | 9 | 0 | 0 | F | 57 | 18 | 11 | 0 | In head | 2 | 63.3 | 50 | |
| M | 60 | 2 | 0 | 0 | M | 71 | 22 | 3 | 0 | In head | 6 | 68.4 | 30 | |
| F | 75 | 18 | 13 | 4 | M | 71 | 11 | 5 | 0 | L&R | 10 | 32.2 | 20 | |
| M | 66 | 8 | 0 | 0 | M | 64 | 11 | 0 | 0 | L&R | 20 | 30 | 20 | |
| M | 74 | 2 | 0 | 1 | F | 72 | 17 | 3 | 0 | R | 13 | 50.6 | 35 | |
| M | 70 | 11 | 14 | 0 | M | 49 | 10 | 4 | 2 | L&R | 2 | 57.5 | 50 | |
| – | – | – | – | – | F | 58 | 15 | 1 | 1 | L&R | 40 | 18.7 | 25 | |
| Mean | 8 M / 3 F | 68.5 | 9.6 | 3.8 | 1 | 7 M / 5 F | 65.8 | 14.6 | 4.3 | 1.1 | – | 15.5 | 43.7 | 32.8 |
| SD | – | – | 5.54 | 5.34 | 1.55 | – | – | 4.9 | 3.36 | 1.31 | – | 20.4 | 18.32 | 19 |
Audiological profile
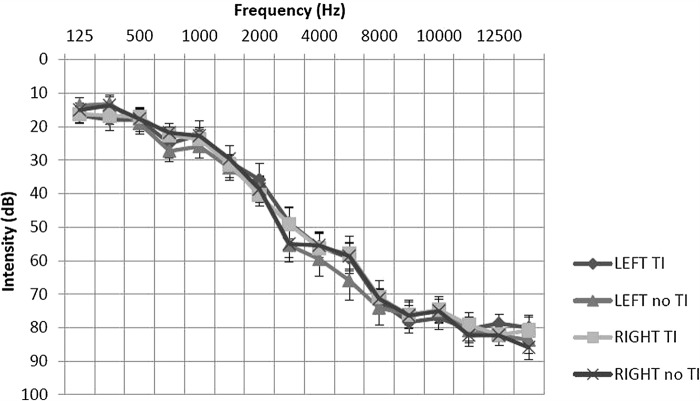
Participants had an extended frequency hearing test (125–14000 Hz) prior to scanning. Participants with unilateral or asymmetrical hearing loss (as indicated by a between-ear air conduction threshold difference of 15 dB at two or more consecutive frequencies) were excluded from the study. Those with hyperacusis, as indicated by a score of ≥ 29 on the hyperacusis questionnaire (Khalfa et al, 2002) were also excluded. Post-hoc t-tests of average hearing thresholds revealed no significant differences between or within participant groups. The general hearing status of both groups could be described as a bilateral, mild to moderately severe sloping sensorineural hearing loss, typical of presbyacusis (see Figure 1).
Figure 1.
Mean average hearing thresholds for tinnitus and no tinnitus groups. Post hoc t-tests of average hearing thresholds found no significant differences between or within participant groups (P > 0.05). Error bars represent one standard error of the mean.
Behavioural profile
All participants completed the hyperacusis questionnaire (HQ: Khalfa et al, 2002), the Beck anxiety inventory (BAI: Beck et al, 1998) and the Beck depression inventory – fast screen (BDI: Beck et al, 2000). Tinnitus participants also completed the tinnitus handicap questionnaire (THQ: Kuk et al, 1990) and the tinnitus case history questionnaire (TCHQ: Langguth et al, 2007). Questionnaire scores are given in Table 1. For the tinnitus group, BAI and BDI scores were not significantly different from the control group. On average, BAI and BDI scores were minimal in severity for both groups. For the tinnitus group, HQ scores were significantly higher (P = 0.031) than the control group. However, the mean HQ score for the two groups were comparable to the mean score of the general population (i.e. 15) and no participant in either group had a HQ score of > 28, which according to Khalfa et al (2002) indicates the presence of hyperacusis.
fMRI acquisition
Data were obtained from a Philips Achieva 3T MR scanner (Philips Medical Systems, The Netherlands) using an eight-channel SENSE receiver head coil. Whole brain functional images were acquired for each participant using an echo-planar image sequence, during a five-minute period of wakeful rest using a multi-echo sequence for optimal detectability of subcortical activity (echo times: 20, 45 ms, interscan interval 2.7 s, 36 slices, 0 mm slice gap, FOV = 240, voxel size 3 × 3× 3 mm, 112 acquisitions). The participants had no explicit task to perform, rather they were instructed to keep still and alert with their eyes closed. During scans, participants wore ear plugs as well as circum-aural headphones which employed active noise control to reduce noise generated by the scanner (Hall et al, 2009). A five minute T1-weighted anatomical image was also acquired for each participant (160 slices, FOV = 256, voxel size 1 × 1× 1 mm).
Preprocessing steps
Functional MRI data were preprocessed using statistical parametric mapping software SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Images were realigned, co-registered with the participant's high resolution anatomical scan, normalized to the Montreal Neurological Institute (MNI152) template, and spatially smoothed (4 mm full-width at half maximum).
Analyses approach summary
Analysis of resting-state functional data in this present study involved two stages. Firstly, group ICA was used to extract the auditory component of interest. This largely incorporated bilateral auditory cortex. Four seed ROIs within the auditory component were then selected: bilateral primary auditory cortex and nonprimary auditory cortex within the lateral part of planum temporale. Bivariate correlation and partial correlation analyses were then used to assess levels of functional connectivity between each ROI. With the exception of the partial correlation analysis, all steps followed Kim et al (2012).
Group independent component analysis (ICA) and auditory component selection
Group ICA was performed using the Group ICA for fMRI Toolbox v1.8 (GIFT, http://mialab.mrn.org/software/gift/index.html) in MATLAB version 7.14. GIFT applies ICA as an unbiased, whole-brain analysis method of blind source signal separation, on either single or group level data. It allows the extraction of functionally related, spatially independent brain sources (referred to as components), each with an associated time course and spatial map.
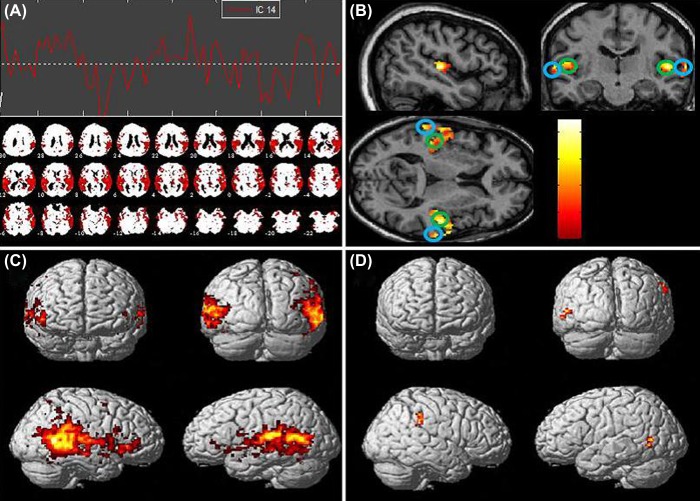
Group ICA was first used to estimate the number of components using concatenated data from both tinnitus and no tinnitus groups. Of the 23 components identified, the component which most resembled the auditory network (component 14) was visually selected (see Figure 2, A). To support this selection, the independent components (ICs) were spatially sorted by performing a correlation analysis against a spatial template of the auditory network. The auditory template was taken from the SPM anatomy toolbox v1.8 (http://www.fz-juelich.de/inm/inm-1/DE/Forschung/_docs/SPMAnatomyToolbox/SPMAnatomyToolbox_node.html Eickhoff et al, 2005) which incorporated bilateral primary auditory cortex and nonprimary auditory cortex. Component 14 was found to be most highly correlated with the auditory template (Figure 2, A) and will henceforth be putatively referred to as the ‘auditory network’ component. Within SPM8, a one-sample t-test was used to derive the auditory network functional connectivity maps for the tinnitus group (n = 12), no tinnitus group (n = 11) and both groups combined (n = 23). A further two-sample t-test was used to assess between group differences in the auditory functional connectivity maps.
Figure 2. (A) Auditory network component and time-course. (B) Whole group one-sample t-test of auditory component, masked with auditory template (P < 0.05, uncorrected). Primary auditory cortex regions (circled in green). Nonprimary auditory cortex regions (circled in blue). (C) Whole group one-sample t-test of auditory network component (P < 0.001, uncorrected). (D) Tinnitus > No tinnitus two-sample t-test (P < 0.01, uncorrected, 48 voxel extent threshold) showing increased connectivity in the right supramarginal gyrus and increased connectivity in the left posterior middle temporal gyrus.
Defining and constructing regions of interest (ROIs)
Regions of interest were functionally defined using concatenated data from both tinnitus and no tinnitus groups. Within SPM8, a one-sample t-test was performed on the extracted auditory network component. Results were masked using the same auditory template used previously and thresholded at P < 0.05, uncorrected. Voxel co-ordinates for peak activity in bilateral primary auditory and nonprimary auditory cortices were extracted and used as the centre co-ordinate for each of the four spherical auditory ROIs (5-mm radius; co-ordinates of peak activity of the four ROIs are indicated on Figure 2, B). The ROIs were constructed within the MarsBar toolbox (http://marsbar.sourceforge.net/) and used in the functional connectivity analyses.
Correlation analysis
As in Kim et al's study (2012), the Functional Connectivity Toolbox v12.1 (SPM8, http://web.mit.edu/swg/software.htm) was used to compute Fisher-transformed bivariate correlation coefficients (beta values) between the low frequency BOLD fluctuations of each ROI pair. To reduce any possible confounding sources of noise, cerebrospinal fluid motion, participant motion parameters, and white matter signals were used as nuisance covariates. The BOLD signal was also band-pass filtered (0.009–0.08 Hz) to facilitate exclusion of respiratory or myogenic artifacts (Cole et al, 2010).
Partial correlation analysis was also performed to exclusively assess functional connectivity relationships between selected ROI pairs at the same time as accounting for the influence of activity from the other two ROIs. Fisher-transformation was also applied to the partial correlation coefficients to ensure measures were approximately normal in distribution. A two-sample t-test was then used to evaluate group level differences between all Fisher-transformed bivariate and partial correlation coefficients generated from each auditory ROI pair. Heterogeneous inter-hemispheric auditory ROI pairs, e.g. left primary auditory cortex to right nonprimary auditory cortex, were not investigated as commissural projections in primary and nonprimary auditory cortices arise predominantly from contralateral homotopic regions (Lee & Winer, 2008). Bonferonni corrections were applied to all correlations to control for family-wise error.
Results
Independent component analysis (ICA)
A one-sample t-test of the auditory network component combined across both tinnitus and no tinnitus control groups (P < 0.001, uncorrected) revealed robust functional connectivity between bilateral auditory cortical areas (Figure 2, C). A two-sample t-test of the auditory network component adopting the same statistical thresholding as Kim et al (2012) (i.e. P < 0.01, uncorrected for multiple comparisons, 48 voxel extent threshold) showed increased functional connectivity in the right supramarginal gyrus and left posterior middle temporal gyrus for the tinnitus group (Figure 2, D). However, after correcting for multiple comparisons using the more stringent family-wise error (FWE) corrected statistical thresholding, these areas of enhanced functional connectivity did not survive. These results differ from Kim et al (2012) who reported increased functional connectivity in tinnitus participants between the auditory network and the left amygdala and between the auditory network and dorsomedial prefrontal cortex. No suprathreshold clusters of voxels were found in the auditory network for the ‘reverse contrast’ two-sample t-test comparison (no tinnitus > tinnitus).
Correlation analyses
Average bivariate correlation coefficients were not significantly different between the tinnitus group and controls (Figure 3, A). For the partial correlation analysis (Figure 3, B), only the average partial correlation coefficient between the left primary auditory cortex and the left auditory association cortex was significantly lower (P = 0.029) in the tinnitus group. However, this did not survive statistical thresholding following Bonferonni adjustment of the alpha level (0.05/4).
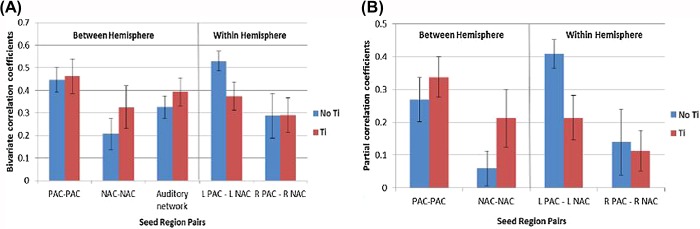
Figure 3. (A) Average bivariate correlation coefficients (beta values) for each paired ROI. The ‘auditory network’ refers to a combined mean average derived from the BOLD time-series of primary auditory and auditory association cortical seed regions. (B) Average partial correlation coefficients for each paired ROI. Error bars represent one standard error of the mean. PAC = primary auditory cortex, NAC = nonprimary auditory cortex, L = left, R = right, Ti = tinnitus group, No Ti = no tinnitus control group.
Discussion
We sought to consolidate early findings regarding resting-state fMRI in chronic subjective tinnitus, by controlling for a number of important factors; namely age, sex, BAI/BDI scores, and audiometric profile. Using methods based on Kim et al (2012), we chose to assess auditory functional connectivity both between and, in addition to Kim et al (2012), within brain hemispheres. We also employed methods of partial correlation which have recently been found to be a powerful analysis approach (Smith et al, 2011), allowing functional connectivity relationships between two chosen auditory regions to be assessed whilst controlling for the effects of the remaining ROIs. In the present study, whole-brain ICA and bivariate correlation analyses resulted in similar patterns of auditory network connectivity between tinnitus and no-tinnitus groups. Our additional methods of partial correlation and exploring connectivity within hemispheres revealed no significant differences between groups indicating that auditory cortical functional connectivity is not modified by the experience of tinnitus.
Connectivity within the auditory cortex network
Kim et al (2012) found a significant reduction in bilateral auditory cortical functional connectivity in their tinnitus participants compared to controls. They hypothesized that this reduction may imply a loss of coherence in spontaneous resting state neural activity between the left and right auditory cortices. While our null result for auditory network functional connectivity contradicts Kim and colleagues, it is supported by reports from Burton et al (2012) and Wineland et al (2012). Interpreting these mixed findings is challenging because of the many methodological differences (participants, analysis etc.). One pertinent factor which may explain the differences in auditory network connectivity between studies is hearing acuity. According to Husain et al (2011), compensatory mechanisms for hearing loss may differ to those of tinnitus, resulting in differences in functional neural responses unless hearing status is carefully controlled as a potential confound. We note that Kim et al (2012) matched hearing between their tinnitus and control groups based on a three point average hearing threshold (500, 1000, and 2000 Hz), leaving high frequency hearing loss unaccounted for. The present study carefully matched hearing status between tinnitus participants and controls across a wide range of frequencies 125–14000 Hz. We acknowledge differences in tinnitus laterality between the present study cohort and that of Kim et al (2012). Kim recruited people with lateralized tinnitus, while our cohort was mixed with a majority experiencing bilateral tinnitus. There have been several sound-evoked fMRI studies investigating tinnitus laterality (Melcher et al, 2000; Lanting et al, 2008; Smits et al, 2007). However, there is no systematic evidence to indicate an effect of tinnitus laterality on the patterns of resting-state brain activity and connectivity.
Connectivity between auditory and emotional networks
De Ridder et al, (2011) suggest that distress associated with tinnitus results from a constant learning process and is reflected by the presence of a non-specific distress network consisting of the anterior cingulate cortex, anterior insula, and the amygdala. In their tinnitus participants, Kim et al (2012) reported reduced functional connectivity between left and right auditory cortices and increased functional connectivity between the auditory network and the left amygdala and dorsomedial prefrontal cortex. Although these data plausibly suggest that tinnitus is associated with increased functional connectivity in brain regions which sub-serve emotion and attention, Kim et al (2012) did not report the degree of tinnitus distress experienced by their participants and the statistical reliability of their findings is also questionable. Our results throw doubt on this interpretation because this finding was not replicated in the present study, although we do acknowledge that our participants had relatively low levels of tinnitus distress (THQ mean score was 43.7 out of 100) and tinnitus annoyance (TCHQ mean score was 32.8%).
Several other recent resting-state fMRI studies have found alterations in networks associated with emotion and attention, with findings tending to indicate this depends on the bothersome nature of the tinnitus symptoms. For example, Maudoux et al (2012b) observed a positive correlation between the resting-state activity of the posterior cingulate/precuneus regions and tinnitus handicap inventory scores (an indicator for emotional distress). Burton et al (2012) found that the right anterior insula and left frontal gyrus of their distressed tinnitus group showed significantly greater functional connectivity with the auditory network than controls, while data from the same research group reported in a separate paper (Wineland et al, 2012) found no differences in functional connectivity in those with non-bothersome tinnitus compared against age- and hearing-matched controls. Although these results might imply that only bothersome tinnitus alters functional connectivity in brain regions related to attention and emotional processing, direct statistical comparisons need to be made between subgroups with low and high levels of tinnitus distress in order to confirm any such claims.
Independent replications of experimental findings in tinnitus represent an important way to validate claims made about the underpinning neural mechanisms of this enigmatic condition, seeking to separate truth from myth. Just as there has recently been a call for an international standard in clinical trial methodology for tinnitus research (Landgrebe et al, 2012), we would argue that the same concerted collaborative efforts would benefit this newly emerging field of resting-state fMRI. Transparency in the details of the methods and analysis and sharing of customized analysis software would help us all as a community to obtain reliable information about the neural circuitry in the tinnitus brain.
Acknowledgements
This research was supported by a Deafness Research UK PhD studentship awarded to the first author (JD). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. These data were presented at the 7th International Tinnitus Research Initiative (TRI) Meeting; Tinnitus: A Treatable Disease, May 15–18, 2013, in Valencia, Spain.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Adjamian P., Magdalena S., Hall D. The mechanisms for tinnitus: Perspectives from human functional neuroimaging. Hearing Research. 2009;253:15–31. doi: 10.1016/j.heares.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Baguley D., McFerran D., Hall D. Tinnitus. The Lancet. 2013;12:08329. doi: 10.1016/S0140-6736(13)60142-7. [DOI] [PubMed] [Google Scholar]
- 3.Beck A.T., Epstein N., Brown G., Steer R.A. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 4.Beck A.T., Steer R.A., Brown G.K. . BDI-fast screen for medical patients: Manual. San Antonio, USA: Psychological Corporation; 2000. [Google Scholar]
- 5.Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc B Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echoplanar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 7.Burton H., Wineland H., Bhattacharya M., Nicklaus J., Garcia K, et al. Altered networks in bothersome tinnitus: A functional connectivity study. BMC Neuroscience. 2012;13:1–15. doi: 10.1186/1471-2202-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole D.M., Smith S., Beckmann C.F. Advances and pitfalls in the analysis and interpretation of resting-state fMRI data. Frontiers in Systems Neuroscience. 2010;4:1–15. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Ridder D., Elgoyhen A.B., Romo R., Langguth B. Phantom percepts: Tinnitus and pain as persisting aversive memory networks. Proceedings of the National Academy of Sciences, USA. 2011;108:8075–8080. doi: 10.1073/pnas.1018466108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eickhoff S., Stephan K.E., Mohlberg H., Grefkes C., Fink G.R, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 11.Field A. Discovering Statistics Using SPSS. 3rd ed. London, UK: SAGE; 2009. [Google Scholar]
- 12.Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 13.Garrity A., Pearlson G., McKiernan K., Lloyd D., Kiehl K, et al. Aberrant ‘default mode’ functional connectivity in schizophrenia. Am J Psychiatry. 2007;163:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 14.Golm D., Schmidt-Samoa C., Dechent P., Kroner-Herwig B. Neural correlates of tinnitus related distress: An fMRI-study. Hear Res. 2013;295:87–99. doi: 10.1016/j.heares.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu J.W., Halpin C., Nam E., Levine R., Melcher J. Tinnitus, diminished sound-level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. J Neurophysiology. 2010;104:3361–3370. doi: 10.1152/jn.00226.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall D.A., Chambers J., Akeroyd M.A., Foster J.R., Coxon R, et al. Acoustic, psychophysical, and neuroimaging measurements of the effectiveness of active cancellation during auditory functional magnetic resonance imaging. J Acoust Soc Am. 2009;125:347–59. doi: 10.1121/1.3021437. [DOI] [PubMed] [Google Scholar]
- 18.Husain F.T., Pajor N.M., Smith J. F., Kim J. H., Rudy S, et al. Discrimination task reveals differences in neural bases of tinnitus and hearing impairment. PLos ONE. 2011;6:1–12. doi: 10.1371/journal.pone.0026639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khalfa S., Dubal S., Veuillet E., Perez-Diaz F., Jouvent R, et al. Psychometric normalization of a hyperacusis questionnaire. ORL J Otorhinolaryngol Relat Spec. 2002;64:436–42. doi: 10.1159/000067570. [DOI] [PubMed] [Google Scholar]
- 20.Kim J., Kim Y., Lee S., Seo J.H., Song H.J, et al. Alteration of functional connectivity in tinnitus revealed by resting-state fMRI?: A pilot study. Int J Audiol. 2012;51, 413–417 doi: 10.3109/14992027.2011.652677. [DOI] [PubMed] [Google Scholar]
- 21.Kuk F.K., Tyler R.S., Russell D., Jordan H. The psychometric properties of a tinnitus handicap questionnaire. Ear Hear. 1990;11, 434–45 doi: 10.1097/00003446-199012000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Landgrebe M., Azevedo A., Baguley D., Bauer C., Cacace A, et al. Methodological aspects of clinical trials in tinnitus: A proposal for an international standard. J Psychosomatic Res. 2012;73:112–121. doi: 10.1016/j.jpsychores.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langguth B., Goodey R., Azevedo A., Bjorne A., Cacace A, et al. Consensus for tinnitus patient assessment and treatment outcome measurement: Tinnitus research initiative meeting, Regensburg, July 2006. Prog Brain Res. 2007;166:525–536. doi: 10.1016/S0079-6123(07)66050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanting C.P., De Kleine E., Bartels H., Van Dijk P. Functional imaging of unilateral tinnitus using fmri. Acta Otolaryngol. 2008;128:415–421. doi: 10.1080/00016480701793743. [DOI] [PubMed] [Google Scholar]
- 25.Lanting C.P., De Kleine E., Van Dijk P. Neural activity underlying tinnitus generation: Results from PET and fMRI. Hear Res. 2009;355:1–13. doi: 10.1016/j.heares.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Lee C., Winer J.A. Connections of cat auditory cortex: II. Commissural system. J Comp Neurol. 2008;507:1901–1919. doi: 10.1002/cne.21614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee M. H., Solowski N., Wineland A., Oluwafunmilola O., Nicklaus J, et al. Functional connectivity during modulation of tinnitus with orofacial mauevers. Otolaryngol Head Neck Surg. 2012;147,:757–762. doi: 10.1177/0194599812450680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maudoux A., Lefebure P., Cabay J.E., Demertzi A., Vanhaudenhuyse A, et al. Auditory resting-state network connectivity in tinnitus: A functional MRI study. PLos ONE. 2012a;7:1–9. doi: 10.1371/journal.pone.0036222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maudoux A., Lefebure P., Cabay J.E., Demertzi A., Vanhaudenhuyse A, et al. Connectivity graph analysis of the auditory resting state network in tinnitus, Brain Res. 2012b;1485:10–21. doi: 10.1016/j.brainres.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Melcher J., Levine R., Bergevin C., Norris B. The auditory midbrain of people with tinnitus: Abnormal sound-evoked activity revisited. Hear Res. 2009;257:63–74. doi: 10.1016/j.heares.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melcher J.R., Sigalovsky I.S., Guinan J.J., Levine R.A. Lateralized tinnitus studied with functional magnetic resonance imaging: abnormal inferior colliculus activation. J Neurophysiol. 2000;83:1058–1072. doi: 10.1152/jn.2000.83.2.1058. [DOI] [PubMed] [Google Scholar]
- 32.Melcher J.R., Knudson I.M., Levine R.A. Subcallosal brain structure: correlation with hearing threshold at supra-clinical frequencies (> 8 kHz), but not with tinnitus. Hear Res. 2012;295:79–86. doi: 10.1016/j.heares.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Narayanan A., White C.A., Saklayen S., Scaduto M.J., Carpenter A.L, et al. Effect of Propranolol on functional connectivity in autism spectrum disorder: A pilot study. Brain Imaging and Behaviour. 2010;4:189–197. doi: 10.1007/s11682-010-9098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogawa S., Lee T., Kay A., Tank D. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers B., Morgan V., Newton A., Gore J. Assessing functional connectivity in the human brain by fMRI. Magnetic Resonance Imaging. 2007;25:1347–1357. doi: 10.1016/j.mri.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith S.M., Miller K.L., Salimi-Khorshidi G., Webster M., Beckmann C.F, et al. Network modelling methods for FMRI. Neuroimage. 2011;54:875–891. doi: 10.1016/j.neuroimage.2010.08.063. [DOI] [PubMed] [Google Scholar]
- 37.Smits M., Kovacs S., De Ridder D., Peeters R., Hecke P, et al. Lateralization of functional magnetic resonance imaging (fMRI) activation in the auditory pathway of patients with lateralized tinnitus. Neuroradiology. 2007;49, 669–679 doi: 10.1007/s00234-007-0231-3. [DOI] [PubMed] [Google Scholar]
- 38.van den Heuvel M.P., Hulshoff Pol H.E. Exploring the brain network: A review on resting-state fMRI functional connectivity. European Neuropsychopharmacology. 2010;20:519–553. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Veer I.M., Beckmann C.F., Van Tol M.J., Ferrarini L., Milles J, et al. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Frontiers in Systems Neuroscience. 2010;41, 1–10 doi: 10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wineland A.M., Burton H., Piccirillo J. Functional connectivity networks in non-bothersome tinnitus. Otolaryngology Head Neck Surg. 2012;147:900–906. doi: 10.1177/0194599812451414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang D., Snyder A.Z., Fox M.D., Sansbury M.W., Shimony J.S, et al. Intrinsic functional relations between human cerebral cortex and thalamus. J. Neurophysiol. 2008;100:1740–1748. doi: 10.1152/jn.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]