Abstract
Human immunodeficiency virus (HIV) type 2, the second AIDS-associated human retrovirus, differs from HIV-1 in its natural history, infectivity, and pathogenicity, as well as in details of its genomic structure and molecular behavior. We report here that HIV-2 inhibits the replication of HIV-1 at the molecular level. This inhibition was selective, dose-dependent, and nonreciprocal. The closely related simian immunodeficiency provirus also inhibited HIV-1. The selectivity of inhibition was shown by the observation that HIV-2 did not significantly downmodulate the expression of the unrelated murine leukemia virus; neither did the murine leukemia virus markedly affect HIV-1 or HIV-2 expression. Moreover, while HIV-2 potently inhibited HIV-1, the reverse did not happen, thus identifying yet another and remarkable difference between HIV-1 and HIV-2. Mutational analysis of the HIV-2 genome suggested that the inhibition follows a complex pathway, possibly involving multiple genes and redundant mechanisms. Introduction of inactivating mutations into the structural and regulatory/accessory genes did not render the HIV-2 provirus ineffective. Some of the HIV-2 gene defects, such as that of tat and rev genes, were phenotypically transcomplemented by HIV-1. The HIV-2 proviruses with deletions in the putative packaging signal and defective for virus replication were effective in inducing the suppressive phenotype. Though the exact mechanism remains to be defined, the inhibition appeared to be mainly due to an intracellular molecular event because it could not be explained solely on the basis of cell surface receptor mediated interference. The results support the notion that the inhibition likely occurred at the level of viral RNA, possibly involving competition between viral RNAs for some transcriptional factor essential for virus replication. Induction of a cytokine is another possibility. These findings might be relevant to the clinical-epidemiological data suggesting that infection with HIV-2 may offer some protection against HIV-1 infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreasson P. A., Dias F., Nauclér A., Andersson S., Biberfeld G. A prospective study of vertical transmission of HIV-2 in Bissau, Guinea-Bissau. AIDS. 1993 Jul;7(7):989–993. [PubMed] [Google Scholar]
- Arya S. K., Beaver B., Jagodzinski L., Ensoli B., Kanki P. J., Albert J., Fenyo E. M., Biberfeld G., Zagury J. F., Laure F. New human and simian HIV-related retroviruses possess functional transactivator (tat) gene. Nature. 1987 Aug 6;328(6130):548–550. doi: 10.1038/328548a0. [DOI] [PubMed] [Google Scholar]
- Arya S. K., Gallo R. C. Human immunodeficiency virus type 2 long terminal repeat: analysis of regulatory elements. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9753–9757. doi: 10.1073/pnas.85.24.9753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya S. K., Guo C., Josephs S. F., Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- Arya S. K. Human and simian immunodeficiency retroviruses: activation and differential transactivation of gene expression. AIDS Res Hum Retroviruses. 1988 Jun;4(3):175–186. doi: 10.1089/aid.1988.4.175. [DOI] [PubMed] [Google Scholar]
- Arya S. K. Human immunodeficiency virus type 2 (HIV-2) trans-activator (Tat): functional domains and the search for trans-dominant negative mutants. AIDS Res Hum Retroviruses. 1993 Sep;9(9):839–848. doi: 10.1089/aid.1993.9.839. [DOI] [PubMed] [Google Scholar]
- Arya S. K. Human immunodeficiency virus type-2 gene expression: two enhancers and their activation by T-cell activators. New Biol. 1990 Jan;2(1):57–65. [PubMed] [Google Scholar]
- Arya S. K., Sadaie M. R. Fusogenicity of mutant and chimeric proviruses derived from molecular clones of cytopathic and noncytopathic human immunodeficiency virus type 2. J Acquir Immune Defic Syndr. 1993 Nov;6(11):1205–1211. [PubMed] [Google Scholar]
- Cocchi F., DeVico A. L., Garzino-Demo A., Arya S. K., Gallo R. C., Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995 Dec 15;270(5243):1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- Dayton A. I., Sodroski J. G., Rosen C. A., Goh W. C., Haseltine W. A. The trans-activator gene of the human T cell lymphotropic virus type III is required for replication. Cell. 1986 Mar 28;44(6):941–947. doi: 10.1016/0092-8674(86)90017-6. [DOI] [PubMed] [Google Scholar]
- Dufoort G., Couroucé A. M., Ancelle-Park R., Bletry O. No clinical signs 14 years after HIV-2 transmission via blood transfusion. Lancet. 1988 Aug 27;2(8609):510–510. doi: 10.1016/s0140-6736(88)90154-7. [DOI] [PubMed] [Google Scholar]
- Emerman M., Guyader M., Montagnier L., Baltimore D., Muesing M. A. The specificity of the human immunodeficiency virus type 2 transactivator is different from that of human immunodeficiency virus type 1. EMBO J. 1987 Dec 1;6(12):3755–3760. doi: 10.1002/j.1460-2075.1987.tb02710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. A., Moreau J., Odehouri K., Legg H., Barboza A., Cheng-Mayer C., Levy J. A. Characterization of a noncytopathic HIV-2 strain with unusual effects on CD4 expression. Science. 1988 Jun 10;240(4858):1522–1525. doi: 10.1126/science.2836951. [DOI] [PubMed] [Google Scholar]
- Evans L. A., Moreau J., Odehouri K., Seto D., Thomson-Honnebier G., Legg H., Barboza A., Cheng-Mayer C., Levy J. A. Simultaneous isolation of HIV-1 and HIV-2 from an AIDS patient. Lancet. 1988 Dec 17;2(8625):1389–1391. doi: 10.1016/s0140-6736(88)90586-7. [DOI] [PubMed] [Google Scholar]
- Fisher A. G., Feinberg M. B., Josephs S. F., Harper M. E., Marselle L. M., Reyes G., Gonda M. A., Aldovini A., Debouk C., Gallo R. C. The trans-activator gene of HTLV-III is essential for virus replication. 1986 Mar 27-Apr 2Nature. 320(6060):367–371. doi: 10.1038/320367a0. [DOI] [PubMed] [Google Scholar]
- Gao F., Yue L., Robertson D. L., Hill S. C., Hui H., Biggar R. J., Neequaye A. E., Whelan T. M., Ho D. D., Shaw G. M. Genetic diversity of human immunodeficiency virus type 2: evidence for distinct sequence subtypes with differences in virus biology. J Virol. 1994 Nov;68(11):7433–7447. doi: 10.1128/jvi.68.11.7433-7447.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
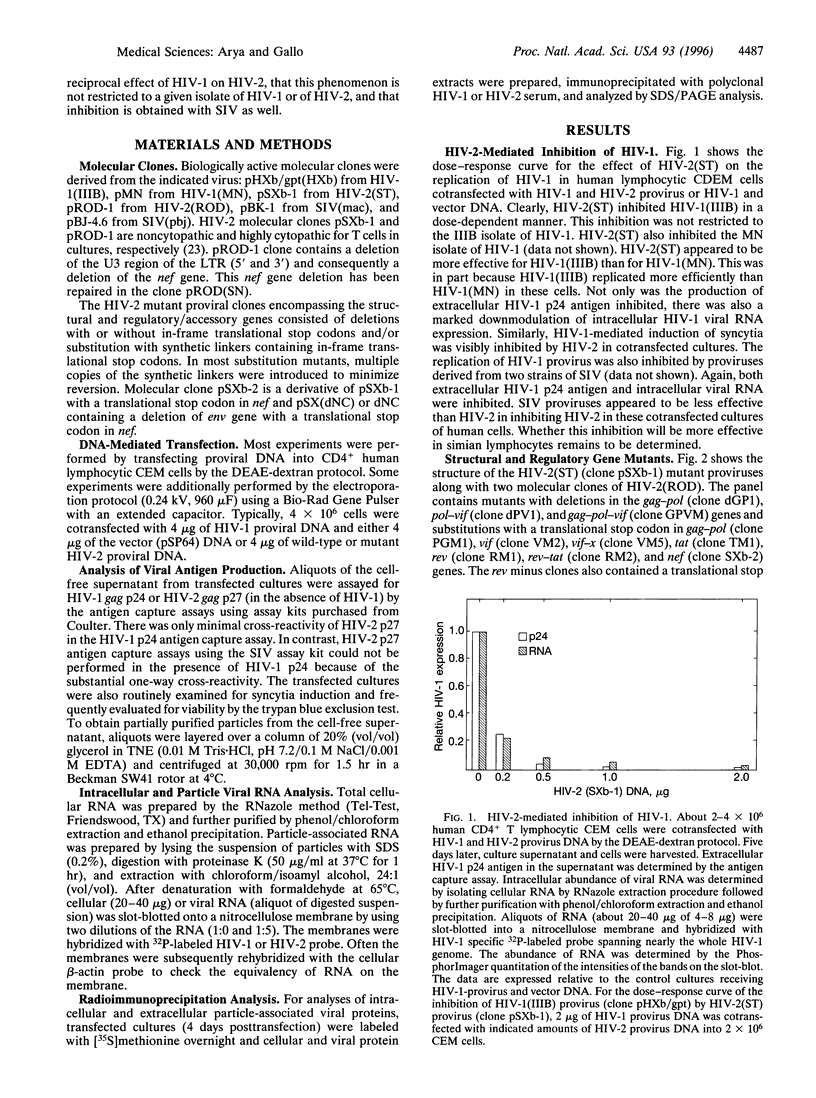
- Garzino-Demo A., Gallo R. C., Arya S. K. Human immunodeficiency virus type 2 (HIV-2): packaging signal and associated negative regulatory element. Hum Gene Ther. 1995 Feb;6(2):177–184. doi: 10.1089/hum.1995.6.2-177. [DOI] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Hoxie J. A., Brass L. F., Pletcher C. H., Haggarty B. S., Hahn B. H. Cytopathic variants of an attenuated isolate of human immunodeficiency virus type 2 exhibit increased affinity for CD4. J Virol. 1991 Sep;65(9):5096–5101. doi: 10.1128/jvi.65.9.5096-5101.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet T., Cheynier R., Meyerhans A., Roelants G., Wain-Hobson S. Genetic organization of a chimpanzee lentivirus related to HIV-1. Nature. 1990 May 24;345(6273):356–359. doi: 10.1038/345356a0. [DOI] [PubMed] [Google Scholar]
- Ivey-Hoyle M., Culp J. S., Chaikin M. A., Hellmig B. D., Matthews T. J., Sweet R. W., Rosenberg M. Envelope glycoproteins from biologically diverse isolates of immunodeficiency viruses have widely different affinities for CD4. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):512–516. doi: 10.1073/pnas.88.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanki P. J., Travers K. U., MBoup S., Hsieh C. C., Marlink R. G., Gueye-NDiaye A., Siby T., Thior I., Hernandez-Avila M., Sankalé J. L. Slower heterosexual spread of HIV-2 than HIV-1. Lancet. 1994 Apr 16;343(8903):943–946. doi: 10.1016/s0140-6736(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Kumar P., Hui H. X., Kappes J. C., Haggarty B. S., Hoxie J. A., Arya S. K., Shaw G. M., Hahn B. H. Molecular characterization of an attenuated human immunodeficiency virus type 2 isolate. J Virol. 1990 Feb;64(2):890–901. doi: 10.1128/jvi.64.2.890-901.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak R. A., Calnan B. J., Frankel A. D., Sharp P. A. HIV-1 Tat protein trans-activates transcription in vitro. Cell. 1990 Nov 16;63(4):791–802. doi: 10.1016/0092-8674(90)90145-5. [DOI] [PubMed] [Google Scholar]
- Marlink R. G., Ricard D., M'Boup S., Kanki P. J., Romet-Lemonne J. L., N'Doye I., Diop K., Simpson M. A., Greco F., Chou M. J. Clinical, hematologic, and immunologic cross-sectional evaluation of individuals exposed to human immunodeficiency virus type-2 (HIV-2). AIDS Res Hum Retroviruses. 1988 Apr;4(2):137–148. doi: 10.1089/aid.1988.4.137. [DOI] [PubMed] [Google Scholar]
- Marlink R., Kanki P., Thior I., Travers K., Eisen G., Siby T., Traore I., Hsieh C. C., Dia M. C., Gueye E. H. Reduced rate of disease development after HIV-2 infection as compared to HIV-1. Science. 1994 Sep 9;265(5178):1587–1590. doi: 10.1126/science.7915856. [DOI] [PubMed] [Google Scholar]
- Putkonen P., Walther L., Zhang Y. J., Li S. L., Nilsson C., Albert J., Biberfeld P., Thorstensson R., Biberfeld G. Long-term protection against SIV-induced disease in macaques vaccinated with a live attenuated HIV-2 vaccine. Nat Med. 1995 Sep;1(9):914–918. doi: 10.1038/nm0995-914. [DOI] [PubMed] [Google Scholar]
- Rayfield M., De Cock K., Heyward W., Goldstein L., Krebs J., Kwok S., Lee S., McCormick J., Moreau J. M., Odehouri K. Mixed human immunodeficiency virus (HIV) infection in an individual: demonstration of both HIV type 1 and type 2 proviral sequences by using polymerase chain reaction. J Infect Dis. 1988 Dec;158(6):1170–1176. doi: 10.1093/infdis/158.6.1170. [DOI] [PubMed] [Google Scholar]
- Simon F., Matheron S., Tamalet C., Loussert-Ajaka I., Bartczak S., Pépin J. M., Dhiver C., Gamba E., Elbim C., Gastaut J. A. Cellular and plasma viral load in patients infected with HIV-2. AIDS. 1993 Nov;7(11):1411–1417. doi: 10.1097/00002030-199311000-00002. [DOI] [PubMed] [Google Scholar]
- Travers K., Mboup S., Marlink R., Guèye-Nidaye A., Siby T., Thior I., Traore I., Dieng-Sarr A., Sankalé J. L., Mullins C. Natural protection against HIV-1 infection provided by HIV-2. Science. 1995 Jun 16;268(5217):1612–1615. doi: 10.1126/science.7539936. [DOI] [PubMed] [Google Scholar]
- Zagury J. F., Franchini G., Reitz M., Collalti E., Starcich B., Hall L., Fargnoli K., Jagodzinski L., Guo H. G., Laure F. Genetic variability between isolates of human immunodeficiency virus (HIV) type 2 is comparable to the variability among HIV type 1. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5941–5945. doi: 10.1073/pnas.85.16.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]