Abstract
The incidence of fungal infections due to C. parapsilosis and closely related cryptic species (-psilosis complex) has increased in the last few years, but differences in virulence among these species have not been widely studied. Fifteen clinical isolates of C. parapsilosis, C. orthopsilosis, and C. metapsilosis, including the type strains, were used to evaluate their virulence in Galleria mellonella larvae. Fluctuations in the hemocyte density and in the phagocytic activity were also tested. Differences in the median survival for these species were demonstrated at 37 °C (2.6 ± 1.02, 2.3 ± 0.92, and 4.53 ± 1.65 d for C. parapsilosis, C. orthopsilosis, and C. metapsilosis, respectively). Galleria mellonella hemocytes phagocytosed C. metapsilosis strains more effectively than did for C. orthopsilosis and C. parapsilosis (P < 0.05). The phagocytosis rate was lower for C. parapsilosis than for C. orthopsilosis (P < 0.05). The hemocyte density was increased in larvae infected with C. metapsilosis compared with those infected with C. parapsilosis or C. orthopsilosis (P < 0.05). Moreover, in vitro studies of virulence factors such as pseudohyphae production and hydrolytic enzyme secretion showed that the capability of C. metapsilosis strains to produce those virulence factors was reduced. Infections due to -psilosis complex species produced tissue damage in G. mellonella and pseudohyphae could be also observed during infection with C. parapsilosis.
Keywords: Candida parapsilosis, Candida orthopsilosis, Candida metapsilosis, virulence, Galleria mellonella
Introduction
Infections due to Candida parapsilosis are the second most common cause of candidemia in some European countries and Latin America.1-3 However, in some invertebrate models, C. parapsilosis has shown reduced virulence compared with other Candida species.4 In 2005, Tavanti et al. described two new species related to C. parapsilosis as a cause of Candida infections: C. orthopsilosis and C. metapsilosis.5 The prevalence of these species is increasing, and C. orthopsilosis has been recently described as the fifth cause of fungemia in Spain over C. krusei.6 Antifungal susceptibility patterns in these species showed reduced in vitro susceptibility to echinocandins although it has been reported that minimum inhibitory concentrations (MICs) to caspofungin for C. orthopsilosis and C. metapsilosis are lower than those for C. parapsilosis sensu stricto.7
Virulence of the C. parapsilosis complex has been studied using different models. An in vitro infection model using microglial cells pointed out C. metapsilosis as the least virulent member of the -psilosis complex.8 Besides, virulence of C. parapsilosis, C. orthopsilosis, and C. metapsilosis was also assessed in epithelial and epidermal tissue models. Candida metapsilosis was found to be the least pathogenic in that it produced fewer alterations and yeasts could rarely be observed attached to the epithelium.9 Despite known differences in virulence, it is unclear what virulence factors (biofilm development, lipases, or aspartyl proteinases) may play a role in the final outcome.10-15
Murine models are the gold standard for virulence studies; however, ethical considerations associated with mammalian models have made invertebrate models (Caenorhabditis elegans, Drosophila melanogaster, Galleria mellonella, Dictyostelium discoideum, etc.) an attractive alternative.16 Several articles have shown the utility of G. mellonella as a model to study fungal infection.17,18 G. mellonella provides important advantages such as larval maintenance at 25–37 °C, direct delivery of exact inoculum by injection, and mortality assessment without special equipment requirements.17,19 Moreover, some of the fungal virulence factors described to be important in mammalian infections have also been found in G. mellonella.17,19,20
An additional advantage of G. mellonella is that its innate response is conserved in mammals. Hemocytes are important in the larva’s cellular defense against bacteria and fungi since they act as phagocytic cells.17,21,22 Fluctuations in the hemocyte density and in the phagocytosis rate have been related to the ability of the fungus to kill larvae, although several pathogens have developed strategies to avoid phagocytic killing.23,24 Currently, these two parameters, hemocyte density and phagocytosis rate, have been used to evaluate virulence of Cryptococcus neoformans, Fusarium oxysporum, Aspergillus fumigatus, C. albicans, C. krusei, Histoplasma capsulatum, Paracoccidioides lutzii, and C. tropicalis.25-31
In the present study, we demonstrate the ability of the species belonging to C. parapsilosis complex to produce lethal infection in G. mellonella. Additionally, hemocyte density, phagocytosis activity, pseudohyphae formation, and production of hydrolytic enzymes were also evaluated using five clinical strains of each of the species including their type strains.
Results
Differences among C. parapsilosis sensu lato (s.l.) virulence in Galleria mellonella are not due to differences in their growth rate
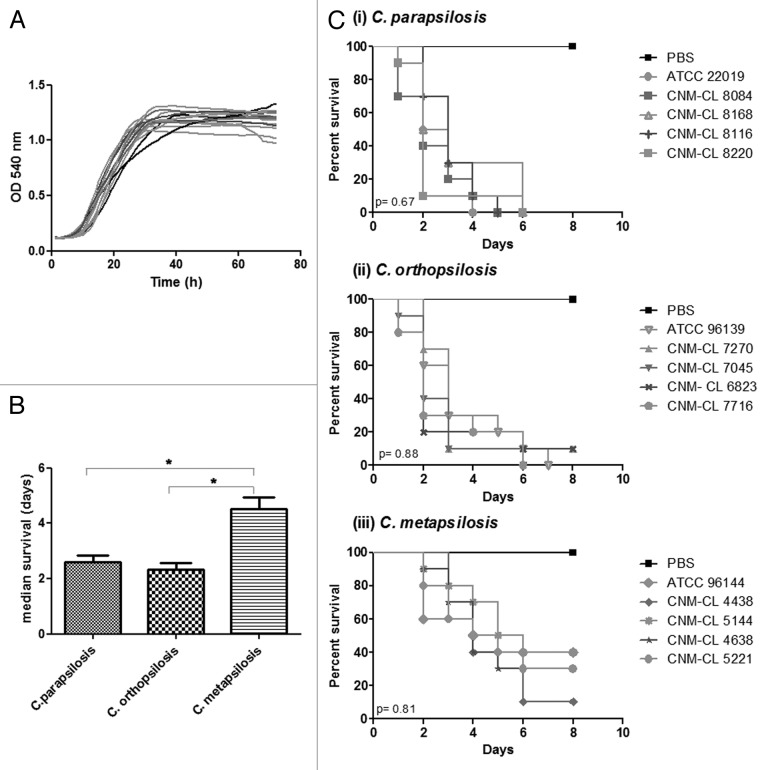
In the present study we compared different isolates of C. parapsilosis, C. metapsilosis, and C. orthopsilosis for their capability to kill G. mellonella. Five strains per species were evaluated. Inoculum standardization was assessed with the type strains and a 2 × 106 yeasts/larva inoculum was assessed to compare differences among species. No significant differences in growth rates at 37 °C were detected for any species (P > 0.05) (Fig. 1A).
Figure 1. (A) Growth curves of C. parapsilosis strains at 37 °C. (B) Differences in the median survival of G. mellonella larvae infected with C. parapsilosis species. (C) Survival curves of G. mellonella infected with five different strains of C. parapsilosis, C. orthopsilosis, and C. metapsilosis at a final concentration of 2 × 106 yeasts/larva and incubated at 37 °C. P value below 0.05 is indicated with asterisk.
The median survival for larvae infected with C. metapsilosis strains was significantly higher than that of larvae infected with C. orthopsilosis and C. parapsilosis (P < 0.05). However, no differences in the median survival were detected when we compared C. orthopsilosis and C. parapsilosis infection (Fig. 1B, P > 0.05). No differences in the median survival were found within strains belonging to the same species (Fig. 1C, P > 0.05).
Hemocyte density in Galleria mellonella differs in C. parapsilosis, C. orthopsilosis, and C. metapsilosis
Galleria mellonella larvae were infected with 106 yeasts/larva and hemocytes were enumerated after 2 h of infection at 37 °C (Fig. 2A). All the species induced an increase in the hemocytes present in the hemolymph of G. mellonella compared with controls (P < 0.05). Hemocyte population was significantly higher in larvae infected with C. metapsilosis than in those infected with C. parapsilosis or C. orthopsilosis (P < 0.05). Moreover, the number of hemocytes in the hemolymph of larvae infected with C. orthopsilosis was higher than that recorded in larvae infected with C. parapsilosis (P < 0.05). No differences in hemocyte density were observed when larvae were infected with different strains of the same species (Fig. 2B).
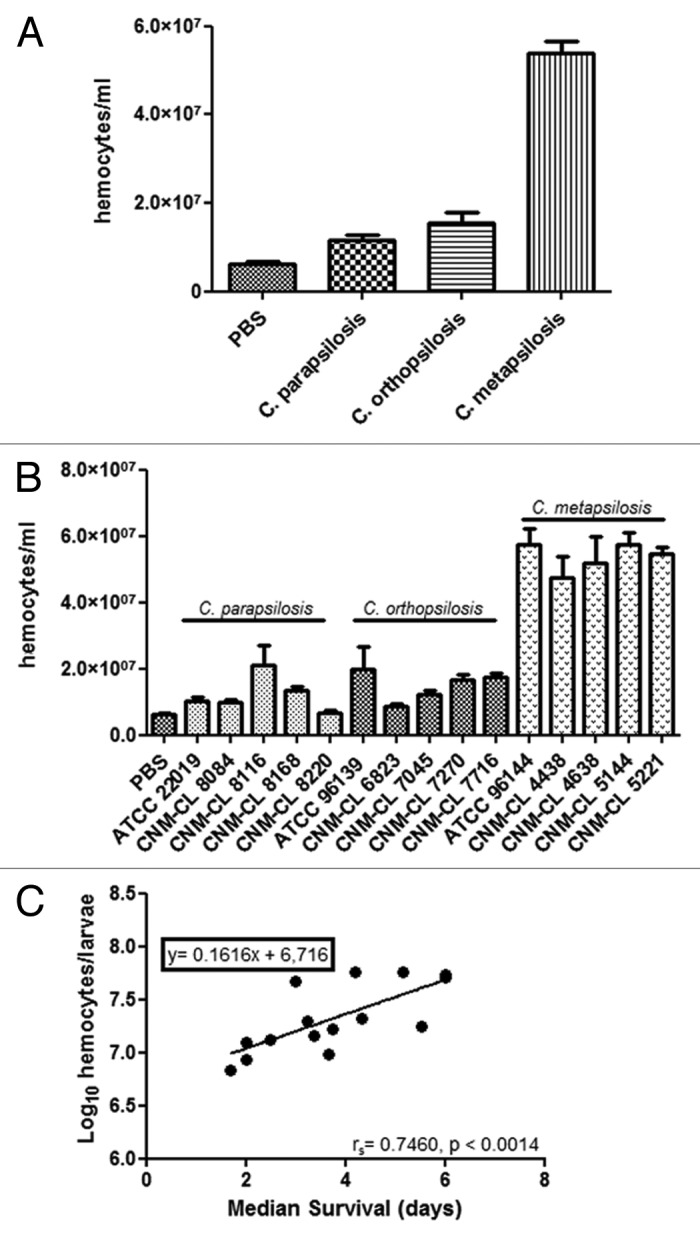
Figure 2. (A) Changes in the global hemocyte density during C. parapsilosis species infection. (B) Differences in the hemocyte density in G. mellonella due to infection with different C. parapsilosis strains. (C) Correlation between median survival and hemocyte density in G. mellonella larvae infected with C. parapsilosis strains. Significant differences (P < 0.05) in hemocyte density relative to that in the PBS treated larvae are indicated with an asterisk.
A Spearman correlation coefficient (rS) was determined by comparing the median survival and the hemocyte density data for each of the strains. From these rS values, a Spearman correlation test was performed to determine whether those parameters varied regularly. A direct correlation between hemocytes and median survival was found (rS = 0.7460) (Fig. 2C). Candida metapsilosis showed the highest median survival and the highest amount of hemocytes in the hemolymph in G. mellonella.
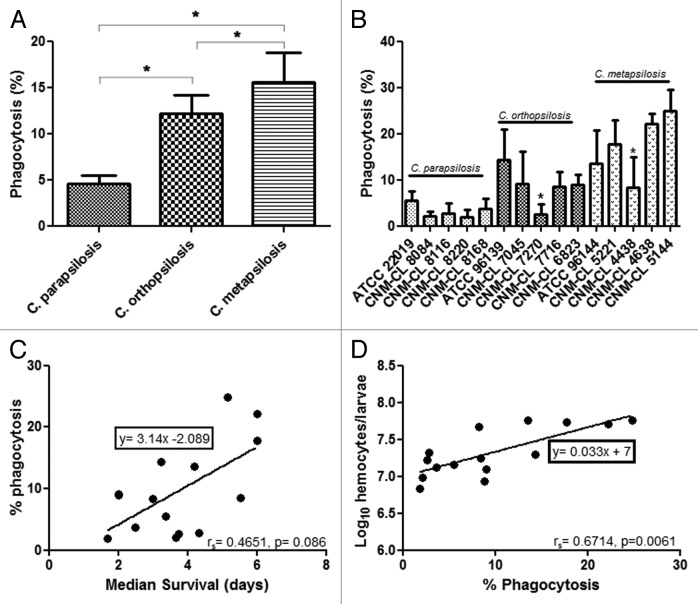
C. metapsilosis is more effectively phagocytosed by G. mellonella hemocytes than C. parapsilosis and C. orthopsilosis
To investigate whether the differences observed in virulence could be associated with differences in the efficiency of the innate immune system against these species, experiments of phagocytosis in vivo were performed. Larvae were infected with the type strains of each species and then incubated for 2 and 4 h. No differences in the phagocytosis efficiency were detected between 2 h and 4 h and thus phagocytosis at 2 h was determined for all of the strains. After 2 h of infection, all C. metapsilosis strains were more effectively phagocytosed by G. mellonella hemocytes than C. parapsilosis and C. orthopsilosis. Differences in the percentage of phagocytosis were statistically significant when compared among the species (P < 0.05) (Fig. 3A). No differences in the phagocytosis efficiency were detected intraspecies, except for C. orthopsilosis strain CNM-CL 7270 and C. metapsilosis strain CNM-CL 4438 that showed very low phagocytosis rates (Fig. 3B).
Figure 3. (A) Percentage of phagocytosis at 2 h post infection with C. parapsilosis. (B) Average of phagocytosis percentages of C. parapsilosis, C. orthopsilosis, and C. metapsilosis. (C) Correlation between median survival and percentage of phagocytosis during C. parapsilosis sensu lato infection. (D) Correlation between percentage of phagocytosis and hemocyte density during C. parapsilosis sensu lato infection. P value less than 0.05 is indicated with asterisk.
A positive correlation (rS = 0.6714) was detected between percentage of phagocytosis and median survival (Fig. 3C). Candida metapsilosis showed the highest phagocytosis efficiency and the highest median survival (4.53 d). Percentage of phagocytosis and hemolymph density showed also a positive correlation (rS = 0.4651) between C. metapsilosis strains with the highest phagocytosis efficiency and the highest values for hemocyte population after infection (Fig. 3C).
Pseudohyphae formation and secretion of hydrolytic enzymes
Secretion of hydrolytic enzymes and pseudohyphae production results are summarized in Table 1. All C. parapsilosis strains produced pseudohyphae after 24 h, while only 2 out of 5 strains of C. orthopsilosis were able to produce pseudohyphae after 48 h. In contrast, none of the C. metapsilosis strains produced hyphae or pseudohyphae after 48 h of incubation.
Table 1. Enzymatic profiles and pseudohyphae production of the strains included in this study.
| Species | Protease | Esterase | Urease | Hemolysin | 24 h pseudohyphae | 48 h pseudohyphae |
|---|---|---|---|---|---|---|
| C. parapsilosis | 5/5 | 1/5 | 5/5 | 2/5 | 5/5 | 5/5 |
| C. orthopsilosis | 3/5 | 3/5 | 5/5 | 0/5 | 1/5 | 2/5 |
| C. metapsilosis | 1/5 | 4/5 | 5/5 | 2/5 | 0/5 | 0/5 |
Regarding the secretion of hydrolytic enzymes, we found that all C. parapsilosis strains secreted proteases. Curiously, most of the C. metapsilosis strains showed esterase activity. However, in vitro production of hydrolytic enzymes varied among C. orthopsilosis strains and therefore no correlation with other virulence traits could be established for this group.
Candida parapsilosis sensu stricto induced pseudohyphae formation in G. mellonella
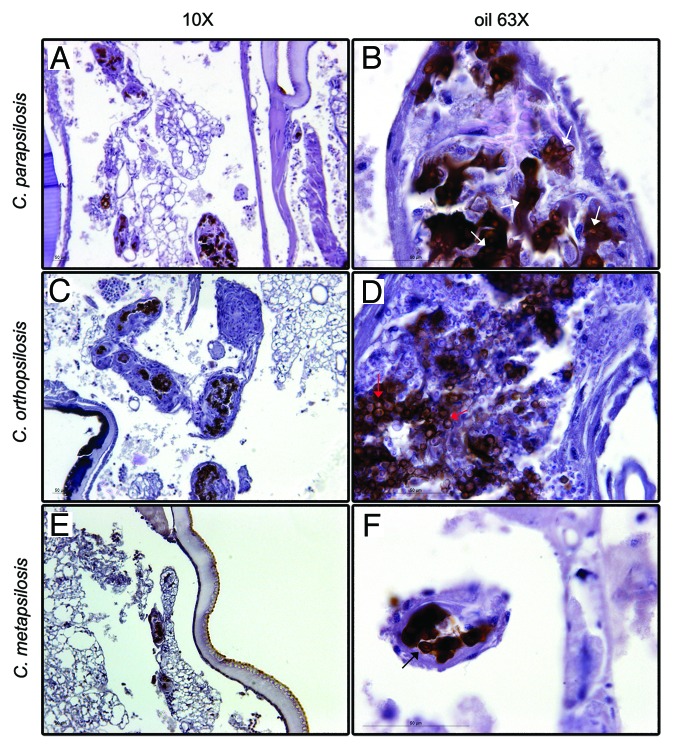
Histopathology studies of larvae infected with C. parapsilosis, C. orthopsilosis, and C. metapsilosis type strains were performed at days 1, 2, and 3 post-infection (Fig. 4). At day 3 post-infection, C. parapsilosis was spread all over the fat body of G. mellonella larvae and both yeasts and pseudohyphae forms could be found (white arrows, Fig. 4B). These pseudohyphae were formed by 2–3 blastoconidias and resemble those observed during C. krusei infection of G. mellonella.28 In larvae infected with C. orthopsilosis, infection was spread all over the body fat of the larvae similar to the pattern observed in C. parapsilosis infection. However, only yeast forms were observed (red arrows, Fig. 4D). Finally, infection of larvae with C. metapsilosis resulted in focalized infection, and only yeast forms could be observed during the infection (black arrows, Fig. 4F). Yeasts were always found surrounded by tissue in a granuloma-like structure. Curiously, these structures varied in size during infection. At day 2 post-infection, granuloma-like structures were bigger than at day 3 (data not shown).
Figure 4. Histopathology of G. mellonella infection infected with 2 × 106 yeasts/larva of C. parapsilosis (A and B), C. orthopsilosis (C and D), and C. metapsilosis (E and F) at day 3 post infection. Panels show images taken with low magnification (A, C, and E) and high magnification (B, D, and F).
Discussion
Since the description of cryptic species of C. parapsilosis, several molecular tools to differentiate them have been described, but virulence studies remain scarce. In the present work, we compared C. metapsilosis, C. parapsilosis, and C. orthopsilosis pathogenicity using Galleria mellonella as a model host. Galleria mellonella has been used to study virulence of some Candida spp., Cryptococcus spp., Fusarium spp., and Aspergillus spp, showing strong correlation with mammal models.17,20,25-33
In agreement with previous studies in other models, differences in virulence for C. parapsilosis complex were detected in G. mellonella. Candida metapsilosis was the least virulent of the -psilosis complex7-9,34 with a median survival longer than that one observed with the other two species (4.53 d vs. 2.6 and 2.3 d for C. parapsilosis and C. orthopsilosis respectively). However, these differences could not be associated to differences in their growth rate.35
Macrophages play an important role in the immune response by phagocytosing and killing microorganisms.36-38 The ability of macrophages to internalize medically important fungal species as C. neoformans, C. krusei, Aspergillus spp., C. albicans, and also species belonging to C. parapsilosis spp. complex has already been described.28,36,39-43 The Galleria mellonella immune system consists mainly of hemocytes, which have phagocytic activity44 and play an important role against pathogens.45,46 Changes in the hemocyte density during infection can be used as a parameter of the response of the larvae to the infection.44 We showed that these hemocytes can internalize C. parapsilosis species after 2 and 4 h of incubation as previously described for other fungal species.26,28 Interestingly, although C. metapsilosis is the least phagocytosed of the -psilosis complex by microglial cells,8 we have observed higher percentages of phagocytosis for C. metapsilosis strains compared with C. parapsilosis and C. orthopsilosis in our model. In fact, we observed the highest counts of hemocytes after 2 h of infection with C. metapsilosis compared with the other two species, suggesting a higher immune stimulation and therefore giving a possible explanation to the longer survival with this species.
A reduction in the number of phagocytic cells in larvae inoculated with C. parapsilosis and C. orthopsilosis species compared with C. metapsilosis infected larvae was observed. These two species reportedly develop pseudohyphae during the infection, as observed in histological preparations of the infected larvae. We demonstrated the capability of C. parapsilosis strains to develop pseudohyphae in vitro, while this characteristic was limited or absent in C. orthopsilosis and C. metapsilosis (Table 1). Pseudohyphae formation could lead to the death of the phagocytic population which could be the reason for the low numbers of hemocytes found in larvae infected with these species.8,34,42
The development of hyphae or pseudohyphae has been linked to virulence in some Candida species such as C. albicans, C. krusei, and C. tropicalis.25,27,28 The reduced virulence of C. parapsilosis compared with C. albicans has been associated to the ability of the later to produce hyphal forms that are more adherent compared with the pseudohyphal forms produced by C. parapsilosis.47,48 Other virulence traits, such as secretion of hydrolytic enzymes or pseudohyphae production, have been widely used to analyze differences in virulence among species belonging to the -psilosis complex.42 No correlation between the production of hemolysins or urease and the development of pseudohyphae could be established for any of the -psilosis species. However, all C. parapsilosis strains showed proteinase activity and were able to develop pseudohyphae, which could lead to the evasion of the phagocytosis observed in vivo. In contrast, no pseudohyphae formation was observed in any of the C. metapsilosis and only one strain showed proteinase activity. In addition, most C. metapsilosis produced esterase as described in Ge et al.49 while only one C. parapsilosis was able to produce this enzyme. Finally, C. orthopsilosis strains were the most heterogeneous group regarding the production of both hydrolytic enzymes and pseudohyphae, and therefore no correlation could be established.42 Other unknown virulence factors might be involved in the pathogenesis of this last species.
We were able to correlate the degree of damage on the histological preparations of G. mellonella to the virulence showed for each species, as described for C. tropicalis infections.27 C. metapsilosis induced minor alterations in G. mellonella compared with both C. parapsilosis and C. orthopsilosis. Candida parapsilosis produced pseudohyphae and severe tissue damage in the larvae with numerous areas of infection. Occasional pseudohyphal structures were also detected in G. mellonella infected with C. orthopsilosis, although the main structures observed were clustered yeast cells surrounded by tissue structures. Several studies have also shown that pseudohyphae production is not common in C. orthopsilosis.13,42 Differences in virulence have been described between C. orthopsilosis and C. parapsilosis although the incidence varies between studies.1,2,6,8,12,50 Our results suggest that initial phagocytosis does not predict the final outcome of C. orthopsilosis infection since despite being easily phagocytized by hemocytes, C. orthopsilosis could cause disseminated infection.
Our work demonstrates that G. mellonella is a good alternative model to study virulence in species belonging to the -psilosis complex. The results are in agreement with previous work that pointed out C. metapsilosis as the least virulent of the C. parapsilosis species complex.8 Differences in the production of virulence factors in vitro, such as pseudohyphae production or proteinase and esterase activity, could be linked to the differences observed in phagocytosis in vivo. Moreover, for the first time, virulence has been associated with differences in hemocyte influx in response to the infection, phagocytosis efficiency and the capability of undergoing morphological changes that can lead to tissue damage.
Materials and Methods
Yeast strains and culture conditions
Fifteen strains of C. parapsilosis complex isolated from blood cultures were used in this study. Candida parapsilosis ATCC 22019 (T), C. orthopsilosis ATCC 96139 (T), and C. metapsilosis ATCC 96144 (T) were used as controls. Yeast isolates were identified to species level by sequencing the ITS (Internal Transcribed Spacers) region of the rDNA. Candida parapsilosis cultures were grown in liquid Sabouraud (SAB) overnight at 30 °C with shaking (150 rpm).
Growth curves
Growth curves in SAB were determined for all the strains of C. parapsilosis complex. Suspensions of C. parapsilosis at 105 yeasts/ml were prepared in SAB and 200 µl were inoculated in a 96-well plate and incubated for 72 h with moderate shaking. Optical density at 540 nm was measured every 30 min in an iEMS Spectrophotometer (Thermofisher) and plotted using Graph Pad Prism 5 software. Growth curves were performed in triplicate at 37 °C. Differences in the curves were calculated by non-parametric Kruskal–Wallis test using Graph Pad Prism 5 software.
Galleria mellonella survival experiments
Galleria mellonella caterpillars in the final instar larval stage were obtained by R.J. Mous Livebait. For experiments, larvae weighing between 0.3 and 0.5 g were selected. Prior to experiments larvae were incubated in the dark at 37 °C overnight.
Galleria mellonella larvae were cleaned with ethanol 70% and Hamilton syringes were disinfected with diluted bleach and ethanol 70%, and washed several times with PBS. All inoculum were prepared in PBS with ampicillin at final concentration of 2 µg/ml. Inoculum standardization was conducted with the three type strains and an inoculum of 2 × 108 yeasts/ml was selected as the most appropriate to monitor virulence. Larvae were inoculated with 10 µl of a 2 × 108 yeasts/ml (2 × 106 yeasts per larva). Untouched larvae and larvae inoculated with PBS-ampicillin were used as uninfected controls. Galleria mellonella larvae were incubated at 37 °C and experiments were performed at least three times independently for all strains. Larval death was determined by visual inspection daily. Killing curves were analyzed by Kaplan–Meier method using Graph Pad Prism 5 software. Differences in the median survival among species were evaluated by Mann–Whitney U test.
Determination of hemocyte density in G. mellonella larvae
Five G. mellonella larvae were inoculated with 106 yeasts/larva of each of the 15 strains tested in the virulence experiments. Larvae inoculated with 10 µl of PBS served as controls. After inoculation, larvae were incubated in dark at 37 °C for 2 h. Then, hemolymph was obtained by insertion of a lancet into the hemocell and diluted 1:1 in PBS to prevent melanization. Density was assessed by counting the hemocytes using a hemocytometer. Statistical analysis was performed by one-way ANOVA to test differences among strains and t test was performed to see differences among species by using Graph Pad Prism 5 software.
Phagocytosis assay
Strains were grown on SAB overnight at 30 °C with shaking (150 rpm). Cells were stained with Calcofluor white at 10 mg/ml for 30 min at 37 °C and washed twice in PBS. Three caterpillars were inoculated with 106 yeasts/larvae for each of the strains and incubated for 2 h at 37 °C. After incubation, hemolymph was recovered and washed with PBS to remove the non-internalized yeasts. The hemocytes were placed on coverslips and then allowed to adhere for 30 min. Samples were mounted in Fluoromount G and images were taken to quantify the phagocytosis with a Leica DMI 3000B fluorescent microscope. A minimum of 100 hemocytes per group were scored. Experiments were performed in triplicate on different days. Statistical differences among strains and species were calculated using one-way ANOVA and t test respectively using Graph Pad Prism 5 software.
Statistical correlations between median survival, percentage of phagocytosis and hemocyte density data were assessed by determining nonparametric Spearman correlations and linear regression analysis using Graph Pad Prism 5 software.
Filamentation assay
Cell suspensions were prepared at 108 yeasts/ml in PBS. Two hundred microliters were inoculated into 10 ml of YPD and incubated at 37 °C up to 48 h. Cultures were observed under a Leica 300B microscope at both 24 and 48 h.
Production of extracellular hydrolytic enzymes
The production of secreted aspartyl proteinases (sap), esterases, hemolysins, and urease were determined at 37 °C as previously described.51,52
Plate assays containing each specific test medium were used for each of the determinations. An aliquot of 5 µl of yeast suspension (107 cells/ml) or 10 µl for hemolysis assay was spotted on each medium. The plates were incubated for 10 d at 37 °C.
For aspartil proteinase activity determination, the media was prepared with 1% bactoagar (Difco, DF214030) 0.1% KH2PO4, 0.5% MgSO4, 1% glucose (pH 5.0), and 0.16% of bovine serum albumin. Aspartil proteinase production was detected using 1.25% w/v amidoblack (90% metanol and 10% acetic acid).
Esterase activity was determined using Tween 80 opacity test medium containing 10 g Bacto peptone (Difco, DF211677), 5 g NaCl2, 0.1 g CaCl2, 15 g bacto agar (Difco, DF214030) in 1 l distilled water; pH was adjusted at 6.8 and then the media was autoclaved. When the medium was cooled, 5 ml of Tween 80 (Sigma, P8074) were added. The production of lipolytic enzymes was detected as a precipitation halo around the colonies.
Hemolysin and urease activities were determined by using blood sheep agar (Difco, DF240920) and Christensen medium (BD, 221097) respectively according to manufacturer’s instruction.
Histological examination
Histopathology studies were assessed to determine the course of the infection in G. mellonella. Tissue sections were obtained on the first, second, and third day after infection with C. parapsilosis s.l. type strains. Larvae inoculated with PBS were included as a control. Samples were processed according to a protocol previously described.27 Periodic acid Schiff (PAS) staining was performed and sections were microscopically examined. Histological sections were observed under a Leica 3000B microscope with 10× and 63× objectives. Pictures were taken with a Leica DFC 300FX camera using Leica Microsystems Software.
Disclosure of Potential Conflict Of Interest
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This project was supported by Fondo de Investigación Sanitaria: PI12/02376, MPY1003/13. S.G. is supported by a research fellowship from the “Fondo de Investigaciones Sanitarias” of the Spanish Ministry of Science and Innovation (FI10/00464). R.G.R. is supported by a FPI fellowship (BES-2009-015913) from the Spanish Ministry of Science and Innovation. A.A.I. has a research contract from the Spanish Network for Research in Infectious Diseases (REIPI RD06/0008), supported by Plan Nacional de I+D+i 2008–2011 and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI RD12/0015)—co-financed by European Development Regional Fund “A way to achieve Europe” ERDF. We are indebted to Julie M Wolf and Radames JB Cordero for useful comments on the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/26973
References
- 1.Almirante B, Rodríguez D, Cuenca-Estrella M, Almela M, Sanchez F, Ayats J, Alonso-Tarres C, Rodriguez-Tudela JL, Pahissa A. Epidemiology, risk factors, and prognosis of Candida parapsilosis bloodstream infections: case-control population-based surveillance study of patients in Barcelona, Spain, from 2002 to 2003. J Clin Microbiol. 2006;44:1681–5. doi: 10.1128/JCM.44.5.1681-1685.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lockhart SR, Iqbal N, Cleveland AA, Farley MM, Harrison LH, Bolden CB, Baughman W, Stein B, Hollick R, Park BJ, et al. Species identification and antifungal susceptibility testing of Candida bloodstream isolates from population-based surveillance studies in two U.S. cities from 2008 to 2011. J Clin Microbiol. 2012;50:3435–42. doi: 10.1128/JCM.01283-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nucci M, Queiroz-Telles F, Alvarado-Matute T, Tiraboschi IN, Cortes J, Zurita J, Guzman-Blanco M, Santolaya ME, Thompson L, Sifuentes-Osornio J, et al. Latin American Invasive Mycosis Network Epidemiology of candidemia in Latin America: a laboratory-based survey. PLoS One. 2013;8:e59373. doi: 10.1371/journal.pone.0059373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chamilos G, Lionakis MS, Lewis RE, Lopez-Ribot JL, Saville SP, Albert ND, Halder G, Kontoyiannis DP. Drosophila melanogaster as a facile model for large-scale studies of virulence mechanisms and antifungal drug efficacy in Candida species. J Infect Dis. 2006;193:1014–22. doi: 10.1086/500950. [DOI] [PubMed] [Google Scholar]
- 5.Tavanti A, Davidson AD, Gow NA, Maiden MC, Odds FC. Candida orthopsilosis and Candida metapsilosis spp. nov. to replace Candida parapsilosis groups II and III. J Clin Microbiol. 2005;43:284–92. doi: 10.1128/JCM.43.1.284-292.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pemán J, Cantón E, Miñana JJ, Florez JA, Echeverria J, Ortega DN, Alarcón JM, Fontanals D, Sard BG, Moreno BB, et al. el Grupo de Estudio FUNGEMYCA [Changes in the epidemiology of fungaemia and fluconazole susceptibility of blood isolates during the last 10 years in Spain: results from the FUNGEMYCA study] Rev Iberoam Micol. 2011;28:91–9. doi: 10.1016/j.riam.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Spreghini E, Orlando F, Tavanti A, Senesi S, Giannini D, Manso E, Barchiesi F. In vitro and in vivo effects of echinocandins against Candida parapsilosis sensu stricto, Candida orthopsilosis and Candida metapsilosis. J Antimicrob Chemother. 2012;67:2195–202. doi: 10.1093/jac/dks180. [DOI] [PubMed] [Google Scholar]
- 8.Orsi CF, Colombari B, Blasi E. Candida metapsilosis as the least virulent member of the ‘C. parapsilosis’ complex. Med Mycol. 2010;48:1024–33. doi: 10.3109/13693786.2010.489233. [DOI] [PubMed] [Google Scholar]
- 9.Gácser A, Schäfer W, Nosanchuk JS, Salomon S, Nosanchuk JD. Virulence of Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis in reconstituted human tissue models. Fungal Genet Biol. 2007;44:1336–41. doi: 10.1016/j.fgb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Bertini A, De Bernardis F, Hensgens LA, Sandini S, Senesi S, Tavanti A. Comparison of Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis adhesive properties and pathogenicity. Int J Med Microbiol. 2013;303:98–103. doi: 10.1016/j.ijmm.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Duarte TR, Oliveira SS, Macrae A, Cedrola SM, Mazotto AM, Souza EP, Melo AC, Vermelho AB. Increased expression of keratinase and other peptidases by Candida parapsilosis mutants. Braz J Med Biol Res. 2011;44:212–6. doi: 10.1590/S0100-879X2011007500011. [DOI] [PubMed] [Google Scholar]
- 12.Horváth P, Nosanchuk JD, Hamari Z, Vágvölgyi C, Gácser A. The identification of gene duplication and the role of secreted aspartyl proteinase 1 in Candida parapsilosis virulence. J Infect Dis. 2012;205:923–33. doi: 10.1093/infdis/jir873. [DOI] [PubMed] [Google Scholar]
- 13.Melo AS, Bizerra FC, Freymüller E, Arthington-Skaggs BA, Colombo AL. Biofilm production and evaluation of antifungal susceptibility amongst clinical Candida spp. isolates, including strains of the Candida parapsilosis complex. Med Mycol. 2011;49:253–62. doi: 10.3109/13693786.2010.530032. [DOI] [PubMed] [Google Scholar]
- 14.Cornet M, Sendid B, Fradin C, Gaillardin C, Poulain D, Nguyen HV. Molecular identification of closely related Candida species using two ribosomal intergenic spacer fingerprinting methods. J Mol Diagn. 2011;13:12–22. doi: 10.1016/j.jmoldx.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vermelho AB, Mazotto AM, de Melo AC, Vieira FH, Duarte TR, Macrae A, Nishikawa MM, da Silva Bon EP. Identification of a Candida parapsilosis strain producing extracellular serine peptidase with keratinolytic activity. Mycopathologia. 2010;169:57–65. doi: 10.1007/s11046-009-9231-7. [DOI] [PubMed] [Google Scholar]
- 16.Desalermos A, Fuchs BB, Mylonakis E. Selecting an invertebrate model host for the study of fungal pathogenesis. PLoS Pathog. 2012;8:e1002451. doi: 10.1371/journal.ppat.1002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs BB, O’Brien E, Khoury JB, Mylonakis E. Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence. 2010;1:475–82. doi: 10.4161/viru.1.6.12985. [DOI] [PubMed] [Google Scholar]
- 18.Junqueira JC. Galleria mellonella as a model host for human pathogens: recent studies and new perspectives. Virulence. 2012;3:474–6. doi: 10.4161/viru.22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mylonakis E. Galleria mellonella and the study of fungal pathogenesis: making the case for another genetically tractable model host. Mycopathologia. 2008;165:1–3. doi: 10.1007/s11046-007-9082-z. [DOI] [PubMed] [Google Scholar]
- 20.Mylonakis E, Moreno R, El Khoury JB, Idnurm A, Heitman J, Calderwood SB, Ausubel FM, Diener A. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect Immun. 2005;73:3842–50. doi: 10.1128/IAI.73.7.3842-3850.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukherjee K, Raju R, Fischer R, Vilcinskas A. Galleria Mellonella as a Model Host to Study Gut Microbe Homeostasis and Brain Infection by the Human Pathogen Listeria Monocytogenes. Adv Biochem Eng Biotechnol. 2013 doi: 10.1007/10_2013_203. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 22.Mukherjee K, Altincicek B, Hain T, Domann E, Vilcinskas A, Chakraborty T. Galleria mellonella as a model system for studying Listeria pathogenesis. Appl Environ Microbiol. 2010;76:310–7. doi: 10.1128/AEM.01301-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joyce SA, Gahan CG. Molecular pathogenesis of Listeria monocytogenes in the alternative model host Galleria mellonella. Microbiology. 2010;156:3456–68. doi: 10.1099/mic.0.040782-0. [DOI] [PubMed] [Google Scholar]
- 24.Senior NJ, Bagnall MC, Champion OL, Reynolds SE, La Ragione RM, Woodward MJ, Salguero FJ, Titball RW. Galleria mellonella as an infection model for Campylobacter jejuni virulence. J Med Microbiol. 2011;60:661–9. doi: 10.1099/jmm.0.026658-0. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs BB, Eby J, Nobile CJ, El Khoury JB, Mitchell AP, Mylonakis E. Role of filamentation in Galleria mellonella killing by Candida albicans. Microbes Infect. 2010;12:488–96. doi: 10.1016/j.micinf.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García-Rodas R, Casadevall A, Rodríguez-Tudela JL, Cuenca-Estrella M, Zaragoza O. Cryptococcus neoformans capsular enlargement and cellular gigantism during Galleria mellonella infection. PLoS One. 2011;6:e24485. doi: 10.1371/journal.pone.0024485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mesa-Arango AC, Forastiero A, Bernal-Martínez L, Cuenca-Estrella M, Mellado E, Zaragoza O. The non-mammalian host Galleria mellonella can be used to study the virulence of the fungal pathogen Candida tropicalis and the efficacy of antifungal drugs during infection by this pathogenic yeast. Med Mycol. 2013;51:461–72. doi: 10.3109/13693786.2012.737031. [DOI] [PubMed] [Google Scholar]
- 28.Scorzoni L, de Lucas MP, Mesa-Arango AC, Fusco-Almeida AM, Lozano E, Cuenca-Estrella M, Mendes-Giannini MJ, Zaragoza O. Antifungal efficacy during Candida krusei infection in non-conventional models correlates with the yeast in vitro susceptibility profile. PLoS One. 2013;8:e60047. doi: 10.1371/journal.pone.0060047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomaz L, García-Rodas R, Guimarães AJ, Taborda CP, Zaragoza O, Nosanchuk JD. Galleria mellonella as a model host to study Paracoccidioides lutzii and Histoplasma capsulatum. Virulence. 2013;4:139–46. doi: 10.4161/viru.23047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slater JL, Gregson L, Denning DW, Warn PA. Pathogenicity of Aspergillus fumigatus mutants assessed in Galleria mellonella matches that in mice. Med Mycol. 2011;49(Suppl 1):S107–13. doi: 10.3109/13693786.2010.523852. [DOI] [PubMed] [Google Scholar]
- 31.Navarro-Velasco GY, Prados-Rosales RC, Ortíz-Urquiza A, Quesada-Moraga E, Di Pietro A. Galleria mellonella as model host for the trans-kingdom pathogen Fusarium oxysporum. Fungal Genet Biol. 2011;48:1124–9. doi: 10.1016/j.fgb.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Coleman JJ, Muhammed M, Kasperkovitz PV, Vyas JM, Mylonakis E. Fusarium pathogenesis investigated using Galleria mellonella as a heterologous host. Fungal Biol. 2011;115:1279–89. doi: 10.1016/j.funbio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glavis-Bloom J, Muhammed M, Mylonakis E. Of model hosts and man: using Caenorhabditis elegans, Drosophila melanogaster and Galleria mellonella as model hosts for infectious disease research. Adv Exp Med Biol. 2012;710:11–7. doi: 10.1007/978-1-4419-5638-5_2. [DOI] [PubMed] [Google Scholar]
- 34.Silva S, Henriques M, Oliveira R, Azeredo J, Malic S, Hooper SJ, Williams DW. Characterization of Candida parapsilosis infection of an in vitro reconstituted human oral epithelium. Eur J Oral Sci. 2009;117:669–75. doi: 10.1111/j.1600-0722.2009.00677.x. [DOI] [PubMed] [Google Scholar]
- 35.Junqueira JC, Fuchs BB, Muhammed M, Coleman JJ, Suleiman JM, Vilela SF, Costa AC, Rasteiro VM, Jorge AO, Mylonakis E. Oral Candida albicans isolates from HIV-positive individuals have similar in vitro biofilm-forming ability and pathogenicity as invasive Candida isolates. BMC Microbiol. 2011;11:247. doi: 10.1186/1471-2180-11-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levitz SM. Macrophage-Cryptococcus interactions. Immunol Ser. 1994;60:533–43. [PubMed] [Google Scholar]
- 37.Lewis LE, Bain JM, Lowes C, Gillespie C, Rudkin FM, Gow NA, Erwig LP. Stage specific assessment of Candida albicans phagocytosis by macrophages identifies cell wall composition and morphogenesis as key determinants. PLoS Pathog. 2012;8:e1002578. doi: 10.1371/journal.ppat.1002578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis LE, Bain JM, Lowes C, Gow NA, Erwig LP. Candida albicans infection inhibits macrophage cell division and proliferation. Fungal Genet Biol. 2012;49:679–80. doi: 10.1016/j.fgb.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brakhage AA, Bruns S, Thywissen A, Zipfel PF, Behnsen J. Interaction of phagocytes with filamentous fungi. Curr Opin Microbiol. 2010;13:409–15. doi: 10.1016/j.mib.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 40.García-Rodas R, González-Camacho F, Rodríguez-Tudela JL, Cuenca-Estrella M, Zaragoza O. The interaction between Candida krusei and murine macrophages results in multiple outcomes, including intracellular survival and escape from killing. Infect Immun. 2011;79:2136–44. doi: 10.1128/IAI.00044-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonçalves SS, Amorim CS, Nucci M, Padovan AC, Briones MR, Melo AS, Colombo AL. Prevalence rates and antifungal susceptibility profiles of the Candida parapsilosis species complex: results from a nationwide surveillance of candidaemia in Brazil. Clin Microbiol Infect. 2010;16:885–7. doi: 10.1111/j.1469-0691.2009.03020.x. [DOI] [PubMed] [Google Scholar]
- 42.Sabino R, Sampaio P, Carneiro C, Rosado L, Pais C. Isolates from hospital environments are the most virulent of the Candida parapsilosis complex. BMC Microbiol. 2011;11:180. doi: 10.1186/1471-2180-11-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Segal BH. Role of macrophages in host defense against aspergillosis and strategies for immune augmentation. Oncologist. 2007;12(Suppl 2):7–13. doi: 10.1634/theoncologist.12-S2-7. [DOI] [PubMed] [Google Scholar]
- 44.Bergin D, Brennan M, Kavanagh K. Fluctuations in haemocyte density and microbial load may be used as indicators of fungal pathogenicity in larvae of Galleria mellonella. Microbes Infect. 2003;5:1389–95. doi: 10.1016/j.micinf.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 45.Boman HG, Hultmark D. Cell-free immunity in insects. Annu Rev Microbiol. 1987;41:103–26. doi: 10.1146/annurev.mi.41.100187.000535. [DOI] [PubMed] [Google Scholar]
- 46.Kavanagh K, Reeves EP. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol Rev. 2004;28:101–12. doi: 10.1016/j.femsre.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Grubb SE, Murdoch C, Sudbery PE, Saville SP, Lopez-Ribot JL, Thornhill MH. Adhesion of Candida albicans to endothelial cells under physiological conditions of flow. Infect Immun. 2009;77:3872–8. doi: 10.1128/IAI.00518-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grubb SE, Murdoch C, Sudbery PE, Saville SP, Lopez-Ribot JL, Thornhill MH. Candida albicans-endothelial cell interactions: a key step in the pathogenesis of systemic candidiasis. Infect Immun. 2008;76:4370–7. doi: 10.1128/IAI.00332-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ge YP, Lu GX, Shen YN, Liu WD. In vitro evaluation of phospholipase, proteinase, and esterase activities of Candida parapsilosis and Candida metapsilosis. Mycopathologia. 2011;172:429–38. doi: 10.1007/s11046-011-9440-8. [DOI] [PubMed] [Google Scholar]
- 50.Lattif AA, Mukherjee PK, Chandra J, Swindell K, Lockhart SR, Diekema DJ, Pfaller MA, Ghannoum MA. Characterization of biofilms formed by Candida parapsilosis, C. metapsilosis, and C. orthopsilosis. Int J Med Microbiol. 2010;300:265–70. doi: 10.1016/j.ijmm.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Treviño-Rangel RdeJ, González JG, González GM. Aspartyl proteinase, phospholipase, esterase and hemolysin activities of clinical isolates of the Candida parapsilosis species complex. Med Mycol. 2013;51:331–5. doi: 10.3109/13693786.2012.712724. [DOI] [PubMed] [Google Scholar]
- 52.Littman ML. An improved method for detection of urea hydrolysis by fungi. J Infect Dis. 1957;101:51–61. doi: 10.1093/infdis/101.1.51. [DOI] [PubMed] [Google Scholar]