Abstract
Non-typhoidal Salmonella (NTS) infections are emerging as leading problem worldwide and the variations in host immune status append to the concern of NTS. Salmonella enterica serovar Typhimurium is one of the causative agents of NTS infections and has been extensively studied. The inactivation of Salmonella pathogenicity island 2 (SPI2) encoded type-III secretion system 2 (TTSS2) has been reported rendering the strain incapable for systemic dissemination to host sites and has also been proposed as live-attenuated vaccine. However, infections from TTSS2-deficient Salmonella have also been reported. In this study, mutant strain MT15 was developed by inactivation of the hemolysin expression modulating protein (hha) in TTSS2-deficient S. Typhimurium background. The MT15 strain showed significant level of attenuation in immune-deprived murine colitis model when tested in iNos−/−, IL10−/−, and CD40L−/− mice groups in C57BL/6 background. Further, the mutation in hha does not implicate any defect in bacterial colonization to the host gut. The long-term infection of developed mutant strain conferred protective immune responses to suitably immunized streptomycin pre-treated C57BL/6 mice. The immunization enhanced the CD4+ and CD8+ cell types involved in bacterial clearance. The serum IgG and luminal secretory IgA (sIgA) was also found to be elevated after the due course of infection. Additionally, the immunized C57BL/6 mice were protected from the subsequent lethal infection of Salmonella Typhimurium. Collectively, these findings implicate the involvement of hemolysin expression modulating protein (Hha) in establishment of bacterial infection. In light of the observed attenuation of the developed mutant strain, this study proposes the possible significance of SPI2-deficient hha mutant as an alternative live-attenuated vaccine strain for use against lethal Salmonella infections.
Keywords: Salmonella Typhimurium, hha, immunocompromised host, C57BL/6, inflammation
Introduction
Salmonella is a bacteria belonging to Enterobacteriaceae family. The serovars of Salmonella enterica are facultative intracellular pathogens with efficient colonizing capacity and competence of causing disease in a wide host range. Food products, including poultry, egg, milk, and pork constitutes an important source of Salmonella infection in humans.1Salmonella enterica serovar Typhimurium (hereafter referred to as S. Typhimurium) is a broad host range serovar that infects humans, cattle, mice, and chickens, and is one of the major causes of foodborne human salmonellosis.2,3 In humans, S. enterica causes diseases ranging from localized gastroenteritis to disseminated systemic fever.
The pathogenesis of Salmonella has been extensively studied in the mouse host.4-7 In susceptible mice, Salmonella causes an acute systemic disease with limited intestinal manifestations.8 The experimental model to study the Salmonella enterocolitis has been developed in streptomycin-pretreated C57BL/6 mice and used with broad scientific acceptance.9 The Salmonella pathogenicity islands (SPIs) 1 and 2 are the two major virulence determinants of S. enterica. They encode type III secretion systems (T3SS) that form syringe-like structure on the surface of Salmonella and enable the injection of effector proteins directly into the cytosol of eukaryotic cells to establish the infection.10,11 These effectors ultimately manipulate the cellular functions of the infected host and facilitate the progression of the infection.
A critical step in initiation of salmonellosis is the ability to invade the intestinal cells of the host. The entry process occurs by rearrangement of the cellular membrane in the form of actin-ruffles engulfing the bacteria.11 The principle behind successful infection by Salmonella includes bacterial invasion and survival within nonphagocytic cells, as well as replication within macrophages.12,13 Host cell invasion depends on the production of SPI1 type III secretion system (TTSS1) that injects effector proteins. The effector proteins trigger rearrangements of the actin cytoskeleton that lead to transient membrane ruffling and bacterial uptake.14,15 Most of the genes that are required for invasion are located at SPI1 genome patch of Salmonella;16,17 however, others proteins important for bacterial survival within the macrophages are located primarily in the SPI2 island. The expression of invasion associated effector proteins is a collective result due to coordinated functioning of SPI1 associated genes. The regulation of SPI1 and SPI2 gene expression involves numerous transcriptional regulators located both inside and outside these pathogenicity islands. Many of the regulatory proteins were reported influencing the expression for invasion associated proteins. Few such proteins include activators HilA, InvF, HilD, HilC, and RtsA and nucleoid-associated proteins H-NS and Hha.5,18 The activators act in a cascade starting with homologous regulators HilD/HilC/RtsA (Hil activators), followed by the key regulator HilA. It has been shown that two HilD/HilC sites at the hilA promoter overlap with upstream binding sites for repressors Hha/H-NS. Hha is a small nucleoid-associated protein (8 kDa) involved in the negative modulation of virulence genes rather than of housekeeping genes in gram-negative bacteria. Hha was originally shown to increase the cytoplasmic expression of hemolysin in Escherichia coli19 and has also been shown to negatively regulate invasion associated genes in Salmonella.20
Virulence gene regulation in bacterial pathogens is a highly coordinated process involving extracellular sensors and transcription factors that are activated in response to specific environmental cues. In S. Typhimurium, the virulence factors required for intracellular growth are encoded on a large pathogenicity island SPI2, which encodes a type III secretion system (TTSS) 2 and a two-component regulatory system called SsrA–SsrB that activates type III system in the intracellular environment.1 The specific environmental context required for SPI2 activation implied the existence of a repressing system to silence intracellular virulence genes in SPI2 in the absence of an activating environmental signal. The ssaV gene is an important structural component of TTSS2 encoded by SPI2. SsaV is a structural gene encoding part of the secretion apparatus of TTSS2 complex. SPI2 mutations are attenuating in mice, and there is evidence that SPI2 is required for survival and growth within macrophages, which normally mediate the systemic spread of the organisms. Growing evidence suggests that the roles of SPI1 and SPI2 are not as segregated as previously thought, including the finding that SPI2 is also activated inside the intestinal lumen. However, some of the reports also claim that SPI2-deficient Salmonella can also disseminate, though with slow rate, to systemic sites. Also, it has been shown in our previous finding that such Salmonella can cause significant lethal infections in various immunocompromised host groups.21,22 Systemic spread of S. Typhimurium ssaV mutant, in host system, implicates the involvement of additional proteins favoring the survival of the bacterium.
In this report, we investigated the impact of hha mutation in TTSS2-deficient S. Typhimurium with respect to systemic colonization and reactivity to immunocompromised hosts. Our results show that TTSS2-deficient S. Typhimurium colonizes in the systemic sites of murine colitis models of immunocompromised hosts; however, the access to systemic sites can further be restricted when deletion of hemolysin expression modulating protein Hha is accompanied. Also, we have tried to demonstrate the preliminary observations to establish TTSS2-deficient hha mutant of S. Typhimurium as live-attenuated vaccine strain safe to be used in immunocompromised mice hosts.
Results
TTSS2-deficient S. Typhimurium hha mutant (MT15) showed reduced systemic loads in immune-deprived hosts
Salmonella Typhimurium with inactive TTSS2 has been proved to be incompetent in disseminating to host systemic sites to cause lethal infection23 and thus established as vaccine candidate while being able to elicit protective immune responses in C57BL/6 mice. In our study, we evaluated the pathogenicity of SPI2-deficient S. Typhimurium in various isogenic immunocompromised mice of C57BL/6 genetic background; we observed that it causes lethal infections in immune-deprived mice groups. We hypothesized that such dissemination to systemic sites by previously proposed vaccine strain can further be reduced by incorporating additional mutation in the genes possibly involved either in bacterial colonization or systemic replication. Thus, we targeted the hha gene of S. Typhimurium because of its above discussed properties (see Introduction) and contribution in controlling the TTSS1 associated proteins. We designed a double mutant (MT15) deficient in SsaV structural protein of TTSS2 and Hha hemolysis expression modulating protein of S. Typhimurium.
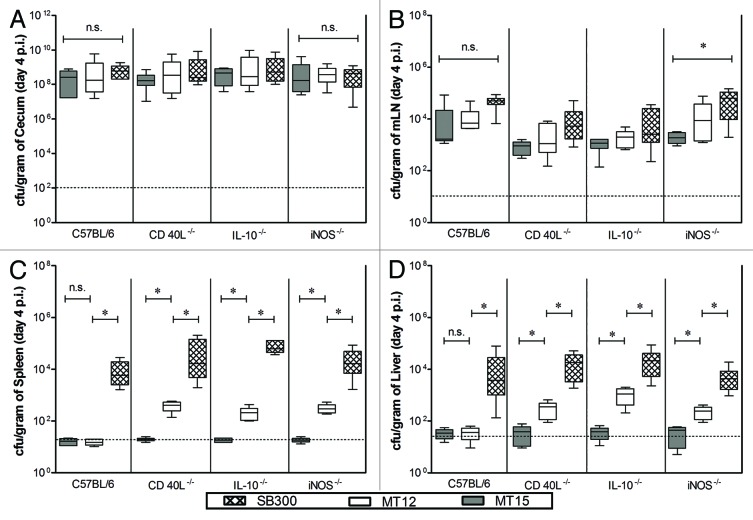
The attenuation characteristics of developed S. Typhimurium strains were assessed in C57BL/6 and its immune-deficient mice lines devoid of iNos−/−, IL10−/−, and CD40L−/−. Group of mice (n = 6 each group) were administered with wild-type strain of S. Typhimurium (SB300), MT12 (ΔssaV; SPI2-deficient strain), and MT15 (hha and ΔssaV; SPI2-deficient hha mutant strain). The systemic bacterial dissemination and intestinal colonization was analyzed at day 4 post infection (p.i.). All the strains showed efficient colonization in the gut of various mice lines used in the study (Fig. 1A) and we observed no significant differences between the cecal colonization efficacies of subjected mutants among the various mice groups. The bacterial densities in mesenteric lymph node (mLN) of C57BL/6 mice were comparable within the group; however, the bacterial load of MT12 (ΔssaV; SPI2-deficient strain) and MT15 (hha and ΔssaV; SPI2-deficient hha mutant strain) were significantly different when compared with SB300 (wild-type) load in mLN of all immune deprived mice groups (Fig. 1B). It was observed that the strain devoid of SPI2 (MT12) failed to colonize the systemic sites (liver and spleen) of the C57BL/6 mice group; however showed significant colonization to such systemic organs when tested in immunocompromised mice (Fig. 1C and D). In comparison to this, the MT15 (SPI2-deficient hha mutant) was stringently attenuated in parental C57BL/6 mice and other immune-deprived mice tested in the study (Fig. 1C and 1D). Collectively, data suggested that additional inactivation of hha in SPI2-deficient S. Typhimurium would render the strain incapable of colonizing systemic sites in wild-type mice as well as various immune-deprived host mice tested.
Figure 1. Comparison of M15 fitness in immunocompromised and wild-type C57BL/6 mice. Groups of various immunocompromised mice (iNos−/−, IL10−/−, and CD40L−/− in C57BL/6 background along with wild-type mice as control group) were infected with 5 × 107 cfu of Salmonella Typhimurium (wild-type, SB300; ssaV mutant, MT12; ssaV and hha double mutant, MT15) by oral gavage. Mice were sacrificed at 4 d p.i. and bacterial colonization was assessed. Graph represents colonization of S. Typhimurium strains at different host sites: (A) cecum, (B) mesenteric lymph nodes, (C) spleen, and (D) liver. Broken lines in the graphs shows minimum detection limit. n.s., not significant; *statistically significant (P < 0.05, t test).
S. Typhimurium MT15 mutant colonizes efficiently to cecal tissues
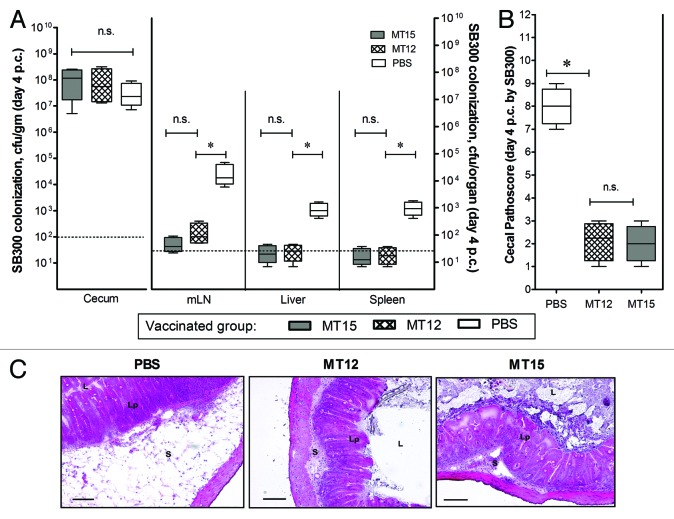
Intestinal colonization and elicitation of mucosal immune response is an important feature of potent Salmonella mucosal vaccine claimed elsewhere. Since the TTSS2-deficient strain was also claimed to be capacitive of efficient cecal colonization and effective luminal immune response elicitation,24 it was important to check the effect of hha mutation on these attributes, especially the intestinal colonization and conferring immune response. Keeping in consideration, wild-type C57BL/6 mice groups (n = 10 mice in each group) were infected/vaccinated with S. Typhimurium MT12 (ΔssaV; TTSS2-deficient strain) and MT15 (hha and TTSS2-deficient strain). The PBS-treated mice served as control group. We assessed the CFU counts of administered Salmonella strains in fecal shedding of infected mice at different time points through the course of infection. Subjected strains reached the cecal densities of 108 to 109 cfu/g within 24 h of infection. These strains maintained their cecal densities through the course of 30 d infection with a slight decrease during the last 10 d of infection period. However, the fecal shedding of strain MT12 and MT15 was comparable until day 21 p.v. which was suggestive of their efficient gut-colonization efficiency. The bacterial densities of MT15 strain in the feces were slightly reduced at day 30 p.v. (P < 0.05) when compared with SPI2-deficient MT12 strain (Fig. 2A). To substantiate the observations, the bacterial densities in cecal tissues were also assessed from infected mice groups by euthanizing some of the mice (n = 5) from each group. Equivalent cecal load of MT15 and MT12 strains were reported in cecum at day 30 p.v. These strains disseminated at comparable rate to mLN of infected wild-type C57BL/6 hosts (Fig. 2B); however, they failed to infiltrate the systemic organs like spleen and liver. During the infection, the PBS-treated control mice group had no signs of Salmonella infiltrations. To facilitate appropriate statistical analysis, the bacterial counts for PBS-treated control group were kept at minimum detection limit. Collectively, competent colonization in the draining lymph node and gut associated mucosa by TTSS2-deficient hha mutant (MT15) was suggestive of efficient cecal colonization and possibility of exerting effective immunogenic response in C57BL/6 hosts by the developed MT15 strain.
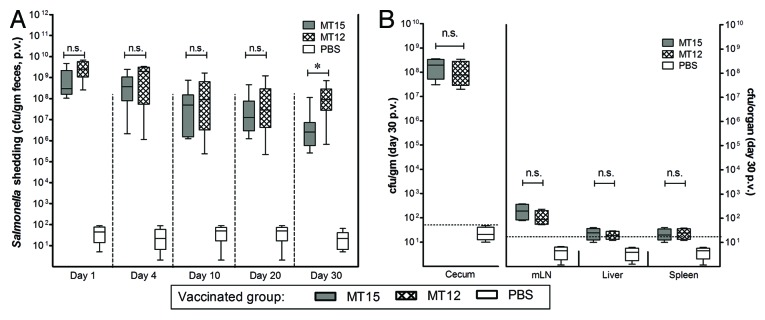
Figure 2. Deletion of hha does not impair long-term cecal colonization of MT15. Groups of C57BL/6 mice were vaccinated with 5 × 107 cfu of Salmonella Typhimurium MT12 (ssaV mutant; n = 10) and MT15 (ssaV and hha double mutant; n = 10) by oral gavage. Control mice group was treated with PBS (n = 10). (A) Fecal shedding of different Salmonella strains as analyzed by plating at various time-points. (B) Mice from each vaccinated group were sacrificed (n = 5) at day 30 post-vaccination (p.v.) to assess the bacterial densities at different host sites. Graph shows the enumeration of bacterial loads at various host sites. Broken lines in the graphs shows minimum detection limit. n.s., not significant; *statistically significant (P < 0.05, t test).
MT15 strain confers protective immune response
Since the parental TTSS2-deficient S. Typhimurium strain (MT12) was previously anticipated as an effective vaccine candidate,24 we were interested to study the vaccine prospective of developed MT15 strain and to compare the same with its parental strain MT12. To evaluate this, the vaccinated mice groups were administered orally with 20 mg of ampicillin in order to clear any colonized test bacterial strains and regrown gut flora from the gut of the remaining mice (n = 5) of each vaccinated group (discussed in previous section). After 24 h of ampicillin treatment, these mice groups were challenged with S. Typhimurium strain (wild-type; SB300) through oral gavage. These mice were kept under observation and euthanized at day 4 post-challenge (p.c.). Various organ sites were assessed for the bacterial densities and the cecal pathology was gauged. We observed an efficient colonization of the wild-type SB300 challenge bacterial strain to mice gut. These bacterial densities were equivalent with that of the bacterial densities from the control mice group (PBS; P > 0.05). The challenged wild-type Salmonella SB300 had better access to systemic sites of the non-vaccinated PBS control mice group in comparison to the mice groups those were previously vaccinated with MT15 and MT12 (Fig. 3A). Effective infiltration of wild-type Salmonella to systemic sites of the PBS-treated mice group was suggestive for absence of any Salmonella-specific immune response to counter lethal infections of Salmonella. On the other hand, the challenged wild-type SB300 strain failed to colonize to various systemic sites in the mice groups vaccinated with MT12 and MT15; the observation was suggestive of development of successful immunity in vaccinated mice groups (Fig. 3A). Assessed cecal pathoscores and representative HE-stained cecal sections of MT15- and MT12-vaccinated mice groups supported in favor of above observations (Fig. 3B and C). Collectively, presented observations were supportive of the fact that additional deletion of hha in TTSS2-deficient Salmonella Typhimurium does not impair its ability to confer protective immune responses in vaccinated C57BL/6 mice.
Figure 3. Analysis of vaccination efficacy of M15 S. Typhimurium strain. Vaccinated C57BL/6 mice groups (PBS, n = 5; MT12, n = 5; MT15, n = 5) were ampicillin-treated (25 mg by gavage), challenged with wild-type SB300 (ampr, smr) after 24 h of treatment, and euthanized at day 4 post-challenge (p.c.). (A) Colonization of SB300 at various host sites after challenge. (B) Enumeration of cecal pathology in terms numerical pathoscore was determined as described in material and methods section. Broken lines in the graphs shows minimum detection limit. n.s., not significant; *statistically significant (P < 0.05, t test). (C) Representative HE-stained cecal sections obtained from SB300-challenged mice groups pre-immunized with MT12, MT15, and PBS (control). Bar, 200 µm. S, submucosal edema; Lp, lamina propria; L, lumen.
Vaccination by MT15 enhances T-cell activation
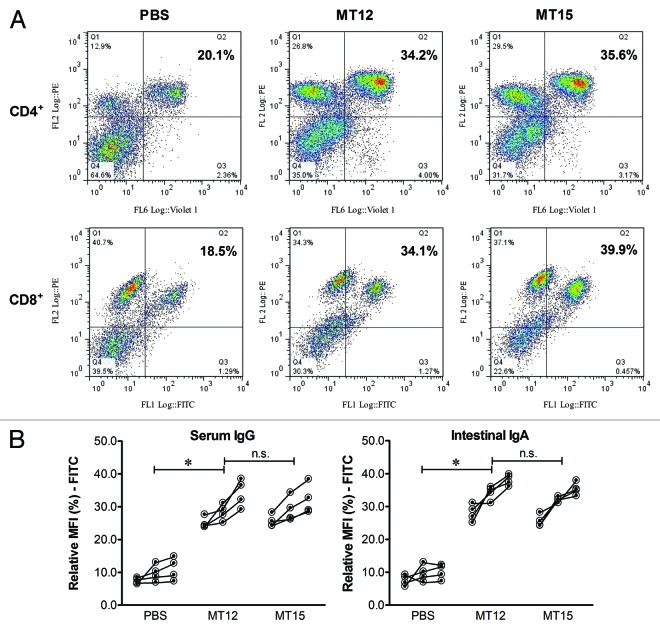
Induction of immune responses depends on the ability of mucosal immunogen to access the gut-associated lymphoid tissues (GALT) of the host to activate the CD4+ and CD8+ cell populations. To analyzing this feature, the impact of S. Typhimurium strain MT12 (SPI2-deficient) and MT15 (SPI2-deficient hha double mutant) on different cell populations was evaluated and the changes in the CD4+ and the CD8+ cells counts in the mLN was analyzed. The cell populations were analyzed by flowcytometry and were found to be almost equally populated in the MT12 and MT15 vaccinated mice groups but significantly enhanced in comparison to the PBS-treated mice cell counts (compare Q2 of FACS plots in Fig. 4A). This clearly indicates that MT15 strain has an ability to induce cell mediated immune response in the vaccinated mice.
Figure 4. Analysis of host immune response together with assessment of CD4/8+ cell population in immunized mice. The mesenteric lymph nodes (mLN) from remaining mice vaccinated with MT12, MT15, and PBS were collected in 500 µl of RPMI medium (with 10% FCS) at day 30 p.v. The homogenates were prepared and cellular fractions were collected. After processing, cells were reacted with CD4- and CD8-specific antibodies and FACS analyzed for cell densities. (A) Representative FACS graphs depicting CD4+ (pacific blue-labeled) and CD8+ (FITC-labeled) cell populations (Q2 in each graph) in various immunized mice groups. (B) Assessment of antibody response (serum IgG and intestinal IgA) by bacterial FACS. Different dilutions of serum and gut-wash from vaccinated mice were reacted with prewashed overnight statically-grown wild-type S. Typhimurium followed by incubation with FITC-conjugated respective antibodies to mouse IgG or IgA. Cellular fraction was collected, washed, and resuspended for FACS analysis. Figure shows antibody titer for serum IgG and luminal secretory-IgA (sIgA) from collected sample with all tested dilutions.
Immunization with MT15 shows raised serum IgG and luminal sIgA titers
Immunity toward Salmonella infection by Salmonella (SPI2-deficient) strain develops basically through luminal secretory IgA (sIgA). The enhanced immune response can also be reflected in titer of IgG in serum.24 The potential of SPI-2 deficient hha mutant (MT15) to mount immune response was validated by analyzing antibody response of subjected mice groups by evaluating serum-IgG and luminal-IgA titers. Thoroughly washed bacterial cells of S. Typhimurium (SB300) were treated with suitably diluted serum (1:20, 1:60, 1:120) and gut-wash (undiluted, 1:3, 1:9) samples. Treated cells were incubated with appropriately labeled secondary antibodies (see Materials and Methods section). Washed bacterial conjugates were FACS analyzed and the mean fluorescent intensities were derived with the use of Flow Jo software (v 10.0.5). The serum IgG and intestinal IgA titers observed from the diluted samples of MT12 and MT15 vaccinated mice groups were found comparable with no statistical significance (Fig. 4B). However, these antibody titers were found significantly different with observed antibody titers of PBS-treated mice groups. These observations were suggestive of presentation of unhampered immune response by the MT15 vaccinated mice groups in comparison to the host groups immunized with TTSS2-deficient pre-established MT12 S. Typhimurium strain.
Discussion
Non-typhoidal Salmonella (NTS) infections are emerging as serious problem for vital appeal worldwide. To date, no permanent solutions are advised for NTS infections. However, the development of live-attenuated vaccines (LAV) targeting the leading causative agents of NTS is of high importance. Young children (<3 y) and HIV-infected individuals were reported prone to develop fatal outcomes upon NTS infection.25 The Ty21a and the Typhim Vi are the two possible vaccines currently available for the typhoid prevention.26 Experiments on numerous attenuated Salmonella vaccine strains or LAV with protective potential against Salmonella infection have employed S. Typhimurium murine infection models.7 Such LAV strains induce relatively efficient immunogenicity than the killed organisms.27,28 Unfortunately, most of the promising vaccine candidates provide only short-term and incomplete protection and show often an insufficient efficiency in young children.29-31 The mutant of structural protein of TTSS2 (ssaV mutant) has been reported to confer protection against S. Typhimurium infection.24 However, in a recent study, the TTSS2-deficient Typhimurium strain was reported with increased replication within the infected phagocytes and macrophages.32 The biggest challenge in developing live vaccine candidates is the generation of a Salmonella strain that provides both immunogenicity and safety (virulence attenuated) even when used in immune deprived hosts.
In our study, we focused on the bacterial attenuation by inactivating hha in SPI2-deficient background. Though it has been discussed and proved that Salmonella disseminate to the systemic sites even after TTSS2 inactivation, we showed that complete attenuation can be achieved upon additional inactivation of hha that has been reported earlier to be involved in environmental regulation of virulence factors of the bacterium. This can be explained by considering the interaction of hha with other genes as seen (Fig. S1) from STRING, a web-based protein interaction prediction tool.33 Hha is a hemolysin expression modulating protein of E. coli19 and its homolog is present in the genome of S. Typhimurium (STM0473). The direct interaction of Hha and H-NS has been reported to alter the expression if invasion associated proteins of Salmonella.34 Further, the STRING tool shows Hha interaction with Rck, the outer membrane protein of Salmonella that helps in bacterial invasion through PI 3-kinase-Akt signaling-dependent Rac1 activation.35,36 Such proven and other predicted interactions indicate for the possible involvement of Hha in bacterial fitness during the course of infection.
While considering immunity for bacterial infections, the fine-tuning of activation and restraining of host immune system becomes crucial to minimize host injury. The host T cells plays an important role in establishing equilibrium between bacterial clearance and propagation37 and many of the reports advocate for the enhanced T-cell response in infections with Salmonella strains.38,39 The TH cells (CD4+) governs the bacterial clearance from the host tissues possibly by activation of the Salmonella-containing mononuclear cells. However, the TC cells (CD8+) support protection against bacterial infection by clearing intracellular S. Typhimurium from infected macrophages.40 In this study, we observed the induction of CD4/8+ cells in host mice vaccinated with S. Typhimurium for 30 d. Such increase in T-cell counts in suitably immunized mice hosts might be considered to grant efficient defense against subsequent infection with Salmonella.
As predominant antibody class, sIgA in the external secretions plays an important role in immune protection. The sIgA has been reported in impairing pathogens attachments to epithelial cell receptors. The cholera toxin,41 reovirus type 1 Lang,42 and few more exhibit same mechanism. Furthermore, immune exclusion is a suggested mechanism by which sIgA prevents pathogens from attaching, colonizing, and invading mucosal epithelial cells.43 As per our findings, in Salmonella-immunized mice, invasion of S. Typhimurium into the epithelium was significantly impaired. We observed an excellent sIgA response from the immunized mice groups which resulted in protection against subsequent Salmonella infection. This cannot be explained by receptor blocking or immune exclusion, rather the sIgA directly affect bacterial virulence factors to impair epithelial invasion by S. Typhimurium.44 A thorough considerate of the sIgA functions in intestinal immunity would be appreciable with respect to designing of competent mucosal vaccines.
As conclusion, the developed double mutant of Salmonella Typhimurium strain MT15 confers protection by inducing proficient mucosal and serum immune responses against Salmonella Typhimurium infections. This strain (MT15) was found attenuated in various immunocompromised host mice tested in this study. Based on the available reports, it can be speculated that deletion of hha in strain MT15 possibly alters the Salmonella proteins responsible for its entry to the host cells and thus suppresses the MT15 infection profile. Overall, the study addresses involvement of hha in bacterial infection and dissemination and it could be a potential target in developing live-attenuated vaccine particularly benefiting immune-deprived hosts. Further, to have a relative investigation of the niche supporting the attenuation properties of MT12 and MT15 in experimental hosts with respect to their genetic background would be interesting. Additionally, detailed study on involvement of secretory IgA toward the protection against Salmonella challenges through MT15 vaccination could possibly reveal unexplored immunological links in conferring protective immunity by such genetically modified strain.
Material and Methods
Bacterial strains used in the study
Bacterial strains (Table 1) were cultured in Luria–Bertani medium (LB) supplemented with 0.3 M NaCl for 12 h at 37 °C. For infection experiments, all the bacterial strains were diluted 1:20 in fresh LB medium and sub-cultured for another 4 h under mild aeration until an optical density of 0.6 was achieved. Harvested bacterial cells were washed in ice-cold phosphate buffered saline (PBS) and about 5 × 107 CFU were suspended in 50 μl cold PBS for in vivo experiments. All strains were tested for growth attenuation.
Table 1. Bacterial strains, plasmids, and primers used in study.
| Strains | Genetic information | Background | References |
|---|---|---|---|
| SB300 | Salmonella Typhimurium; Smr | Wild type | 24 |
| M1525 | Salmonella Enteritidis P125109 wild type; Smr | Wild type | 48 and 49 |
| MT12 | S. Typhimurium ΔssaV; Smr | SB300 | This study |
| MT14 | S. Typhimurium hha::aphT; Smr, Kmr | SB300 | This study |
| MT15 | S. Typhimurium hha::aphT, ΔssaV; Smr, Kmr | MT12 | This study |
| Plasmids | Relevant genotype (S) and/or phenotype (S) | Resistance | References |
| pKD4 | Template plasmid; FRT-aphT-FRT | Kmr | 45 |
| pKD46 | Red recombinase expression plasmid; ParaB; oriR101 | Ampr | 45 |
| pCP20 | FLP recombinase expression plasmid | Cmr, Ampr | 45 |
| pM973 | bla PssaH gfpmut2 plasmid with oripMB1 | Ampr | 22 |
| Primers | Sequence (5′ to 3′) | ||
| Fw-hha | AGGCAGATAA CACCTGCGTG TTCTCTAAAA AGTAATGTAG CGTGATGTGT AGGCTGGAGC TGCTT | ||
| Rw-hha | GTTAGTTTGT CTTGTTAAAA ATTATTACAA TCATAGGTAG AATTTATATG AATATCCTCC TTAGT | ||
| Conf-hha | GTGATCTATA TCACTGTTCT ATAATAGCC | ||
| Fw-ssaV | TCATCGACAA ATAAAATTTC TGGAGTCGCA ATGCGTTCAT GGTTATGTGT AGGCTGGAGC TGCTT | ||
| Rw-ssaV | ATTTCAGCCT CAGACGTTGC ATCAATTCAT TCTTCATTGT CCGCCATATG AATATCCTCC TTAGT | ||
| Conf-ssaV | GCAATGAGTT GTTCTCCACC | ||
Development of genetic mutant strains
Deletion mutants of Salmonella Typhimurium genes hha (MT15; hha::aphT) and ssaV (MT12; ΔssaV) were developed using red-recombinase plasmid pKD46, template plasmid pKD4, and pCP20 flip-recombinase plasmid as per the standard lambda-red recombinase system protocol45,46 using the primers listed in Table 1. The stable double mutant MT15 (hha::aphT, ΔssaV) was generated by transducing the hha::aphT mutation into the recipient MT12 (ΔssaV) strain. All the mutations were confirmed by PCR using the hha- and ssaV-specific confirmatory primers listed in Table 1.
Ethical statement
All the animal experiments were performed in strict accordance with guidelines laid by the Institutional Animal Ethics Committee (IAEC) of National Center for Cell Sciences (NCCS) Pune, India. This study was approved by the IAEC of NCCS; Permit Number: 7/1999/CPCSEA-09/03/1999. All efforts were made to minimize suffering of animals during experimentation.
In vivo animal experimentation for evaluation of bacterial dissemination
For in vivo animal experimentations, individually ventilated cages were used to house the experimental animals as described previously.9 Specific pathogen-free (SPF) mice were used for all in vivo experimentations. C57BL/6, iNos−/− (B6.129P2-Nos2tm1Lau/J), CD40L−/− (B6.129S2-Cd40lgtm1Imx/J), and IL10−/− (B6.129P2-IL10tm1cgn/J) mice from Jackson Laboratories were bred in the C57BL/6 background at the animal facility of NCCS, Pune. Mice (n = 6 each group) were pretreated with 50 mg of streptomycin, intragastrically, before infecting with any of the mentioned Salmonella strain. After 24 h of antibiotic treatment, mice were infected with 5 × 107 CFU of the corresponding bacterial strain (i.e., MT12, MT15, and SB300) with the help of oral gavage. During the course of infection the bacterial loads in the cecum, mesenteric lymph nodes (MLNs), and other systemic sites were enumerated by plating the tissue homogenates on MacConkey agar plates supplemented with appropriate antibiotics (streptomycin, 50 µg/ml; kanamycin, 50 µg/ml; ampicillin, 20 µg/ml; chloramphenicol, 20 µg/ml).
Evaluation of protective efficacy of mutant strains
C57BL/6 mice pretreated with streptomycin were used for vaccination by different Salmonella strains as established previously.9 Concisely, three mice groups (n = 10) were vaccinated (immunized) with mutant MT12 and MT15 S. Typhimurium strains; the PBS-treated mice group served as the control. For accounting fecal shedding of bacterial strains, the fecal sample from all the mice of each group was collected at various time points and its suitably diluted homogenates were grown on MacConkey agar supplemented with antibiotics. At day 30 post-vaccination (p.v.), the bacterial load in various host sites along with cecum contents were determined by sacrificing some of the mice (n = 5) from each group. For statistical analysis, samples with no bacterial count were adjusted to the minimal detectable levels. Remaining mice (n = 5) from each vaccinated group were treated orally with ampicillin (25 mg) to clear the residual Salmonella or other microbial flora from mice gut. Mice groups were further challenged (infected to see the efficacy of vaccination) after 24 h of ampicillin treatment by introducing 200 cfu of wild-type S. Typhimurium SB300 strain harboring pM973 (conferring ampicillin resistance), through oral gavage.47 The colonization efficiency of challenge strain SB300 at various host sites was assessed at day 4 post-challenge (p.c.). For cryosectioning, mice tissues were collected in an Optimum Cutting Temperature compound (OCT, Sakura Finetek Inc.), snap frozen in liquid nitrogen and stored at −80 °C. Cecal inflammation of the infected tissue was scored as described in the next section.
Evaluation of cecal inflammation
Cryopreserved O.C.T. (Sakura Finetek Inc.) embedded segments of the ileum, cecum, and colon were sectioned at −30 °C to the thickness of 4 µm and collected on glass slides and air-dried. The tissue sections were stained with hematoxylin and eosin (H&E) stains and the pathology was evaluated on the basis of previously described scoring system for the quantitative analysis of cecal inflammation.9,47,48 All the cecal sections were scored independently on the basis of pathological changes that include epithelial ulceration (0–3), sub-mucosal edema (0–3), loss of goblet cells (0–3), and polymorphonuclear leukocyte infiltration (0–4) with a summation score ranging between 0–13. The individual total score of any cecal tissue reflected the degree of inflammation. The independent pathoscores of each tissue sample were averaged. The combined pathological scores represents the inflammation levels that included intact intestine without any sign of inflammation (pathoscore 0); minimal sign of inflammation (pathoscore 1–2), which is commonly found in the ceca of specific pathogen-free mice and generally not considered as a pathological feature; slight inflammation as a minimal sign of tissue pathology (pathoscore 3–4); moderate inflammation (pathoscore 5–8); and significant inflammation (pathoscore 9–13).49
Assessment of CD4/8+ cellular counts by flow cytometry
For evaluation of cellular response the mesenteric lymph node (mLN) from the vaccinated mice groups was collected at day 30 post-vaccination. The mLN were collected in 500 µl of RPMI medium (Lonza) supplemented with 10% fetal calf serum (FCS; Lonza). Homogenates of mLNs were centrifuged at 140× g for 8 min at 4 °C. The cellular fractions were resuspended in 1 ml RPMI-FCS medium. Around 106 cells were resuspended in 50 µl of FACS buffer and incubated with different CD4/8+ cell specific antibodies (PacificBlue anti-mouse αCD4, BD PharMingen; FITC anti-mouse αCD8, BD PharMingen) for 90 min at room temperature. Finally, the cells were washed and assessed by FACS Canto (BD Biosciences) and analyzed using FlowJo software (v10.0.5).
Evaluation of serum and gut antibody response
The serum IgG and luminal secretory IgA levels were measured by FACS for assessment of the mucosal immune responses as described previously.23 Serum and gut washes from vaccinated mice groups were collected at the termination of the vaccination period, i.e., day 30 p.v. For FACS analysis of Salmonella-specific IgG and IgA response, the wild-type S. Typhimurium (SB300) was grown overnight in static LB broth at 37 °C. The bacterial cells were harvested and washed twice with 1% BSA and 0.05% sodium azide prepared in sterile PBS. Finally, the cells were suspended in PBS to have bacterial density of 107 cfu/ml. For assessing serum IgG response, the inactivated mice serum was diluted to 1:20, 1:60, and 1:120. Similarly, for evaluation of Salmonella-specific secreted luminal IgA, the clear supernatants of luminal contents from vaccinated mice groups were treated as undiluted, 1:3, and 1:9 dilutions in PBS. Further, a 25 μl of diluted serum samples and the gut washes were mixed separately with 25 μl bacterial suspension of Salmonella Typhimurium (SB300) in PBS and incubated for 1 h at 4 °C followed by washing with PBS. The washed bacterial cells were reacted with FITC-conjugated monoclonal anti-mouse IgG and IgA antibodies (Abcam) for serum and gut-wash samples respectively, and incubated for 1 h at 4 °C. Finally, cells were washed twice (in PBS with 1% BSA, 0.05% sodium azide) and resuspended in PBS (with 2% PFA). Samples were processed in FACS Canto analyzer (BD Biosciences).
Statistical analysis
Statistical analyses were performed using t test and other suitable statistical analysis parameters (Prism 5; GraphPad Software) when needed. Probability with P < 0.05 was considered statistically significant.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We acknowledge the staff members of animal facility (NCCS) for their efficient support in taking care of all animals during the course of experimentation. We also concede efforts of Bhaskar Saha for his critical input in manuscript preparation. This work was supported by the grant from Department of Biotechnology, Government of India (BT/PR14489/Med/29/207/2010).
Glossary
Abbreviations:
- p.i.
post-infection
- p.c.
post-challenge
- p.v.
post-vaccination
- PBS
phosphate-buffered saline
- CFU
colony forming units
- TTSS
type-III secretion system
- SPI
Salmonella pathogenicity island
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/27605
References
- 1.DuPont HL. The growing threat of foodborne bacterial enteropathogens of animal origin. Clin Infect Dis. 2007;45:1353–61. doi: 10.1086/522662. [DOI] [PubMed] [Google Scholar]
- 2.Thorns CJ. Bacterial food-borne zoonoses. Rev Sci Tech. 2000;19:226–39. doi: 10.20506/rst.19.1.1219. [DOI] [PubMed] [Google Scholar]
- 3.Hedberg C. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:840–2. doi: 10.3201/eid0506.990624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol. 2008;6:53–66. doi: 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- 5.Altier C. Genetic and environmental control of salmonella invasion. J Microbiol. 2005;43:85–92. [PubMed] [Google Scholar]
- 6.Suar M, Jantsch J, Hapfelmeier S, Kremer M, Stallmach T, Barrow PA, Hardt WD. Virulence of broad- and narrow-host-range Salmonella enterica serovars in the streptomycin-pretreated mouse model. Infect Immun. 2006;74:632–44. doi: 10.1128/IAI.74.1.632-644.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coburn B, Grassl GA, Finlay BB. Salmonella, the host and disease: a brief review. Immunol Cell Biol. 2007;85:112–8. doi: 10.1038/sj.icb.7100007. [DOI] [PubMed] [Google Scholar]
- 8.Santos RL, Zhang S, Tsolis RM, Kingsley RA, Adams LG, Bäumler AJ. Animal models of Salmonella infections: enteritis versus typhoid fever. Microbes Infect. 2001;3:1335–44. doi: 10.1016/S1286-4579(01)01495-2. [DOI] [PubMed] [Google Scholar]
- 9.Barthel M, Hapfelmeier S, Quintanilla-Martínez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Rüssmann H, Hardt WD. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun. 2003;71:2839–58. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waterman SR, Holden DW. Functions and effectors of the Salmonella pathogenicity island 2 type III secretion system. Cell Microbiol. 2003;5:501–11. doi: 10.1046/j.1462-5822.2003.00294.x. [DOI] [PubMed] [Google Scholar]
- 11.Galán JE. Salmonella interactions with host cells: type III secretion at work. Annu Rev Cell Dev Biol. 2001;17:53–86. doi: 10.1146/annurev.cellbio.17.1.53. [DOI] [PubMed] [Google Scholar]
- 12.Clark MA, Jepson MA, Simmons NL, Hirst BH. Preferential interaction of Salmonella typhimurium with mouse Peyer’s patch M cells. Res Microbiol. 1994;145:543–52. doi: 10.1016/0923-2508(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 13.Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J Exp Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darwin KH, Miller VL. Molecular basis of the interaction of Salmonella with the intestinal mucosa. Clin Microbiol Rev. 1999;12:405–28. doi: 10.1128/cmr.12.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernandez LD, Hueffer K, Wenk MR, Galán JE. Salmonella modulates vesicular traffic by altering phosphoinositide metabolism. Science. 2004;304:1805–7. doi: 10.1126/science.1098188. [DOI] [PubMed] [Google Scholar]
- 16.Galán JE, Curtiss R., 3rd Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci U S A. 1989;86:6383–7. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lostroh CP, Lee CA. The Salmonella pathogenicity island-1 type III secretion system. Microbes Infect. 2001;3:1281–91. doi: 10.1016/S1286-4579(01)01488-5. [DOI] [PubMed] [Google Scholar]
- 18.Olekhnovich IN, Kadner RJ. DNA-binding activities of the HilC and HilD virulence regulatory proteins of Salmonella enterica serovar Typhimurium. J Bacteriol. 2002;184:4148–60. doi: 10.1128/JB.184.15.4148-4160.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nieto JM, Carmona M, Bolland S, Jubete Y, de la Cruz F, Juárez A. The hha gene modulates haemolysin expression in Escherichia coli. Mol Microbiol. 1991;5:1285–93. doi: 10.1111/j.1365-2958.1991.tb01902.x. [DOI] [PubMed] [Google Scholar]
- 20.Fahlen TF, Wilson RL, Boddicker JD, Jones BD. Hha is a negative modulator of transcription of hilA, the Salmonella enterica serovar Typhimurium invasion gene transcriptional activator. J Bacteriol. 2001;183:6620–9. doi: 10.1128/JB.183.22.6620-6629.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Periaswamy B, Maier L, Vishwakarma V, Slack E, Kremer M, Andrews-Polymenis HL, McClelland M, Grant AJ, Suar M, Hardt WD. Live attenuated S. Typhimurium vaccine with improved safety in immuno-compromised mice. PLoS One. 2012;7:e45433. doi: 10.1371/journal.pone.0045433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vishwakarma V, Pati NB, Chandel HS, Sahoo SS, Saha B, Suar M. Evaluation of Salmonella enterica serovar Typhimurium TTSS-2 deficient fur mutant as safe live-attenuated vaccine candidate for immunocompromised mice. PLoS One. 2012;7:e52043. doi: 10.1371/journal.pone.0052043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Endt K, Maier L, Käppeli R, Barthel M, Misselwitz B, Kremer M, Hardt WD. Peroral ciprofloxacin therapy impairs the generation of a protective immune response in a mouse model for Salmonella enterica serovar Typhimurium diarrhea, while parenteral ceftriaxone therapy does not. Antimicrob Agents Chemother. 2012;56:2295–304. doi: 10.1128/AAC.05819-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Endt K, Stecher B, Chaffron S, Slack E, Tchitchek N, Benecke A, Van Maele L, Sirard JC, Mueller AJ, Heikenwalder M, et al. The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal Salmonella diarrhea. PLoS Pathog. 2010;6:e1001097. doi: 10.1371/journal.ppat.1001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandomando I, Macete E, Sigaúque B, Morais L, Quintó L, Sacarlal J, Espasa M, Vallès X, Bassat Q, Aide P, et al. Invasive non-typhoidal Salmonella in Mozambican children. Trop Med Int Health. 2009;14:1467–74. doi: 10.1111/j.1365-3156.2009.02399.x. [DOI] [PubMed] [Google Scholar]
- 26.Pulickal AS, Pollard AJ. Vi polysaccharide-protein conjugate vaccine for the prevention of typhoid fever in children: hope or hype? Expert Rev Vaccines. 2007;6:293–5. doi: 10.1586/14760584.6.3.293. [DOI] [PubMed] [Google Scholar]
- 27.Chuttani CS, Prakash K, Vergese A, Gupta P, Chawla RK, Grover V, Agarwal DS. Ineffectiveness of an oral killed typhoid vaccine in a field trial. Bull World Health Organ. 1973;48:754–5. [PMC free article] [PubMed] [Google Scholar]
- 28.Levine MM, Herrington D, Murphy JR, Morris JG, Losonsky G, Tall B, Lindberg AA, Svenson S, Baqar S, Edwards MF, et al. Safety, infectivity, immunogenicity, and in vivo stability of two attenuated auxotrophic mutant strains of Salmonella typhi, 541Ty and 543Ty, as live oral vaccines in humans. J Clin Invest. 1987;79:888–902. doi: 10.1172/JCI112899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy JR, Grez L, Schlesinger L, Ferreccio C, Baqar S, Muñoz C, Wasserman SS, Losonsky G, Olson JG, Levine MM. Immunogenicity of Salmonella typhi Ty21a vaccine for young children. Infect Immun. 1991;59:4291–3. doi: 10.1128/iai.59.11.4291-4293.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Black RE, Levine MM, Ferreccio C, Clements ML, Lanata C, Rooney J, Germanier R, Chilean Typhoid Committee Efficacy of one or two doses of Ty21a Salmonella typhi vaccine in enteric-coated capsules in a controlled field trial. Vaccine. 1990;8:81–4. doi: 10.1016/0264-410X(90)90183-M. [DOI] [PubMed] [Google Scholar]
- 31.Lin FY, Ho VA, Khiem HB, Trach DD, Bay PV, Thanh TC, Kossaczka Z, Bryla DA, Shiloach J, Robbins JB, et al. The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N Engl J Med. 2001;344:1263–9. doi: 10.1056/NEJM200104263441701. [DOI] [PubMed] [Google Scholar]
- 32.Grant AJ, Morgan FJ, McKinley TJ, Foster GL, Maskell DJ, Mastroeni P. Attenuated Salmonella Typhimurium lacking the pathogenicity island-2 type 3 secretion system grow to high bacterial numbers inside phagocytes in mice. PLoS Pathog. 2012;8:e1003070. doi: 10.1371/journal.ppat.1003070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C, et al. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808–15. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olekhnovich IN, Kadner RJ. Role of nucleoid-associated proteins Hha and H-NS in expression of Salmonella enterica activators HilD, HilC, and RtsA required for cell invasion. J Bacteriol. 2007;189:6882–90. doi: 10.1128/JB.00905-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiedemann A, Rosselin M, Mijouin L, Bottreau E, Velge P. Involvement of c-Src tyrosine kinase upstream of class I phosphatidylinositol (PI) 3-kinases in Salmonella Enteritidis Rck protein-mediated invasion. J Biol Chem. 2012;287:31148–54. doi: 10.1074/jbc.M112.392134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mijouin L, Rosselin M, Bottreau E, Pizarro-Cerda J, Cossart P, Velge P, Wiedemann A. Salmonella enteritidis Rck-mediated invasion requires activation of Rac1, which is dependent on the class I PI 3-kinases-Akt signaling pathway. FASEB J. 2012;26:1569–81. doi: 10.1096/fj.11-189647. [DOI] [PubMed] [Google Scholar]
- 37.Johanns TM, Ertelt JM, Rowe JH, Way SS. Regulatory T cell suppressive potency dictates the balance between bacterial proliferation and clearance during persistent Salmonella infection. PLoS Pathog. 2010;6:e1001043. doi: 10.1371/journal.ppat.1001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yrlid U, Svensson M, Håkansson A, Chambers BJ, Ljunggren HG, Wick MJ. In vivo activation of dendritic cells and T cells during Salmonella enterica serovar Typhimurium infection. Infect Immun. 2001;69:5726–35. doi: 10.1128/IAI.69.9.5726-5735.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McSorley SJ, Asch S, Costalonga M, Reinhardt RL, Jenkins MK. Tracking salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity. 2002;16:365–77. doi: 10.1016/S1074-7613(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 40.Gautreaux MD, Deitch EA, Berg RD. T lymphocytes in host defense against bacterial translocation from the gastrointestinal tract. Infect Immun. 1994;62:2874–84. doi: 10.1128/iai.62.7.2874-2884.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Apter FM, Lencer WI, Finkelstein RA, Mekalanos JJ, Neutra MR. Monoclonal immunoglobulin A antibodies directed against cholera toxin prevent the toxin-induced chloride secretory response and block toxin binding to intestinal epithelial cells in vitro. Infect Immun. 1993;61:5271–8. doi: 10.1128/iai.61.12.5271-5278.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hutchings AB, Helander A, Silvey KJ, Chandran K, Lucas WT, Nibert ML, Neutra MR. Secretory immunoglobulin A antibodies against the sigma1 outer capsid protein of reovirus type 1 Lang prevent infection of mouse Peyer’s patches. J Virol. 2004;78:947–57. doi: 10.1128/JVI.78.2.947-957.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brandtzaeg P. Role of secretory antibodies in the defence against infections. Int J Med Microbiol. 2003;293:3–15. doi: 10.1078/1438-4221-00241. [DOI] [PubMed] [Google Scholar]
- 44.Mantis NJ, Forbes SJ. Secretory IgA: arresting microbial pathogens at epithelial borders. Immunol Invest. 2010;39:383–406. doi: 10.3109/08820131003622635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–5. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lesic B, Rahme LG. Use of the lambda Red recombinase system to rapidly generate mutants in Pseudomonas aeruginosa. BMC Mol Biol. 2008;9:20. doi: 10.1186/1471-2199-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hapfelmeier S, Stecher B, Barthel M, Kremer M, Müller AJ, Heikenwalder M, Stallmach T, Hensel M, Pfeffer K, Akira S, et al. The Salmonella pathogenicity island (SPI)-2 and SPI-1 type III secretion systems allow Salmonella serovar typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanisms. J Immunol. 2005;174:1675–85. doi: 10.4049/jimmunol.174.3.1675. [DOI] [PubMed] [Google Scholar]
- 48.Suar M, Periaswamy B, Songhet P, Misselwitz B, Müller A, Käppeli R, Kremer M, Heikenwalder M, Hardt WD. Accelerated type III secretion system 2-dependent enteropathogenesis by a Salmonella enterica serovar enteritidis PT4/6 strain. Infect Immun. 2009;77:3569–77. doi: 10.1128/IAI.00511-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vishwakarma V, Periaswamy B, Bhusan Pati N, Slack E, Hardt WD, Suar M. A novel phage element of Salmonella enterica serovar Enteritidis P125109 contributes to accelerated type III secretion system 2-dependent early inflammation kinetics in a mouse colitis model. Infect Immun. 2012;80:3236–46. doi: 10.1128/IAI.00180-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.