The blood system is continuously replenished from a rare population of hematopoietic stem cells (HSCs) that balance self-renewal and differentiation.1 It is believed that HSCs emerge during embryogenesis from a population of hemogenic endothelial cells at sites like the aorta-gonad-mesonephros (AGM) region and placenta.2 Transplantation of HSCs is a widely utilized cell therapy for a range of genetic and acquired disorders. Allogeneic transplantation depends on genetic matching to avoid graft vs. host disease as well as graft rejection, and even matched grafts are still associated with high risk. There are also limited quantities of available material especially in cord blood transplants and for various ethnic groups. Therefore, alternative sources of patient-specific transplantable HSCs are needed. Alternatives can potentially come from the in vitro expansion of existing blood stem cells or from de novo generation from other cell sources. Culture of HSCs in vitro results in substantial expansion of cell numbers, but the expanded cells lose their stem cell properties with time. To date, the developed culture methods using cellular and non-cellular substrates can only sustain transplantable HSCs in culture for a limited period of time and are not comparable to the robust protocols that can expand pluripotent stem cells indefinitely.3 In addition, over 2 decades of efforts to generate transplantable HSCs from pluripotent stem cells have met with very limited success. Recently, studies by Yamanaka and colleagues demonstrated that expression of 4 transcription factors (TFs) reprograms mouse and human fibroblasts into an induced pluripotent state.4,5 In addition, other studies have demonstrated inter-conversion of cell types mediated directly with TFs.6
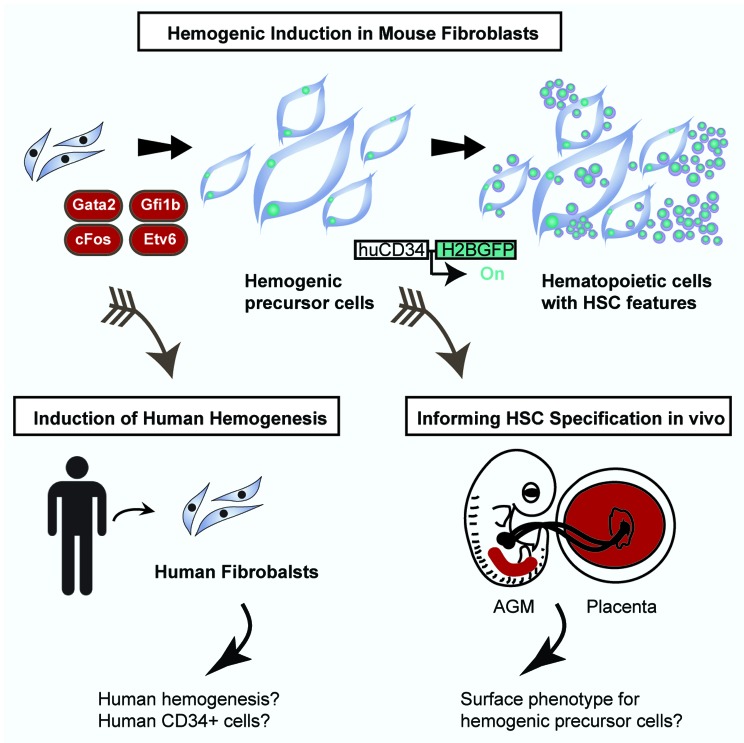
Our study, Pereira et al., reported that a specific combination of TFs can program hemogenesis in fibroblasts.7 The specific combination was identified by a combinatorial screen for HSC-inducing TFs using a human CD34 reporter as read-out. The TFs Gata2, cFos, Gfi1b were found to be the critical combination that sets in motion a hemogenic program, while Etv6 increased the efficiency of the process. This combination of TFs first induces endothelial-like cells with hemogenic potential characterized by activation of a human CD34 reporter, the expression of Sca1 and Prominin1, and a global endothelial gene expression program. Upon additional culture with the continuous expression of the 4 TFs, these cells generate hematopoietic cells with nascent HSC features. Among the induced hematopoietic cells that express the pan-hematopoietic marker CD45, a subpopulation was identified with a long-term repopulating phenotype, Sca1+cKit+CD150+CD48−. In addition, these cells have a gene expression profile very similar to HSCs and progenitors cells isolated from the AGM and placenta.8 The induced hemogenic cells generate hematopoietic colonies after transgene silencing and short-term aggregation culture with mouse placenta. Induction with 4 TFs mimics an endothelial-to-hematopoietic transition, a signature hallmark of HSC specification during development (Fig. 1), thereby, recapitulating developmental hematopoiesis in vitro. Interestingly, this is a unique feature of this system when compared with other cellular conversions, where the target cell type is directly induced and does not transit through an intermediate precursor or progenitor state.6 Hence, these results support the view that HSC specification is a multistep process and underscores the requirement of endothelial precursors and intermediates.
Figure 1. Induction of hemogenesis in mouse fibroblasts and perspectives. Gata2, cFos, Gfi1b, and Etv6 were identified as sufficient to induce a hemogenic program in mouse fibroblasts. First, endothelial precursor cells are induced that generate semi-adherent cells with hematopoietic stem cell features. Future perspectives are highlighted including translation to the human system and comparison with hematopoietic stem cell specification during embryogenesis.
It will be interesting to determine whether such a hemogenic precursor cell defined by the expression of CD34, Sca1, and Prominin1 is present in vivo at hemogenic sites such as the placenta and the AGM region during embryogenesis at the time of HSC specification (Fig. 1). If so, this population could be isolated and further undergo endothelial-to-hematopoietic transition in vitro. This would provide insights in to the process of HSC specification and perhaps reveal specific combination of markers to track the cellular origin of HSC; this goal has been a long-standing challenge in developmental hematopoiesis.2
Some of the direct conversion studies reported to date, such as the induction of cardiomyocytes, hepatocytes, and macrophages do not translate well to the human system.6 This is in contrast to the induction of pluripotency, where the combination of TFs is remarkably well conserved between mouse and human.4,5 It will be interesting to determine whether a similar combination of TFs also induces hemogenesis in human fibroblasts, isolated, for example, from the adult dermis or by using other somatic and embryonic cell sources. For example, would human pluripotent cells be “forced” to differentiate toward an HSC fate by exposing them to such a potent combination of hemogenic TFs? In summary, a combinatorial approach identified the minimal TF network for endothelial-to-hematopoietic transition in mouse fibroblasts. This study provides a platform for the future generation of patient-specific therapeutics and blood products. The sequential and dynamic induction, first generating endothelial-like precursors and then hematopoietic cells permits in vitro studies of the molecular mechanisms mediated by the key TFs Gata2, cFos, Gfi1b, and Etv6. This offers an unprecedented system to understand how key regulatory machinery is sequentially wired, and how the blood stem cell state is established. In the future, directly programmed HSCs from somatic cells could provide an unlimited patient-specific source for cell replacement and genetic correction therapies.
Pereira CF, et al. Cell Stem Cell. 2013;13:205–18. doi: 10.1016/j.stem.2013.05.024.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/27507
References
- 1.Moore KA, et al. Science. 2006;311:1880–5. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 2.Medvinsky A, et al. Development. 2011;138:1017–31. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- 3.Moore KA, et al. Blood. 1997;89:4337–47. [PubMed] [Google Scholar]
- 4.Takahashi K, et al. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K, et al. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Pereira CF, et al. Ann N Y Acad Sci. 2012;1266:7–17. doi: 10.1111/j.1749-6632.2012.06508.x. [DOI] [PubMed] [Google Scholar]
- 7.Pereira CF, et al. Cell Stem Cell. 2013 [Google Scholar]
- 8.McKinney-Freeman S, et al. Cell Stem Cell. 2012;11:701–14. doi: 10.1016/j.stem.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]