The airway epithelium, a pseudostratified layer of cells lining the tracheobronchial tree, is the primary barrier protecting the lungs from environmental pollutants, toxins, allergens, and microbes present in the inhaled air. This function is provided by the unique histologic architecture of the differentiated airway epithelium, composed of 4 principal cell types: ciliated, secretory, intermediate undifferentiated columnar, and basal cells (BC; Fig. 1). Ciliated cells comprise 60–80% of all airway epithelium throughout the airways, except for the terminal and respiratory bronchioles, where the predominant cell population is non-mucous secretory cells producing secretoglobins and other proteins with antimicrobial, anti-inflammatory, and antioxidant properties. In the larger airways, mucus-producing goblet cells are the major secretory cell population, contributing to 5–15% of epithelial cells and, together with ciliated cells, mediating mucociliary clearance of microbes and other foreign particles from the airway surface. The differentiated airway epithelium has tight junctions between adjacent cells that prevent the passage of microbes and xenobiotics across the epithelial layer. The BC population include the stem/progenitor cells capable of self-renewal and generating the differentiated cells of the airway epithlium.1 BC are located immediately above the basement membrane throughout the respiratory tract in humans. In the large airways, BC constitute 10– 20% of epithelial cells; the proportion of BC decreases to <10% in the small airways > 6th generation.
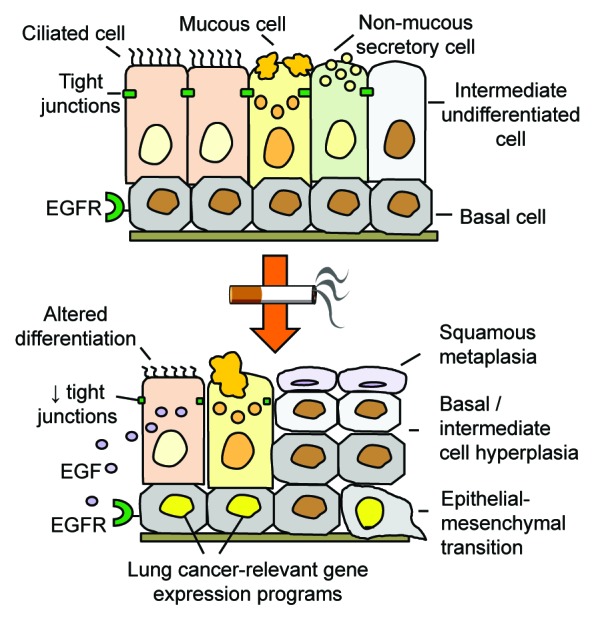
Figure 1. Basal cell (BC) origin of smoking-induced airway remodeling. Cigarette smoking induces expression of epidermal growth factor (EGF) in ciliated cells, which, via interaction with EGF receptor (EGFR) on BC stem/progenitor cells, alters the normal architecture of the airway epithelium and results in smoking-associated pathologic airway epithelial phenotypes relevant to COPD and lung cancer.
In cigarette smokers, the airway epithelial architecture changes dramatically (Fig. 1). The first lesions associated with smoking are characterized by hyperplasia of BC. This is often accompanied by squamous metaplasia, in which differentiated ciliated cells are replaced with keratinocyte-like squamous cells not present in the normal airways. There is also mucous cell hyperplasia, which leads to airway obstruction due to increased secretion of mucus into the airway lumen. In the small airways, increased number of mucus-producing cells is paralleled by the loss of protective non-mucous secretoglobin-secreting cells. Smoking also increases airway permeability, through the broad suppression of the components that maintain junctional barrier assembly and integrity, and induces some features of epithelial–mesenchymal transition (EMT). These phenotypes, all relevant to the development of chronic obstructive pulmonary disease (COPD) and lung cancer, the 2 major smoking-induced diseases, are preceded by dramatic gene expression changes in the airways of clinically “healthy” smokers.2
How does cigarette smoking cause such broad changes in the airway epithelial architecture? This question was central to our recent study.3 Based on the knowledge that BC are the stem/progenitor cells responsible for maintenance of the normally differentiated airway epithelium,1 and our recent advances in isolation and characterization of human airway BC,4 we identified the epidermal growth factor receptor (EGFR) pathway, previously implicated in smoking-induced airway disorders and regulation of stem cell functions in different organs, among those enriched in the human airway BC transciptome.4 We hypothesized that smoking-induced disorganization of the airway epithelium results from EGFR-mediated reprogramming of BC stem/progenitor cell function. In support of this hypothesis, we found that EGF, one of the EGFR ligands, is upregulated in ciliated cells of smokers, which, given decreased junctional barrier integrity caused by smoking, can potentially interact with EGFR-expressing BC. Strikingly, application of EGF to BC differentiating in vitro recapitulated the major pathologic phenotypes observed in the airway epithelium of smokers in vivo, including squamous metaplasia, decreased differentiation toward ciliated and non-mucous secretory lineages, suppression of the junctional barrier integrity accompanied by increased epithelial permeability, and acquisition of the EMT-like features.3 These data support the concept that human airway BC are likely the common cell-of-origin of smoking-induced airway epithelial remodeling (Fig. 1).
In agreement with this concept, in a separate study we found that airway BC of clinically healthy smokers acquire an embryonic stem cell (ESC)-specific gene expression signature.5 Surprisingly, this set of ESC genes is also upregulated in lung cancers in association with smoking status and TP53 mutations.5 Based on the high expression of the BC signature genes, we identified a novel, “BC-high” subtype of lung adenocarcinoma, which exhibited a much more aggressive phenotype and shorter survival.6 This lung adenocarcinoma subtype was more prevalent in smokers, supporting the concept that smoking-induced reprogramming of airway BC is relevant to the pathogenesis of lung cancer. Consistent with these findings, Gomperts and coworkers7 have observed that a subset of BC that express keratin 14, a gene which was induced by EGF in airway BC in our study,3 is enriched in the tumors of smokers with lung cancer.7 In another study, we identified chemokine CXCL14 as a gene induced by smoking in the airway epithelium via EGF-mediated activation of airway BC in association with broad genome-wide changes relevant to both COPD and lung cancer.8
Altogether, multiple lines of evidence support the concept that airway BC stem/progenitor cells are the origin of the earliest molecular and histologic changes in the airway epithelium relevant to the pathogenesis and phenotype of smoking-induced lung diseases. Further understanding of the specific molecular mechanisms underlying reprogramming of airway BC in human lung diseases, including those induced by cigarette smoking, will facilitate development of novel therapeutic approaches to target the development of common and currently incurable chronic lung diseases, such as COPD and lung cancer, at their very early stages.
Shaykhiev R, et al. Proc Natl Acad Sci U S A. 2013;110:12102–7. doi: 10.1073/pnas.1303058110.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/27510
References
- 1.Rock JR, et al. Dis Model Mech. 2010;3:545–56. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaykhiev R, et al. Cell Mol Life Sci. 2011;68:877–92. doi: 10.1007/s00018-010-0500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaykhiev R, et al. Proc Natl Acad Sci U S A. 2013;110:12102–7. doi: 10.1073/pnas.1303058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hackett NR, et al. PLoS One. 2011;6:e18378. doi: 10.1371/journal.pone.0018378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaykhiev R, et al. Stem Cells. 2013;31:1992–2002. doi: 10.1002/stem.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukui T, et al. Eur Respir J. 2013;42:1332–44. doi: 10.1183/09031936.00144012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ooi AT, et al. Cancer Res. 2010;70:6639–48. doi: 10.1158/0008-5472.CAN-10-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaykhiev R, et al. Am J Respir Cell Mol Biol. 2013;49:418–25. doi: 10.1165/rcmb.2012-0396OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


