Abstract
In mammalian cells, DNA double-strand breaks (DSB) can be repaired by 2 main pathways, homologous recombination (HR) and non-homologous end joining (NHEJ). To give access to DNA damage to the repair machinery the chromatin structure needs to be relaxed, and chromatin modifications play major roles in the control of these processes. Among the chromatin modifications, changes in nucleosome composition can influence DNA damage response as observed with the H2A.Z histone variant in yeast. In mammals, p400, an ATPase of the SWI/SNF family able to incorporate H2A.Z in chromatin, was found to be important for histone ubiquitination and BRCA1 recruitment around DSB or for HR in cooperation with Rad51. Recent data with 293T cells showed that mammalian H2A.Z is recruited to DSBs and is important to control DNA resection, therefore participating both in HR and NHEJ. Here we show that depletion of H2A.Z in the osteosarcoma U2OS cell line and in immortalized human fibroblasts does not change parameters of DNA DSB repair while affecting clonogenic ability and cell cycle distribution. In addition, no recruitment of H2A.Z around DSB can be detected in U2OS cells either after local laser irradiation or by chromatin immunoprecipitation. These data suggest that the role of H2A.Z in DSB repair is not ubiquitous in mammals. In addition, given that important cellular parameters, such as cell viability and cell cycle distribution, are more sensitive to H2A.Z depletion than DNA repair, our results underline the difficulty to investigate the role of versatile factors such as H2A.Z.
Keywords: chromatin, H2A.Z, p400, DNA repair, homologous recombination, NHEJ
Introduction
The genome is continuously the target of numerous internal and external agents that produce a large diversity of DNA lesions.1,2 In order to maintain cell viability and avoid the generation of mutations, cells have evolved specific DNA repair processes designed to take into account the different DNA damages.1,2 Among the most deleterious DNA damages, the presence of DNA double-strand breaks (DSB) is a challenge for the cells. To deal with the presence of DSB, mammalian cells use 2 well-characterized DNA repair pathways, homologous recombination (HR), which is dependent on the presence of the intact homologous copy and by the way of the cell cycle, and the non-homologous end joining (NHEJ) pathway, which performs the direct ligation of the 2 DNA ends.3,4
The repair of DNA damage takes place in a chromatin context.5 Chromatin is a complex structure that can undergo numerous modifications in order to give or to repress access to DNA regions and site. Chromatin structure can be modified by post-translational modifications of histones but also by changing the nucleosome positioning or composition.6,7 This last process involves ATP-dependent chromatin remodelers, which use the hydrolysis of ATP as energy source to incorporate histone variants in chromatin.8
Histone variants are critical for DSB management and repair is known for long through studies of the histone variant H2AX, which is quickly phosphorylated in response to DSB induction and gives rise to visible repair foci composed of signaling and repair proteins.9
The only histone H2A variant conserved from yeast to human is the H2A.Z variant. It is incorporated in yeast by the SWR1 enzyme and evicted from nucleosome by the INO80 enzyme.10 In humans H2A.Z variant is incorporated by the p400 or SRCAP enzymes, both related to SWR1. The role of H2A.Z in DSB repair has been studied in yeast,11 and whether it is recruited to DSB is still a matter of debate. Recent data show that it is recruited at a very short period of time during DSB repair, except if the breaks cannot be repaired properly.11 The disruption of the H2A.Z-encoding genes leads to genomic instability, defects in DSB repair, in particular in DNA resection,12 and sensitivity to DNA damaging agents,13 although it is not clear whether it is due to direct effects of H2A.Z around DSBs, or to the global deregulation of genes expression observed upon H2A.Z inactivation10 or to indirect effect resulting from the attempt to replace H2A with H2A.Z in the absence of the latter14
In higher eukaryotes, the role of H2A.Z has mainly been investigated by studying the enzymes able to incorporate H2A.Z in chromatin. In plants, SWR1 is important for the process of HR and for meiosis exit, although in this latter process, it seems to be important for later steps other than HR.15 In mammals, one study showed that p400 is recruited to DSBs and is important for histone ubiquitination around DSB, leading to BRCA1 and 53BP1 recruitment.16 The authors propose that the chromatin remodeling activity of p400 favors the action of ubiquitin ligases on nucleosomal histones. In contrast, we found that p400 depletion does not affect histone ubiquitination but is specifically important at later steps of the process of HR as a co-factor for Rad5117: p400 binds to Rad51, and both proteins are important for chromatin remodeling around DSBs. The reasons for the discrepancies between the 2 studies are not clear.
A recent study has directly investigated the role of H2A.Z around DSB in human 293T cells. The authors found that H2A.Z is recruited to DSBs using ChIP approach in a p400-dependent manner around a unique DSB induced by a Zn finger nuclease.18 Moreover, the depletion of H2A.Z by specific shRNAs led to genomic instability and increased sensitivity to ionizing radiations.18 In addition the authors used GFP-based reporter systems to measure the activity of various repair pathways, and found that H2A.Z depletion impaired both HR and NHEJ. They further demonstrate that H2A.Z depletion leads to an increased DNA resection, and propose that the role of H2A.Z is to block the CtIP-dependent resection.
Here, we investigate the role of H2A.Z in DSB repair in a human osteosarcoma cell line and in human fibroblasts immortalized by the large T antigen from SV40. We did not find any effect of partial H2A.Z depletion on various DSB repair parameters, although H2A.Z depletion led to changes in cell viability and cell cycle phase distribution. In addition, we did not find any evidence for H2A.Z recruitment at DSB. Our data thus suggest that the role of H2A.Z in DSB management in mammals is not ubiquitously observed.
Results
H2A.Z depletion does not impair repair of DSB by homology directed repair or NHEJ
We recently showed that the depletion of the p400 ATPase impairs homology-driven repair of DNA DSBs in RG37 fibroblasts. P400 mediating the incorporation of H2A.Z in chromatin, we examined the potential role of H2A.Z in DSB repair using a siRNA approach.
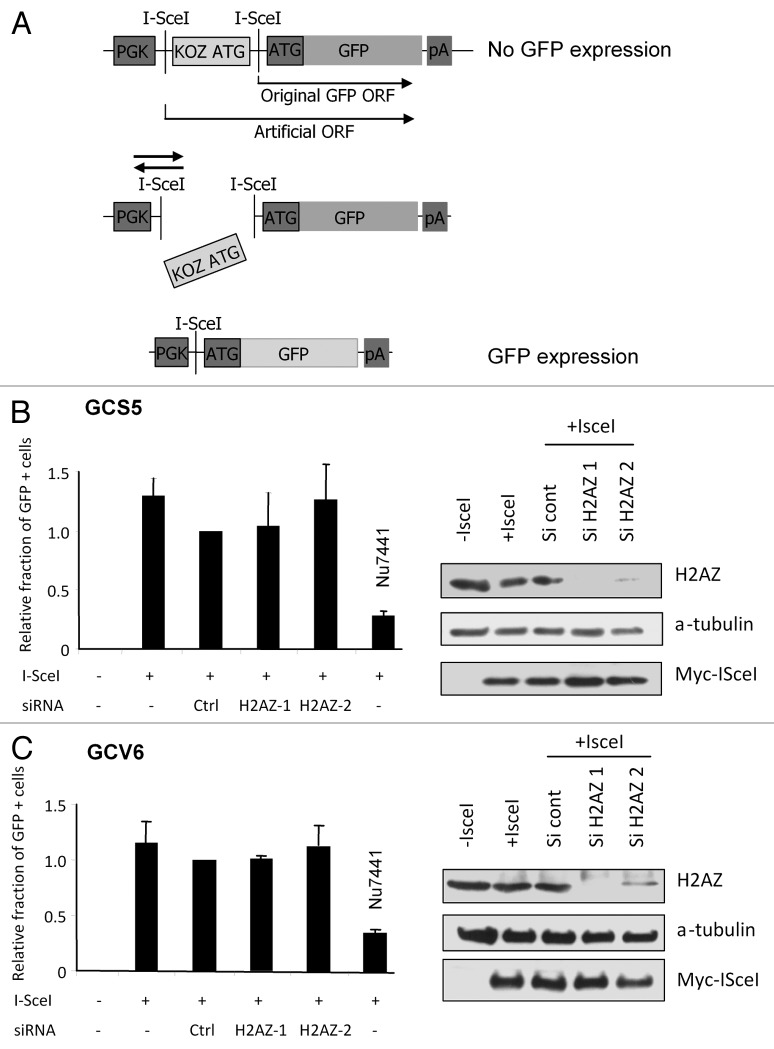
One of the major pathways for repairing DSBs is the non-homologous end joining (NHEJ) process, a DNA repair pathway acting in all cell cycle phases and not requiring sequence homology. To investigate the efficiency of NHEJ-mediated repair, we made use of 2 cell lines derived from normal fibroblasts immortalized by the T antigen from SV40. These cell lines harbor specific substrates allowing to measure NHEJ efficiency by assessing the number of GFP-positive cells following expression of the I-SceI endonuclease.19 I-SceI-mediated digestion leads either to fully cohesive ends (GCS5, upper panel) or partially cohesive ends requiring processing before ligation (GCV6, lower panel).19 Transfection with 2 different H2A.Z siRNAs leads to the decrease of H2A.Z expression, with H2A.Z-1 being more efficient than the H2A.Z-2 (Fig. 1A and B). Moreover, such a depletion of H2A.Z expression leads to a decrease in H2A.Z presence in chromatin (Fig. S2). We observed in both cell lines that depletion of H2A.Z does not influence the repair of DSB by NHEJ, assessed by the percentage of GFP-positive cells (Fig. 1A and B). As a positive control affecting NHEJ activity we treated cells with the DNA–PKcs inhibitor, Nu7441.20 Thus, the depletion of H2A.Z in these cell lines does not affect the repair of I-SceI-induced DNA breaks by NHEJ, irrespective of whether these breaks need to be processed before ligation or not.
Figure 1. Effect of H2A.Z depletion on DSB repair by NHEJ (A) Schematic representation of the NHEJ substrate present in the cell lines. The translation of the GFP gene is suppressed by an upstream, out of frame translation start site (KOZ ATG), the cassette is flanked with 2 I-SceI sites. I-SceI expression induces the release of the Koz ATG and religation of DNA ends allows translation and expression of GFP (B) GCS5 and (C) GCV6 cells were transfected with siRNA (10 nM) then, 24 h later, transfected with I-SceI coding plasmid to induce DSB. Treatment with Nu7441 was performed at 5 µM during 48 h at the time of I-SceI transfection. NHEJ events were counted 48 h later by FACS analysis of GFP-positive cells. Results are the mean +/− sd from 3–5 independent experiments. Western blots monitoring the indicated proteins expression are also shown.
In addition to NHEJ, DSB can also be repaired by homologous recombination (HR), a pathway active in S and G2 in which the genetic information of the sister chromatid serve as a template to repair the DSB. We used a cell line (RG37) derived from the same original immortalized cell line than the GCS5 and GCV6 but harboring a GFP-based substrate, allowing the measurement of HDR (homology directed repair, a process highly related to HR). We transfected RG37 cells with the 2 different siRNA directed against H2A.Z. The H2A.Z-1 siRNA shows again higher efficiency than the H2A.Z-2, both at the protein and mRNA levels (Fig. 2A). As a positive control, we used an siRNA directed against Rad51, a key component of the HR process. Depletion of H2A.Z does not affect the frequency of the homology-directed repair of DSB (assessed by counting the percentage of GFP-positive cells), whereas depletion of Rad51 leads to an approximately 70% decrease and p400 depletion to around 50% as previously observed in the same cellular system17 (Fig. 2B). These results indicate that H2A.Z depletion by siRNA does not influence DSB repair by HR.
Figure 2. Effect of H2A.Z depletion on homology-directed repair of DSB. (A) Schematic representation of the subtrate present in the cells to measure DSB repair mediated by homologous recombination. Two inactive copies of GFP gene are present: one contains the cleavage site for I-SceI; the other one is truncated at the 5′ and 3′ ends. After cleavage intra- or interchromatid use of the truncated form of GFP to repair DSB generates functional GFP (B) RG37 cells were transfected with the different siRNA (10 nM), then, 24 h later, transfected with I-SceI plasmid to induce DSB. HDR efficiency was evaluated 48 h later by FACS analysis. Results are the mean +/− SD from 4 independent experiments. Western blots monitoring the indicated proteins expression are also shown (western blot monitoring the effects of p400 depletion are shown in ref. 17).
Since using siRNA we only achieve a partial H2A.Z depletion, we cannot rule out the possibility that such depletion is not sufficient to observe any effect. However, trying to increase the efficiency of H2A.Z depletion by a second round of siRNA transfection leads to an important cellular toxicity (data not shown), and results could not be interpreted.
H2A.Z depletion influence on cell survival to DNA damage
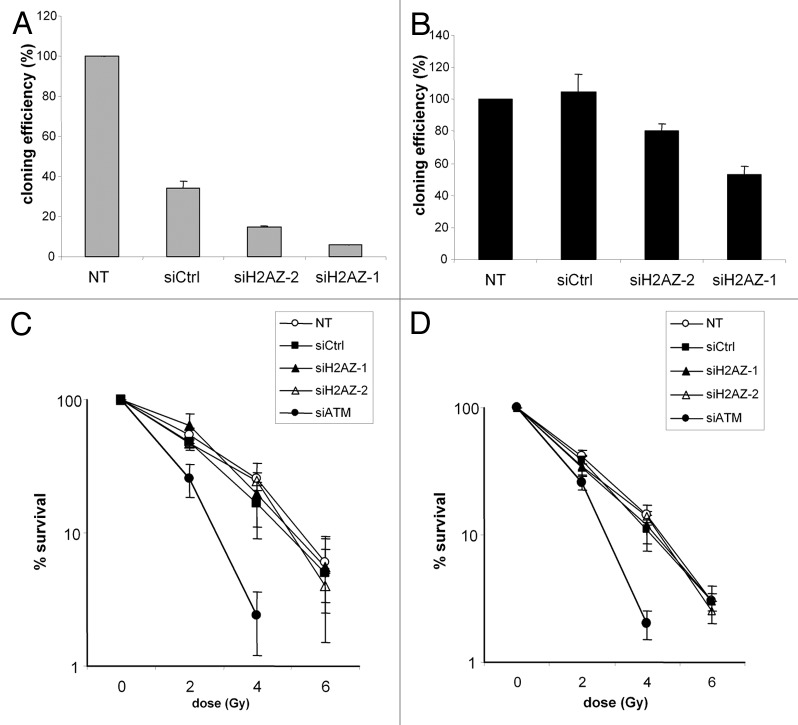
We next analyzed the role of H2A.Z in cell survival following DNA damage by performing clonogenic assay in p53-positive U2OS cells in response to ionizing radiations. We observed that without irradiation, in siCrtl treated-cells the cloning efficiency is 10% (whereas in untreated cells, the cloning efficiency is 30%) and decreases under 5% in H2A.Z-depleted cells (4% in siH2A.Z-2 treated cells and 2% in siH2A.Z-1 treated cells) (Fig. 3A; Fig. S1). Given that these effects are observed with 2 independent siRNAs and are higher with the siRNA leading to the stronger depletion, we conclude from these observations that depletion of H2A.Z per se is detrimental to cell viability and proliferation. The strong effect observed in siCtrl-treated cells is certainly due to the high sensitivity of this cell line to the transfecting reagent, because it is also observed using another control siRNA (data not shown). Moreover, such an effect is not observed in the other cell line (RG37) with the same siRNA and transfecting reagent. These results are in accordance with our previous observation that 2 rounds of transfection with H2A.Z siRNA are very detrimental for cell life. Importantly, they indicate that the depletion of H2A.Z we achieved using both siRNA is sufficient to observe a phenotype (cell clonogenic ability).
Figure 3. Clonogenic survival of IR exposed cells after H2A.Z depletion. U2OS cells transfected with the different siRNA were (A) plated to evaluate clonogenic efficiency or (C) exposed to ionizing radiations 24 h later and clonogenic assay performed. The same protocol was applied with the RG37 cells (B) to evaluate clonogenic efficiency and (D) to examine cell survival after ionizing radiations exposure. Colonies were revealed 10 d later by staining the plates with crystal violet. Results are the mean+/− sd of triplicate.
Strikingly, examination of normalized cell survival showed that H2A.Z depletion did not sensitize cells to ionizing radiations exposure (Fig. 3C), in agreement with the fact that we do not observe any effect of such depletion on the 2 main DSB repair pathways. Similar results were obtained in RG37 cells, the cell line we used for measuring HDR repair in Figure 2 (Fig. 3B and D). However, the effect of H2A.Z depletion on clonogenic efficiency was less pronounced in the RG37 cells than in U2OS cells (Fig. 3B; Fig. S1). The differences of clonogenic efficiency after H2A.Z depletion between the 2 cell lines could result from the different p53 status of each cell line.
We conclude from these experiments that in U2OS osteosarcoma cells and in immortalized fibroblasts, we do not observe any effect of H2A.Z depletion on DSB repair properties in conditions in which cell clonogenicity is affected.
H2A.Z depletion influence on cell cycle progression
The lack of effect of H2A.Z depletion on DSB repair conducted us to evaluate the efficiency of our siRNA on different cellular functions potentially affected by H2A.Z. To this aim, we next investigated the mechanism leading to the decreased clonogenic potential upon H2A.Z depletion. A defect in H2A.Z incorporation at the cell cycle control p21 gene promoter induces the expression of p21 and cell cycle arrest.21 Thus, H2A.Z depletion could have a significant effect on cell cycle progression. We thus analyzed cell cycle distribution of U2OS and RG37 cells following transfection of the H2A.Z siRNAs by flow cytometry. We found that, consistent with the induction of p21, H2A.Z depletion with the 2 H2A.Z siRNA induces an accumulation of U2OS cells in G1 and a decrease in the number of cells in S phase (Fig. 4A). In RG37 cells, we observed an increase number of cells in G2 phase and a decrease in the number of G1 and S phase cells, at least using the H2A.Z-1 siRNA (such an increase was sometimes but not consistently observed for the H2A.Z-2 siRNA, probably because its depleting effect was too low [Fig. 4B]).
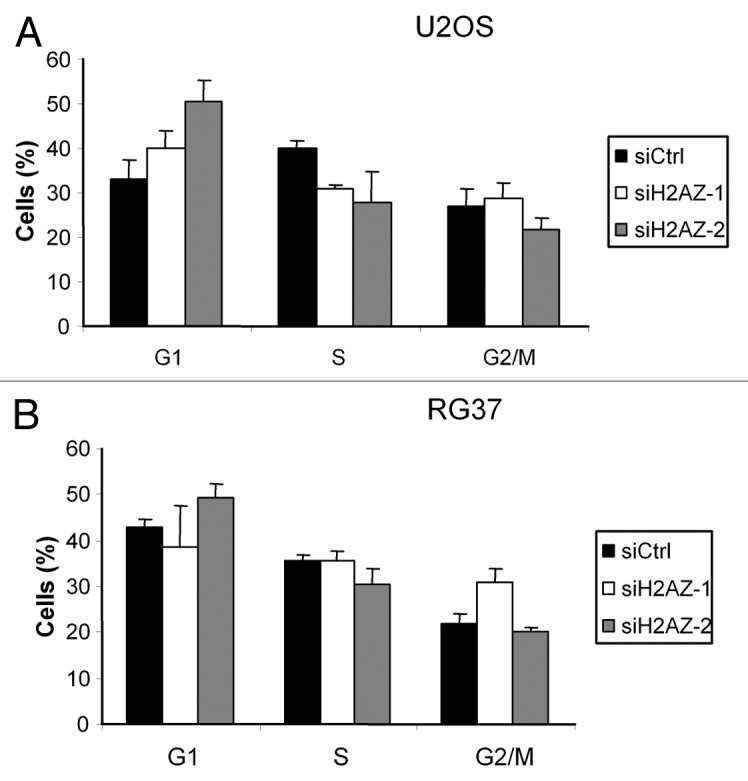
Figure 4. Effect of H2A.Z depletion on cell cycle distribution. Cell cycle distribution was examined by flow cytometry in U2OS (A) and RG37 (B) cells 48 h after siRNA transfection and staining with propidium iodide. Results are the mean +/− sd from 3 independent experiments.
Thus, in these 2 cell lines, H2A.Z depletion affects cell cycle distribution, the difference between them being probably the consequence of their p53 status. Indeed, in U2OS cells, H2A.Z depletion induces a p53-dependent cell cycle arrest in G1 through the induction of p21. In contrast, RG37 cells, being immortalized by T antigen expression that inactivates p53, cannot undergo this p21-dependent cell cycle arrest.
H2A.Z does not accumulate at DNA damage site
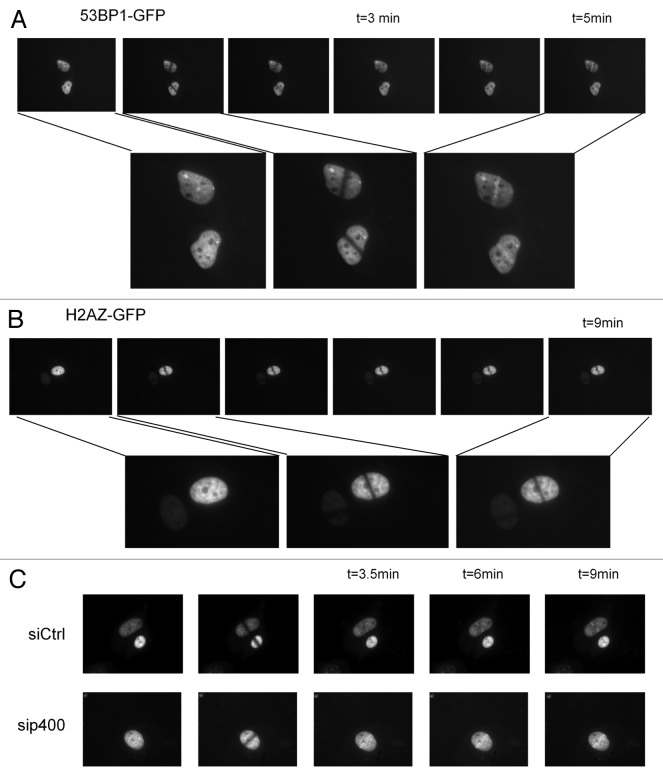
Because we could not find any effect of H2A.Z depletion on DSB repair, we addressed the role of H2A.Z in DSB repair by investigating whether it could be recruited to DSBs. To that purpose, we first used live imaging after laser-induced DNA damage on U2OS cells expressing GFP-tagged H2A.Z to follow whether H2A.Z accumulates at sites of DNA damage. Image recording showed that H2A.Z presents low mobility in the nucleus as observed after photo bleaching, since the bleached area was still observable 9 min following irradiation (Fig. 5B). Strikingly, no recruitment or accumulation was observed at the site of DNA damage at any time points following irradiation (Fig. 5B). This is in strong contrast with what happens in the same conditions using GFP-tagged 53BP1, for which recruitment and accumulation on DNA damaged regions was observed as soon as 5 min post-DNA damage induction, as evidenced by 53BP1 foci formation (Fig. 5A). Thus, no recruitment of H2A.Z can be observed in this experimental setting. In addition, we tested 53BP1-GFP recruitment after p400 depletion following laser induced irradiation in U2OS cells. The results obtained do not show significant alteration of 53BP1 recruitment at damaged areas (Fig. 5C). Similar results were obtained using MEF p400 (+/−) and p400 (+/+) from transgenic mice (data not shown). Such a result is consistent with our previous finding that 53BP1 foci formation upon ionizing radiation exposure is not defective in p400-depleted cells17.
Figure 5. Analysis of H2A.Z recruitment on DNA damage in living cells. Nucleus of U2OS cells transfected with 53BP1-GFP (A) or H2A.Z-GFP (B) plasmids were irradiated with pulsed laser (532 nm) to induce DNA damage then images were collected sequentially. (C) Nucleus of U2OS cells first transfected with siCtrl or sip400 then transfected with 53BP1-GFP plasmid and irradiated to induce DNA damage as in (A). Time after irradiation is indicated above the photographs.
Given that the GFP moiety could affect DSB recruitment of H2A.Z, we also performed chromatin immunoprecipitation (ChIP) experiments in U2OS cells in which sequence-specific DSBs can be induced by the ASiSI restriction enzyme following hydroxyl-tamoxifen (OH-Tam) treatment.22 By this technique, the presence of the phosphorylated form of H2AX was detected after OH–Tam addition around the 3 AsiSI sites we tested, revealing the efficiency of DNA breaks induction and of ChIP analyses (Fig. 6A). Using an antibody directed against endogenous H2A.Z, we detected the presence of H2A.Z near the 3 AsiSI sites as well as from the positive control p21 promoter (data not shown), indicating that the H2A.Z ChIPs are efficient. However, the presence of H2A.Z near the 3 AsiSI sites is similar in the absence or in the presence of AsiSI-induced DNA breaks (Fig. 6A), indicating that, in such experiments, no de novo recruitment of H2A.Z could be detected. Finally, as a control of the efficiency of the ChIP experiment using H2A.Z antibody, we performed ChIP after H2A.Z depletion and we observed a decrease in the H2A.Z signal, confirming that our experimental setting was able to detect changes in H2A.Z levels (Fig. S2).
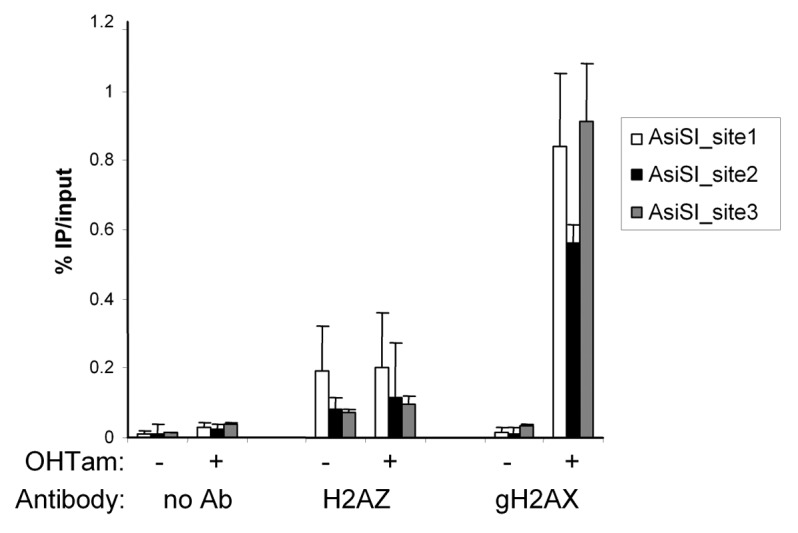
Figure 6. Chromatin immunoprecipitation of H2A.Z at DNA double-strand break. ChIP experiment showing H2A.Z and gH2AX accumulation at DSB in AsiSI-ER-U2OS cells.
Discussion
Here, we investigate the role of the histone variant H2A.Z in DNA double-strand break repair in mammals. We do not observe any defect of DNA DSB repair upon partial H2A.Z depletion. In addition, we do not find any evidence for H2A.Z recruitment around DSBs.
Until recently, work in mammals had focused on the H2A.Z chaperone p400. Depletion of the p400 ortholog in yeast leads to NHEJ defects but not HR defects.13 In contrast, in mammals, depletion of p400 by siRNA or the use of a dominant-negative mutant of p400 impairs homologous recombination-related steps, such as the formation of BRCA1 foci.16,17 We have shown that p400 interacts with the HR-specific protein Rad51 and impairs Rad51-dependent steps, whereas another study showed that p400 regulates histone ubiquitination and thus BRCA1 recruitment.16 However, although both studies clearly demonstrated the involvement of the ATPase activity of p400, the role of H2A.Z in mammals was directly investigated only recently. Our data shows that the phenotype observed upon p400 depletion and upon H2A.Z depletion are very distinct: for example, in the same cell type (RG37), p400 depletion induces a defect in HDR without affecting cell cycle,17 whereas H2A.Z depletion induces changes in cell viability and cell cycle distribution (G2 arrest) without affecting HR (this study). This result indicates that depleting a histone variant chaperone does not necessary give insights into the role of this histone variant. Such discrepancy could be due to H2A.Z-independent roles of p400, for example as a structural component of the Tip60 complex or as chromatin remodeling factor, favoring acetylation of nucleosomes by p400. In addition, as previously demonstrated in yeast for which htz1-specific phenotypes are due to SWR1 becoming detrimental,14 we might suppose that p400 expression and activity could become detrimental to the cell without its substrate (H2A.Z). This could explain the reason why it was not possible to obtain stable clones overexpressing p400 in human cell lines such as U2OS, RG37, and HeLa (unpublished data).
The role of mammalian H2A.Z in DSB repair was recently addressed by Xu et al.18 They showed that H2A.Z expression is important both for HR and NHEJ, H2A.Z depletion leading to a decrease in cell survival upon ionizing radiations exposure. They propose that H2A.Z-containing nucleosomes function as a barrier to block CtIP activity and, consequently, DNA resection. In their model, H2A.Z exchange would be necessary for the acetylation and ubiquitination of histones in order to promote BRCA1 loading but also required for loading of the NHEJ repair protein Ku70/Ku80. Our results stand in contrast with that finding. Indeed, we do not find any repair defect in the absence of H2A.Z, assayed either by cell viability following irradiation or using reporter systems for NHEJ or HR. One major difference between both studies is the way to achieve H2A.Z depletion. Xu et al. used shRNA, leading to a long-term and very strong depletion, as shown by western blot, whereas we only achieved a partial decrease in H2A.Z expression. However, despite this partial decrease, we can observe phenotypes induced by H2A.Z depletion such as basal clonogenic activity and cell cycle defects, indicating that major changes in cell metabolism can be observed upon partial H2A.Z depletion, at least in our cells. Given the strong connections between cell cycle distribution and the repair of DSB, changes in cell cycle distribution preclude any correct interpretation of DSB repair assays. Strikingly, Xu et al. did not find any evidence for an effect of H2A.Z depletion on cell cycle distribution. Clearly, these data indicate that depending on the cell type, depletion of H2A.Z leads to different phenotypes, with cell cycle changes being observed in U2OS and T-immortalized fibroblasts, and defects in DSB repair in HEK-293T cells. The reasons for these differences are still obscure, since 293T and T-immortalized fibroblasts both expressing the large T antigen from SV40 virus, inactivating both p53 and Rb.
Similarly, we did not find any evidence for H2A.Z recruitment to DSB in U2OS cells, either using laser-induced DNA breaks or by chromatin immunoprecipitation following sequence-specific DNA breaks. Local laser-induced irradiation data also indicate that H2A.Z is not very mobile, even following irradiation. This result suggests that the recruitment of H2A.Z to DNA breaks is perhaps restricted to a very short period of time, as proposed in yeast,11 or not very important in quantity or also specific to some cell types. Clearly, understanding why H2A.Z requirement is restricted to some particular DSB is a major issue in the field.
Materials and Methods
Cell culture and transfections
The RG37, GCS5, and GCV6 cell lines have been derived from SV40 T-transformed GM639 human fibroblasts.23 RG37, GCS5, GCV6, and U2OS cells were grown at 37 °C in Dulbecco modified Eagle medium (DMEM) supplemented with antibiotics, 10% FCS (all from Invitrogen). The AsiSI-ER-U2OS stable cell line22 was cultured in DMEM medium supplemented with 10% FCS and antibiotics. When needed, 300 nM of 4OH-tamoxifen was added to culture medium for 4 h. Treatment with the DNAPK inhibitor Nu7441 (Selleckchem) was performed at 5 µM 1 h before transfection with I-SceI and maintained during 48 h until harvesting the cells for GFP detection.
For siRNA transfection, 1 × 105 cells were transfected with siRNA (10 nM) using Interferin (Ozyme) according to the manufacturer’s instructions or 5 × 106 cells were electroporated with siRNAs (10 µM) using an electroporation device (Amaxa AG), according to manufacturer’s specifications (for all other experiments). Plasmids were transfected with Jet-PEI (Ozyme) according to the manufacturer’s indications.
Western blot
Total cell lysates were prepared by the resuspension of the cells directly in Laemmli buffer and sonication. Cells extracts were separated on 10% SDS-PAGE. Proteins were transferred on a nitrocellulose membrane. Primary antibodies as well as peroxidase-conjugated secondary antibodies were used according to standard western blot procedure and peroxidase activity was detected by using the Lumi-LightPLUS Western Blotting Substrate (Roche Diagnostics). The antibodies used were anti H2A.Z from Abcam, gH2AX from Epitomics, α-tubulin from Sigma-Aldrich, anti myc from Roche (9E10).
Assay for homology directed repair (HDR)
RG37 cells were transfected with the different siRNA (10 nM) then 24 h later transfected with I-SceI coding plasmid, and 48 h later cells were washed with PBS and treated with trypsin. Cells were resuspended in PBS and HDR was measured by the quantification of the GFP-positive cells by flow cytometry (FACScalibur, Becton Dickinson). Quantification was performed on 25 × 103 sorted events.
Assay for non-homologous end joining repair
GCS5 and GCV6 cells were transfected with the different siRNA (10 nM) then 24 h later transfected with I-SceI coding plasmid, and 48 h later cells were washed with PBS and treated with trypsin. Cells were resuspended in PBS and NHEJ events were quantify as GFP-positive cells by flow cytometry. Quantification was performed on 25 × 103 sorted events.
Laser microirradiation for the generation of DNA damage and live cell imaging
The system used to perform laser-induced DNA damage is composed of a conventional inverted microscope (DMI6000B; Leica) equipped with a heated stage and covered with an incubation system including a temperature controller and CO2 flow system. DNA damage induction on nucleus was achieved with a frequency-doubled Nd:YAG-pulsed laser at a wavelength of 532 nm; the pulse was estimated to have a duration of 600 ps with a repetition rate of 10 kHz (532-nm Sealed Green Microchip; JDS Uniphase). The guiding of the beam was performed using an L5D head (Roper Industries) coupled to the microscope through the epifluorescence port. The beam is focused with a 100 × NA 1.4 Plan Apo oil immersion objective lens (HCS; Leica). Images were acquired with a cooled charge-coupled device camera (CoolSNAP HQ2). The system is driven by MetaMorph software.
Live cells analysis was performed in a 2-well chamber (Labtek) in 1 ml of optimem medium without red phenol on cells transiently transfected with H2A.Z-GFP or 53BP1-GFP constructs. Images were recorded using the MetaMorph software package (MDS Analytical Technologies).
ChIP experiments
Cells were fixed in 1% formaldehyde (20 min) and glycine added to block the reaction. ChIPs were performed as described24 using 150 µg of chromatin. Nuclei were prepared and sonicated to generate DNA fragments with lengths between 500 and 1000 bp. After pre-clearing and blocking steps, immunoprecipitations were performed overnight with specific antibodies (H2A.Z antibody from Abcam or gH2AX antibody from Millipore) or without antibody as negative control. Recovering of the immune complexes was performed by the incubation of the samples with a mixture of blocked proteinA/protein G beads on a rotating wheel (2 h at 4 °C).After washing, the DNA–protein cross-link was reversed by the addition of Rnase A to the samples and heating at 65 °C. After proteinase K digestion (2 h), DNA was purified and then quantified by qPCR using a Real-Time PCR machine (Bio-Rad Laboratories) according to the manufacturer’s instructions. qPCR reactions were performed in triplicate.
Cell cycle analysis
Cells (U2OS and RG37) were transfected with siRNA (10 nM). Forty-eight hours later cells were harvested and fixed in ice-cold 70% ethanol. DNA was stained with propidium iodide (10 µg/ml) in the presence of RNase (10 µg/ml). Data were collected on a FACScalibur cytometer (Becton Dickinson) and analyzed using Modfit software (Verity Software).
Clonogenic assay
Cells were transfected with siRNA (10 nM), then 24 h later plated in triplicate at the density of 500 to 1000 cells per dish depending on the dose of ionizing radiations exposure. Dishes were irradiated 24 h later at 2, 4, or 6 Gy with a Cs137 source (Biobeam 8000). To examine accurately the cloning efficiency additional experiments were performed in which 2000 cells were plated in dishes 24 h after transfection with the siRNA. The dishes were let in normal culture conditions during 10 d before staining of the colonies by crystal violet solution. Colonies of more than 50 cells were counted.
Supplementary Material
Acknowledgments
This work was supported by grants from EDF (Electricité de France) to YC and from the ARC (Association de Recherche contre le Cancer) to DT (Programme ARC). G-CT-T was supported by a fellowship from the Gabonian government and ANR (Projet blanc 2011 SVSE8 PinGs).
We acknowledge the Toulouse Genotoul TRI facilities (LBCMCP/FRBT) for flow cytometry and fluorescence microscopy. We are grateful to the Non-Invasive Exploration service - US006/CREFRE INSERM/UPS for giving access to the irradiator Biobeam 8000. We thank the Dr BS Lopez (IGR, Villejuif, France) for providing the GCS5 and GCV6 cell lines. We also thank Céline Reyes for her assistance with the living cells Laser irradiation experiments.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/cc/article/ 27143
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/27143
References
- 1.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–74. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 2.Curtin NJ. DNA repair dysregulation from cancer driver to therapeutic target. Nat Rev Cancer. 2012;12:801–17. doi: 10.1038/nrc3399. [DOI] [PubMed] [Google Scholar]
- 3.Hiom K. Coping with DNA double strand breaks. DNA Repair (Amst) 2010;9:1256–63. doi: 10.1016/j.dnarep.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Grabarz A, Barascu A, Guirouilh-Barbat J, Lopez BS. Initiation of DNA double strand break repair: signaling and single-stranded resection dictate the choice between homologous recombination, non-homologous end-joining and alternative end-joining. Am J Cancer Res. 2012;2:249–68. [PMC free article] [PubMed] [Google Scholar]
- 5.Soria G, Polo SE, Almouzni G. Prime, repair, restore: the active role of chromatin in the DNA damage response. Mol Cell. 2012;46:722–34. doi: 10.1016/j.molcel.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Rossetto D, Truman AW, Kron SJ, Côté J. Epigenetic modifications in double-strand break DNA damage signaling and repair. Clin Cancer Res. 2010;16:4543–52. doi: 10.1158/1078-0432.CCR-10-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altmeyer M, Lukas J. To spread or not to spread--chromatin modifications in response to DNA damage. Curr Opin Genet Dev. 2013;23:156–65. doi: 10.1016/j.gde.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Clapier CR, Cairns BR. The biology of chromatin remodeling complexes. Annu Rev Biochem. 2009;78:273–304. doi: 10.1146/annurev.biochem.77.062706.153223. [DOI] [PubMed] [Google Scholar]
- 9.Celeste A, Fernandez-Capetillo O, Kruhlak MJ, Pilch DR, Staudt DW, Lee A, Bonner RF, Bonner WM, Nussenzweig A. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat Cell Biol. 2003;5:675–9. doi: 10.1038/ncb1004. [DOI] [PubMed] [Google Scholar]
- 10.Papamichos-Chronakis M, Watanabe S, Rando OJ, Peterson CL. Global regulation of H2A.Z localization by the INO80 chromatin-remodeling enzyme is essential for genome integrity. Cell. 2011;144:200–13. doi: 10.1016/j.cell.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalocsay M, Hiller NJ, Jentsch S. Chromosome-wide Rad51 spreading and SUMO-H2A.Z-dependent chromosome fixation in response to a persistent DNA double-strand break. Mol Cell. 2009;33:335–43. doi: 10.1016/j.molcel.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Adkins NL, Niu H, Sung P, Peterson CL. Nucleosome dynamics regulates DNA processing. Nat Struct Mol Biol. 2013;20:836–42. doi: 10.1038/nsmb.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Attikum H, Fritsch O, Gasser SM. Distinct roles for SWR1 and INO80 chromatin remodeling complexes at chromosomal double-strand breaks. EMBO J. 2007;26:4113–25. doi: 10.1038/sj.emboj.7601835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morillo-Huesca M, Clemente-Ruiz M, Andújar E, Prado F. The SWR1 histone replacement complex causes genetic instability and genome-wide transcription misregulation in the absence of H2A.Z. PLoS One. 2010;5:e12143. doi: 10.1371/journal.pone.0012143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosa M, Von Harder M, Cigliano RA, Schlögelhofer P, Mittelsten Scheid O. The Arabidopsis SWR1 chromatin-remodeling complex is important for DNA repair, somatic recombination, and meiosis. Plant Cell. 2013;25:1990–2001. doi: 10.1105/tpc.112.104067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Y, Sun Y, Jiang X, Ayrapetov MK, Moskwa P, Yang S, Weinstock DM, Price BD. The p400 ATPase regulates nucleosome stability and chromatin ubiquitination during DNA repair. J Cell Biol. 2010;191:31–43. doi: 10.1083/jcb.201001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Courilleau C, Chailleux C, Jauneau A, Grimal F, Briois S, Boutet-Robinet E, Boudsocq F, Trouche D, Canitrot Y. The chromatin remodeler p400 ATPase facilitates Rad51-mediated repair of DNA double-strand breaks. J Cell Biol. 2012;199:1067–81. doi: 10.1083/jcb.201205059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Y, Ayrapetov MK, Xu C, Gursoy-Yuzugullu O, Hu Y, Price BD. Histone H2A.Z controls a critical chromatin remodeling step required for DNA double-strand break repair. Mol Cell. 2012;48:723–33. doi: 10.1016/j.molcel.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie A, Kwok A, Scully R. Role of mammalian Mre11 in classical and alternative nonhomologous end joining. Nat Struct Mol Biol. 2009;16:814–8. doi: 10.1038/nsmb.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, Thomas HD, Batey MA, Cowell IG, Richardson CJ, Griffin RJ, Calvert AH, Newell DR, Smith GC, Curtin NJ. Preclinical evaluation of a potent novel DNA-dependent protein kinase inhibitor NU7441. Cancer Res. 2006;66:5354–62. doi: 10.1158/0008-5472.CAN-05-4275. [DOI] [PubMed] [Google Scholar]
- 21.Gévry N, Chan HM, Laflamme L, Livingston DM, Gaudreau L. p21 transcription is regulated by differential localization of histone H2A.Z. Genes Dev. 2007;21:1869–81. doi: 10.1101/gad.1545707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iacovoni JS, Caron P, Lassadi I, Nicolas E, Massip L, Trouche D, Legube G. High-resolution profiling of gammaH2AX around DNA double strand breaks in the mammalian genome. EMBO J. 2010;29:1446–57. doi: 10.1038/emboj.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dumay A, Laulier C, Bertrand P, Saintigny Y, Lebrun F, Vayssière JL, Lopez BS. Bax and Bid, two proapoptotic Bcl-2 family members, inhibit homologous recombination, independently of apoptosis regulation. Oncogene. 2006;25:3196–205. doi: 10.1038/sj.onc.1209344. [DOI] [PubMed] [Google Scholar]
- 24.Mattera L, Courilleau C, Legube G, Ueda T, Fukunaga R, Chevillard-Briet M, Canitrot Y, Escaffit F, Trouche D. The E1A-associated p400 protein modulates cell fate decisions by the regulation of ROS homeostasis. PLoS Genet. 2010;6:e1000983. doi: 10.1371/journal.pgen.1000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.