Abstract
Breast-conserving surgery for ductal carcinoma in situ (DCIS) is often combined with irradiation, reducing recurrence rates to 20% within 10 years; however, there is no change in overall survival. Evidence in the invasive breast indicates that breast cancer stem cells (CSCs) are radiotherapy-resistant and are capable of re-initiating a tumor recurrence; hence, targeting CSCs in high risk DCIS patient may improve survival. HER2 is overexpressed in 20% of DCIS and is known to be highly active in breast CSCs; we therefore investigated the effect of Lapatinib on DCIS CSC activity using 2 in vitro culture systems. Two DCIS cell lines DCIS.com (HER2 normal) and SUM225 (HER2 overexpressed) as well as DCIS cells from patient samples (n = 18) were cultured as mammospheres to assess CSC activity and in differentiated 3D-matrigel culture to determine effects within the non-CSCs. Mammosphere formation was reduced regardless of HER2 status, although this was more marked within the HER2-positive samples. When grown as differentiated DCIS acini in 3D-matrigel culture, Lapatinib only reduced acini size in the HER2-positive samples via decreased proliferation. Further investigation revealed lapatinib did not reduce self-renewal activity in the CSC population, but their proliferation was decreased regardless of HER2 status. In conclusion we show Lapatinib can reduce DCIS CSC activity, suggesting that the use of Lapatinib in high-risk DCIS patients has the potential to reduce recurrence and the progression of DCIS to invasive disease.
Keywords: DCIS, HER2, proliferation, Lapatinib, cancer stem cells
Introduction
Ductal carcinoma in situ (DCIS) is a pre-invasive lesion that represents 20% of screen detected breast cancers. Its treatment remains controversial, as a large proportion of patients achieve cure with current treatment strategies of breast-conserving surgery, which is often combined with irradiation. Despite irradiation improving recurrence rates to 20% within 10 years, there is no improvement in overall survival,1-7 suggesting that in these patients current therapeutic strategies do not eliminate DCIS cells that are capable of recurrence. This risk of recurrence is greatest in younger women (<40) who opt for breast-conserving surgery3 and have high-grade, estrogen receptor-negative and HER2-positive DCIS.
We have published evidence of a cancer stem/progenitor population within human primary DCIS and DCIS cell lines8,9 and show increased numbers of these cells in high-grade DCIS. Research in the invasive breast indicates that breast cancer stem cells (CSCs) are chemo- and radiotherapy-resistant,10-14 suggesting CSCs may re-initiate a tumor after treatment. Therefore targeting CSCs may provide a new avenue of treatment for patients with high-risk DCIS to ultimately improve recurrence and survival rates.
HER2 predicts for poor prognosis in DCIS and is overexpressed or amplified in up to 60% of high-grade DCIS lesions.15,16 HER2 has also been found to be overexpressed in breast CSC regardless of HER2 status,17,18 and therefore may be an ideal target to eliminate DCIS CSC in high-risk patients. There are a limited number of human DCIS models to investigate this. We and others have reported in vivo human xenograft models19,20 and 2 pre-clinical in vitro models utilizing 3D matrigel culture and the mammosphere assay that we developed and published in 2007.8,9 Our in vitro models were used within this study to investigate the effects of HER2 inhibition on DCIS CSCs and non-CSC populations. Here we demonstrate that HER2 inhibition reduces the proliferation of HER2-positive differentiated (non-CSC) DCIS cells, whereas the proliferation of an enriched stem/progenitor DCIS population can be reduced regardless of HER2 status, albeit more substantially in the HER2-positive setting, indicating that further investigation into anti-HER2 therapies in DCIS is warranted.
Results
Lapatinib preferentially decreases mammosphere formation in cell lines and patient-derived HER2-positive DCIS
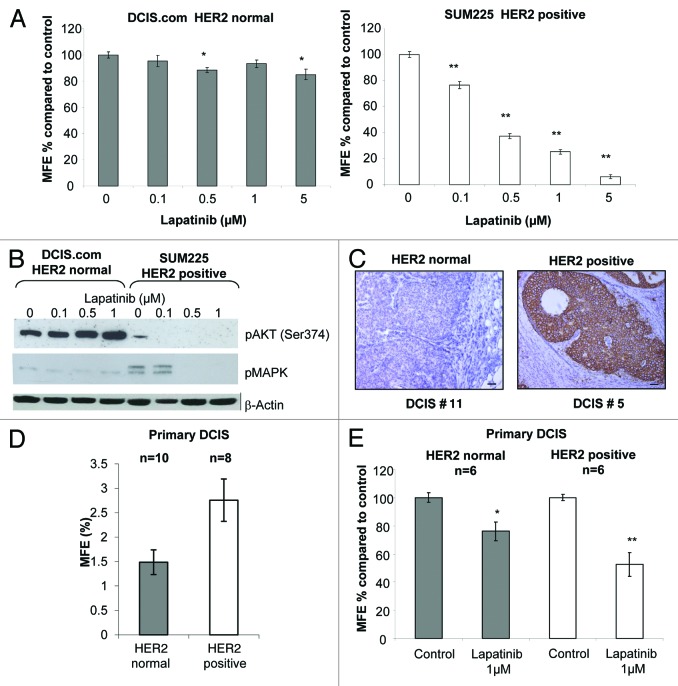
To investigate the effect of EGFR/HER2 inhibition of normal CSC activity we utilized the mammosphere assay. This non-adherent assay allows cells that are anoikis-resistant to self-renew and proliferate to form floating colonies8,9,21 and have been shown to be enriched for tumor-initiating cells.22,23 Lapatinib reduced mammosphere forming efficiency (MFE) in the SUM225 (HER2-positive) cells in a dose-dependent manner, reaching >80% inhibition at 5 µM. In contrast, the effects of Lapatinib on MFE within the HER2 normal DCIS.com cells were not marked with a maximal reduction of 15% with 5 µM (Fig. 1A). As expected western blot analysis showed a reduction of active AKT and ERK1/2 within SUM225 mammospheres treated with Lapatinib (0–1 µM), whereas with no effect was seen in DCIS.com mammospheres (Fig. 1B).
Figure 1. Lapatinib preferentially reduced mammosphere formation in a HER2-positive cell line and primary DCIS samples (A) Graphs showing DCIS.com and SUM225 cells treated with Lapatinib 0.1–5 μM in mammosphere culture. Mammosphere forming efficiency (MFE) was calculated by dividing the number of mammospheres formed by the original number of cells seeded and is expressed as a percentage compared with control. (B) Western blots showing downstream signaling effects of lapatinib (0–1 μM) after 7 d mammosphere culture of DCIS.com and SUM225 cell lines. β-Actin was used as a loading control. (C) Images of representative DCIS samples collected for in vitro culture showing HER2 staining in normal and positive HER2-expressing DCIS samples, scale bar represents 50 μm. (D) Graphs showing the percentage MFE of HER2-positive (n = 10) and normal (n = 8) primary DCIS cells from tissue taken at surgery. (E) Graphs showing the percentage MFE of HER2-positive (n = 6) and normal (n = 6) primary DCIS cells after treatment with lapatinib (1 μM) compared with control conditions. All graphs, mean ± standard error, 3 independent experiments with 3 technical replicates per experiment. n represents number of independent human DCIS samples. Mann–Whitney U test, 2-tailed, * P ≤ 0.02, ** P ≤ 0.0001.
To verify the findings observed in the DCIS cell lines we extracted cells from DCIS tissue from patients undergoing mastectomy. The MFE from the HER2 normal (n = 8) and HER2-positive (n = 10, Table 1; and representative IHC images in Fig. 1C) were compared, and a trend toward the HER2-positive MFE being higher than that of the HER2 normal samples was demonstrated, although this did not reach significance due to a high variance in the samples tested (P = 0.09) (Fig. 1D). Twelve samples (6 HER2 normal and 6 HER2-positive) were treated in the presence and absence of 1 μM Lapatinib; MFE was reduced in both samples, although this was more pronounced in the HER2-positive samples, 48% vs. 24% MFE reduction in HER2-positive vs. HER2 normal, respectively (Fig. 1E). These data suggest that EGFR/HER2 activity is important for mammosphere formation of both HER2-positive and HER2-negative DCIS, this is not unexpected as HER2 normal breast CSCs have been shown to have elevated levels of HER2 compared with the non-CSC population.17
Table 1. Characteristics of DCIS patient samples used for in vitro culture.
| Sample # | ER status | HER2 status |
|---|---|---|
| 1 | un | + |
| 2 | un | + |
| 3 | − | + |
| 4 | + | + |
| 5 | − | + |
| 6 | − | + |
| 7 | + | + |
| 8 | − | + |
| 9 | − | + |
| 10 | − | + |
| 11 | + | − |
| 12 | + | − |
| 13 | − | − |
| 14 | + | − |
| 15 | − | |
| 16 | − | − |
| 17 | + | − |
| 18 | − | − |
ER, estrogen receptor α.
Lapatinib reduces differentiated 3D-matrigel DCIS acini growth in HER2-positive DCIS cell lines and primary DCIS
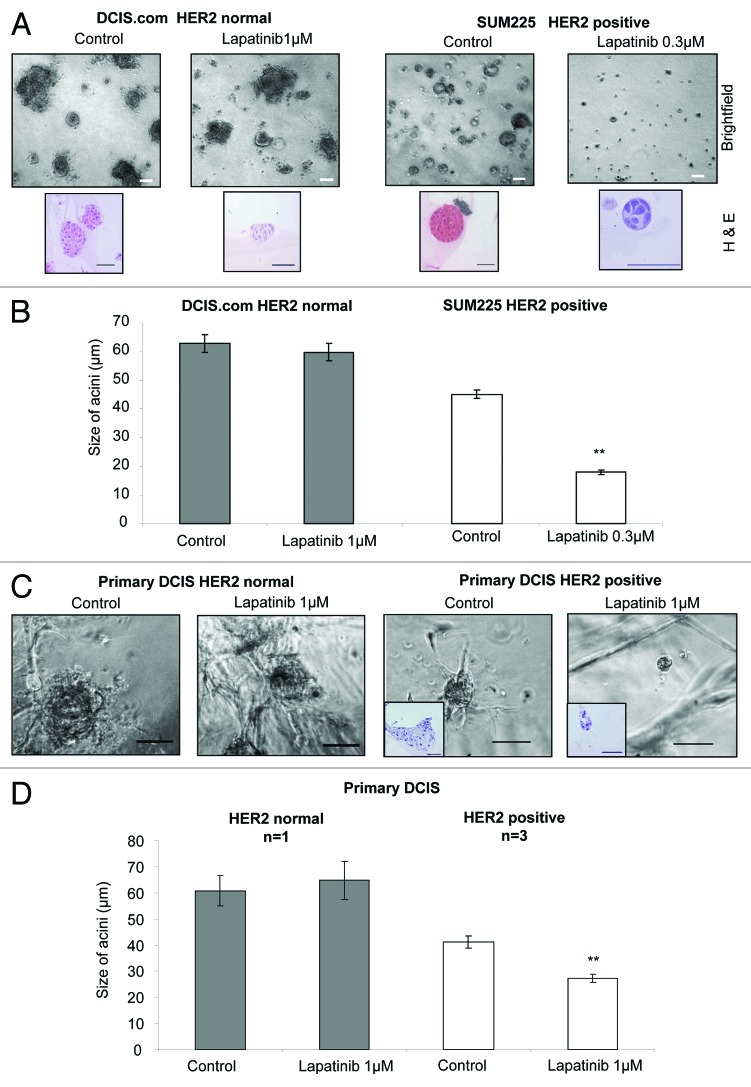
DCIS cell lines and primary DCIS cells can be grown in differentiated 3D-matrigel culture where they recapitulate in vivo-like DCIS acini structures, which are disorganized with occluded lumen (Fig. 2A brightfield and H&E images).8,9 DCIS.com and SUM225 cells were cultured in 3D-matrigel in the presence and absence of Lapatinib at 1 μM or 0.3 μM, respectively. Although the number of acini was not affected, the size of DCIS acini in the SUM225 HER2-positive cell line was significantly reduced after Lapatinib (0.3 μM) treatment compared with control conditions (P < 0.001, control 45 ± 1.5 μm vs treated 18 ± 0.8 μm), whereas no change in acini size was observed in the HER2 normal cell line (DCIS.com), even after treatment with 1 μM Lapatinib (Fig. 2B). We corroborated these results using primary DCIS patient samples cultured as DCIS acini in our 3D-matrigel assay. After treatment with control or Lapatinib 1 μM the number of acini remained unchanged, as did the size of acini in the HER2 normal sample. However, the size of HER2-positive primary DCIS acini were significantly reduced (P < 0.001 control 41 ± 2.4 μm vs. treated 27 ± 01.5 μm Fig. 2C and D). These data indicated that unlike the DCIS CSC population, only the HER2-positive non-CSC cells, grown under these 3D-matrigel conditions, were affected by lapatinib treatment.
Figure 2. Lapatinib reduces acini size of a HER2-positive cell line and primary DCIS samples. (A) Brightfield and Haematoxylin and Eosin images of day 15 acini with control and lapatinib treatment 1 μM and 0.3 μM in DCIS.com or SUM225 cells, respectively. (B) Graph showing size of acini (μm) after 10 d of 3D matrigel culture with 1 μM and 0.3 μM Lapatinib treatment in DCIS.com or SUM225 cells, respectively, 3 independent experiments. (C) Images of HER2 normal and HER2-positive human primary DCIS grown in 3D matrigel culture with control or lapatinib (1 μM) treatment. Inserts shown on HER2-positive sample show Haematoxylin and Eosin staining of a cross section through acini. (D) Graph showing HER2-positive (n = 3) and HER2 normal (n = 1) human primary DCIS cells grown in matrigel in the presence or absence of Lapatinib (1 μM). n represents number of independent human DCIS samples. Scale bar represents 50 μm. Mean ± standard error. Mann–Whitney U test, 2-tailed, * P ≤ 0.02, ** P ≤ 0.0001.
Proliferation of acini and DCIS stem/progenitor cells is reduced after treatment with lapatinib
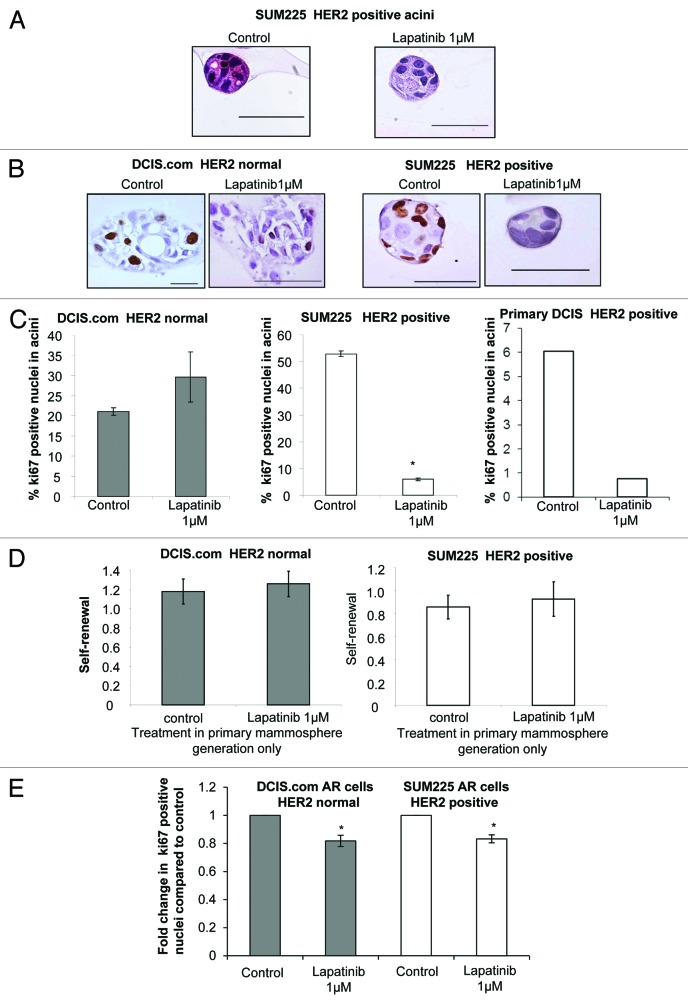
To further investigate the effects of Lapatinib in the differentiated 3D-matrigel culture model we first investigated the morphology of DCIS acini by examining H&E stained cross-sections of the cell line and primary DCIS structures. In all cases, the lumens of the colonies remained solid with no evidence of hollowing even in the Lapatinib-treated SUM225 and primary HER2-positive acini, which were significantly reduced in size compared with controls (Figs. 3A and 2C). Next proliferation was assessed using Ki67 staining of the DCIS acini (Fig. 3B). Data shows that proliferation was significantly reduced in the HER2-positive SUM225 and primary DCIS acini compared with control conditions (SUM225 53 ± 1 vs. 6 ± 0.5 P < 0.0001, primary DCIS 6% vs. 0.8%, Fig. 3C). This reduction in proliferation was not observed in the DCIS.com HER2 normal acini. These data suggest that proliferation of differentiated HER2-positive, but not HER2 normal, cells within the DCIS acini were regulated by EGFR/HER2 signaling, resulting in a reduction in acini size.
Figure 3. Proliferation of acini and DCIS stem/progenitor cells is reduced after treatment with Lapatinib (A) Haematoxylin and Eosin staining of sections through control and Lapatinib 0.3 μM treated SUM225 acini at day 15 of matrigel culture. (B) Images of Ki67 staining of acini formed from control or Lapatinib-treated (1 or 0.3 μM) DCIS.com and SUM225 cell lines. (C) Graphs showing the percentage positive Ki67 nuclei from DCIS.com, SUM225, and a primary DCIS acini grown in control or Lapatinib at 1 μM, respectively. Mean ± standard error, Unpaired t test, 2-tailed, unequal variance, * P < 0.0001. (D) Graphs showing the self-renewal capacity of DCIS.com and SUM225 cells as measured by secondary mammosphere formation from primary generation mammospheres treated with control or Lapatinib (1 μM) (No further treatment is given in secondary mammosphere generation). (E) Graph showing fold change in Ki67-positive cells within the anoikis-resistant (AR) populations of DCIS.com and SUM225 cells in the presence or absence of Lapatinib (1 μM). Mean ± standard error, 3 independent experiments in triplicate, Mann–Whitney U test, 2-tailed *P ≤ 0.0286, **P ≤ 0.001.
Mammosphere formation was reduced in both HER2-positive and HER2 normal cell lines and primary DCIS samples; to investigate the effects of Lapatinib on the mammosphere initiating population we performed secondary generation mammosphere culture, which allows for the assessment of self-renewal after treatment in primary generation mammosphere culture.21 Treatment with lapatinib did not reduce self-renewal regardless of HER status (Fig. 3D). In addition the size of mammospheres was assessed, and no changes were seen in Lapatinib-treated compared with control (data not shown). Finally we investigated the levels of proliferation within an enriched mammosphere-initiating population by selecting the anoikis-resistant (AR) cells after 16 h in non-adherent mammosphere culture. The proliferation within the AR population, as measured by Ki67, was reduced after lapatinib treatment compared with control in both HER2-positive and HER2 normal DCIS cell lines (Fig. 3E). These data suggest that Lapatinib can reduce the proliferation of the progenitor/stem enriched population regardless of HER2 status.
Discussion
We have demonstrated that lapatinib reduced the proliferation of HER2-positive differentiated DCIS cells grown in 3D-matrigel culture, whereas mammosphere formation and proliferation of an enriched stem/progenitor DCIS population can be reduced regardless of HER2 status, albeit more substantially in the HER2-positive setting. We report that lapatinib can reduce mammosphere formation in DCIS cell lines and primary DCIS cells in both HER2-positive and HER2 normal cells. Studies investigating invasive breast cancer have shown inhibition of HER2, with lapatinib, can decrease the CSC populations in patients undergoing chemotherapy treatment in the clinic.12 In addition, our work is keeping with current evidence indicating that breast CSC populations have increased levels of HER2, irrespective of presence of HER2 amplification,17,18 suggesting that the CSC population may be sensitive to HER2 inhibition regardless of HER2 status. Retrospective analysis of HER2 gene amplification on clinical samples from NSABP B31 and N9831 trastuzumab (HER2 humanised monoclonal antibody) adjuvant trials identified that even patients lacking HER2 amplification benefited from treatment, as did patients whose tumors displayed HER2 gene amplification (HER2-positive).24,25 To verify these retrospective findings a randomized prospective phase III trial, NSABP B47, is currently underway. However these data highlight that even a subpopulation of cells with elevated HER2 receptor signaling (non-amplified) may prove to be an important target to decrease recurrence rates in breast cancer and DCIS.
Inhibition of HER2 during growth of differentiated DCIS acini in 3D-matrigel did not affect the number of DCIS acini and their unorganized occluded lumens were maintained. Further analysis revealed that proliferation, as measured by Ki67, was reduced in the HER2-positive cell line and primary DCIS acini, but the cells within the HER2 normal DCIS.com acini were not affected, indicating that lapatinib reduces proliferation in the bulk, differentiated DCIS cells within the acini of HER2-positive cells. Recent data in pre-operative clinical studies with Lapatinib has shown significant inhibition of proliferation in invasive breast cancer and DCIS.26 To note similar Trastuzumab trials in DCIS have not observed any changes in proliferation after a 2-week treatment pre-operatively, implying Trastuzumab may have a different mode of action.27 We have previously used Trastuzumab in a DCIS xenograft model and published that it had no effect on proliferation of HER2-positive DCIS in that setting.28 This suggests our pre-clinical in vitro 3D matrigel culture is mimicking the effects seen in clinical trials, and that small molecular weight inhibitors of HER2 may be more effective than humanized monoclonal antibodies in DCIS.
We demonstrate that treatment of lapatinib in first generation mammospheres, did not affect the self-renewal capacity, as measured by secondary mammosphere formation with no further treatment, although prolonged treatment was not assessed. We specifically investigated change in proliferation within these anoikis-resistant cells, which are enriched for CSCs, rather than the bulk/differentiated DCIS cell within the acini. Here we show lapatinib reduced Ki67 in both the HER2 normal and positive cell lines, suggesting the reduction in mammosphere formation may be caused by decreased proliferation within the CSC population. It would be interesting to determine whether HER2 inhibitors in combination with other therapies are better than single agents as neo-adjuvant trials, in invasive breast cancer, show clinical response was more pronounced in the combination arm (chemotherapy and Trastuzumab) compared with chemotherapy alone.29-33 In the case of DCIS lapatinib may be used in combination with radiotherapy in patients at high risk of recurrence (high-grade, <40 y, ER-negative, HER2-positive), as laboratory evidence suggests that HER2 inhibitors may improve radio-sensitivity.34
In conclusion, we demonstrate that lapatinib can reduce DCIS CSC activity via reduction of proliferation and suggest that further investigation into treatment of high-risk DCIS patients at the time of surgery and radiotherapy with anti-HER2 agents, to target DCIS CSCs, would be warranted.
Materials and Methods
Patient samples
Women were included in the study if they had mammograms showing widespread microcalcification, indicative of DCIS or DCIS surrounding an invasive breast cancer (n = 18). All tissue samples were obtained following therapeutic DCIS surgery, and histopathological confirmation of the diagnosis of DCIS and grade was obtained following subsequent review by a consultant breast pathologist. Approval to remove tissue from pathological samples was granted by the South Manchester Medical Research Ethics Committee.
DCIS tissue was prepared as previously described.8 Briefly, DCIS tissue collected at surgery was dissected and enzymatically digested overnight (16–18 h) at 37 °C in serum-free DMEM (Gibco) containing type I collagenase 200 U/ml (Worthingtons) and 5% penicillin/streptomycin (Sigma). The digest was then filtered to obtain a single cell suspension. HER2 status within these was determined and scored using previously published reagents and methods.35
Cell culture and reagents
Human DCIS cell lines SUM225 (HER2-positive) and MCF10DCIS.com (HER2 negative) were grown adherently in their relevant medium, both were purchased from Asterand. MCF10DCIS.com media - Advanced DMEM/F12, 5% (v/v) horse serum, L-glutamine 2 mM (Gibco). The SUM225 media - Ham’s F12, insulin 5 μg/ml, hydrocortisone 1 μg/ml, HEPES 10 mM, and 5% (v/v) fetal calf serum (Gibco). All cells were maintained in a humidified incubator at 37 °C at an atmospheric pressure of 5% (v/v) carbon dioxide/air. Lapatinib was a gift from GlaxoSmithKline and was dissolved in 100% DMSO to a 10 mM stock solution.
Mammosphere culture
Mammosphere culture was performed as described in references 8, 9, and 21. Briefly, all cell lines or primary samples were seeded at 500 cells/cm2 in polyhema coated tissue culture plates. Mammosphere culture medium comprised DMEM-F12 media supplemented with B27 without vitamin A (Gibco) and EGF 20 ng/ml (Sigma). The mammosphere-forming efficiency (MFE) was calculated as the percentage of MS formed (>60 µm) relative to the original number of single cells seeded (mean % MFE ± standard error [SEM]). Lapatinib (a HER2/EGFR tyrosine kinase inhibitor) treatment was added day 0 and mammospheres were counted day 5 (cell lines) and day 3 (primary DCIS samples) and expressed as percentage MS formation compared with a non-treated DMSO control.
For the growth of secondary MS, primary MS grown with or without treatments, were collected (after counting) by centrifugation (800 rpm) and dissociated enzymatically for 2 min in 0.125% Trypsin-EDTA at 37 °C to obtain a single cell suspension. Cell viability was determined using trypan blue before seeding cells for second-generation mammosphere growth at a density of 500 cell/cm2 with no further treatment. Self-renewal was calculated by dividing the number of secondary mammospheres by the number of primary mammospheres counted.
Western blotting
Lysates from cells containing 50 µg of protein were fractionated by SDS-PAGE and transferred to Hybond nitrocellulose membrane (Amersham). The nitrocellulose was then blocked in Tris-buffered saline (TBS) containing 0.1% Tween-20 and 5% non-fat milk for 1 h at room temperature. The membrane was then incubated with primary antibody for 1 h at room temperature or 4 °C overnight with gentle shaking. The membrane was washed 3 times in TBS containing 0.1% Tween 20 and then incubated with horseradish peroxidase conjugated secondary antibody (1:5000; Dako) for 1 h at room temperature. After washing, immunoreactive proteins were detected by enhanced chemiluminescence (Pierce). Primary antibodies (AKT, Phosphorylated (Ser374) AKT*, MAPK, phosphorylated MAPK* (Cell Signaling), and β-Actin (Sigma) for western blotting were all used at concentrations 1:1000 (*overnight incubation at 4 °C).
Enrichment of fluorescence-activated cell sorting (FACS)
Anoikis-resistant cells (viable cells) collected at 24 h after seeding in non-adherent mammosphere culture (+/− Lapatinib) were collected, stained with 7AAD (BD bioscience) to gate for dead cells, and then fixed in 4% paraformaldehyde for 10 min. The Ki67-FITC conjugated mouse anti-human Ki67 monoclonal antibody (1:200, BD bioscience) was then added for 15 min at room temperature, washed in PBS before FACS analysis Becton Dickinson FACS Calibur. IgG-FITC was used as a control for gating positive Ki67 cells and data interpreted using the WinMIDI 2.8 software.
Matrigel culture
Single-cell suspensions of DCIS cell lines were seeded at a density of 5000 cells/well in the appropriate adherent culture medium containing 2% growth factor-reduced matrigel into 8-well glass chamber slides containing 50 µl growth factor reduced matrigel (BD Bioscience). Lapatinib was added to the matrigel media from day 0, and a DMSO control was used. The resulting acini were grown over a 21-d period, during which the growth medium (with or without Lapatinib) was replaced every 2–3 d. Acini were then counted and sized using a light microscope.
Immunohistochemistry (IHC) Ki67 staining
All slides were dewaxed and rehydrated. Antigen retrieval was achieved using the pre-treatment (PT, Lab Vision) module with 0.01 M citric acid monohydrate buffer, pH 6 at 95 °C for 30 min. Following retrieval, a peroxidase blocking solution (100 µl/section, Dako) was added for 10 min followed by a further 10 min block (3% normal goat serum [Vector Laboratory]/TBS). The primary antibody Ki67 (MIB-1 clone MIB-1, Dako, 1:50 in antibody diluent) was added for 1 h, washed twice for 5 min in TBS (Tris buffered saline) Tween 20. The Dako REAL EnVision Detection System was subsequently used according to the manufacturer’s instruction manual to complete the staining procedure. The slides were counterstained with haematoxylin (Thermo Electron Corporation) and dehydrated back through the graded alcohols. Once dry they were immersed in histoclear (National Diagnostics) for 10 min and mounted with Pertex (Cell Path). Ki67 is a nuclear staining score and ≥250 cells were counted to determine the proliferation percentage.
Acknowledgments
GF and RB are funded by Breast Cancer Campaign Fellowships. RLJ and KW are funded by the Royal College of Surgeons. We would also like to thank GlaxoSmithKline for the gift of Lapatinib and funding RLJ.
Glossary
Abbreviations:
- DCIS
ductal carcinoma in situ
- MFE
mammosphere forming efficiency
- DMEM
Dulbecco modified Eagle medium
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author Contributions
GF, RLJ, and KW performed the experiments. GF, RJL, and NJB wrote the manuscript and all authors read and approved the final manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/27201
References
- 1.Barnes NL, Ooi JL, Yarnold JR, Bundred NJ. Ductal carcinoma in situ of the breast. BMJ. 2012;344:e797. doi: 10.1136/bmj.e797. [DOI] [PubMed] [Google Scholar]
- 2.Bijker N, Meijnen P, Peterse JL, Bogaerts J, Van Hoorebeeck I, Julien JP, Gennaro M, Rouanet P, Avril A, Fentiman IS, et al. EORTC Breast Cancer Cooperative Group. EORTC Radiotherapy Group Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853--a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24:3381–7. doi: 10.1200/JCO.2006.06.1366. [DOI] [PubMed] [Google Scholar]
- 3.Correa C, McGale P, Taylor C, Wang Y, Clarke M, Davies C, Peto R, Bijker N, Solin L, Darby S, Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010:162–77. doi: 10.1093/jncimonographs/lgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher B, Land S, Mamounas E, Dignam J, Fisher ER, Wolmark N. Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin Oncol. 2001;28:400–18. doi: 10.1016/S0093-7754(01)90133-2. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin A, Parker S, Ghersi D, Wilcken N. Post-operative radiotherapy for ductal carcinoma in situ of the breast--a systematic review of the randomised trials. Breast. 2009;18:143–9. doi: 10.1016/j.breast.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Holmberg L, Garmo H, Granstrand B, Ringberg A, Arnesson LG, Sandelin K, Karlsson P, Anderson H, Emdin S. Absolute risk reductions for local recurrence after postoperative radiotherapy after sector resection for ductal carcinoma in situ of the breast. J Clin Oncol. 2008;26:1247–52. doi: 10.1200/JCO.2007.12.7969. [DOI] [PubMed] [Google Scholar]
- 7.Masson S, Bahl A. The management of ductal carcinoma in situ: current controversies and future directions. Clin Oncol (R Coll Radiol) 2013;25:275–82. doi: 10.1016/j.clon.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Farnie G, Clarke RB, Spence K, Pinnock N, Brennan K, Anderson NG, Bundred NJ. Novel cell culture technique for primary ductal carcinoma in situ: role of Notch and epidermal growth factor receptor signaling pathways. J Natl Cancer Inst. 2007;99:616–27. doi: 10.1093/jnci/djk133. [DOI] [PubMed] [Google Scholar]
- 9.Farnie G, Willan PM, Clarke RB, Bundred NJ. Combined inhibition of ErbB1/2 and Notch receptors effectively targets breast ductal carcinoma in situ (DCIS) stem/progenitor cell activity regardless of ErbB2 status. PLoS One. 2013;8:e56840. doi: 10.1371/journal.pone.0056840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diehn M, Clarke MF. Cancer stem cells and radiotherapy: new insights into tumor radioresistance. J Natl Cancer Inst. 2006;98:1755–7. doi: 10.1093/jnci/djj505. [DOI] [PubMed] [Google Scholar]
- 11.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10:R25. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–9. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 13.Phillips TM, McBride WH, Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–85. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 14.Lagadec C, Vlashi E, Della Donna L, Meng Y, Dekmezian C, Kim K, Pajonk F. Survival and self-renewing capacity of breast cancer initiating cells during fractionated radiation treatment. Breast Cancer Res. 2010;12:R13. doi: 10.1186/bcr2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han K, Nofech-Mozes S, Narod S, Hanna W, Vesprini D, Saskin R, Taylor C, Kong I, Paszat L, Rakovitch E. Expression of HER2neu in ductal carcinoma in situ is associated with local recurrence. Clin Oncol (R Coll Radiol) 2012;24:183–9. doi: 10.1016/j.clon.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Rakovitch E, Nofech-Mozes S, Hanna W, Narod S, Thiruchelvam D, Saskin R, Spayne J, Taylor C, Paszat L. HER2/neu and Ki-67 expression predict non-invasive recurrence following breast-conserving therapy for ductal carcinoma in situ. Br J Cancer. 2012;106:1160–5. doi: 10.1038/bjc.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korkaya H, Paulson A, Iovino F, Wicha MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27:6120–30. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magnifico A, Albano L, Campaner S, Delia D, Castiglioni F, Gasparini P, Sozzi G, Fontanella E, Menard S, Tagliabue E. Tumor-initiating cells of HER2-positive carcinoma cell lines express the highest oncoprotein levels and are sensitive to trastuzumab. Clin Cancer Res. 2009;15:2010–21. doi: 10.1158/1078-0432.CCR-08-1327. [DOI] [PubMed] [Google Scholar]
- 19.Holland PA, Knox WF, Potten CS, Howell A, Anderson E, Baildam AD, Bundred NJ. Assessment of hormone dependence of comedo ductal carcinoma in situ of the breast. J Natl Cancer Inst. 1997;89:1059–65. doi: 10.1093/jnci/89.14.1059. [DOI] [PubMed] [Google Scholar]
- 20.Gandhi A, Holland PA, Knox WF, Potten CS, Bundred NJ. Effects of a pure antiestrogen on apoptosis and proliferation within human breast ductal carcinoma in situ. Cancer Res. 2000;60:4284–8. [PubMed] [Google Scholar]
- 21.Shaw FL, Harrison H, Spence K, Ablett MP, Simões BM, Farnie G, Clarke RB. A detailed mammosphere assay protocol for the quantification of breast stem cell activity. J Mammary Gland Biol Neoplasia. 2012;17:111–7. doi: 10.1007/s10911-012-9255-3. [DOI] [PubMed] [Google Scholar]
- 22.Harrison H, Farnie G, Howell SJ, Rock RE, Stylianou S, Brennan KR, Bundred NJ, Clarke RB. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010;70:709–18. doi: 10.1158/0008-5472.CAN-09-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ablett MA, O'Brien CS, Sims AH, Farnie G, Clarke RB. A differential role for CXCR4 in the regulation of normal versus malignant breast stem cell activity. Oncotarget. 2013 doi: 10.18632/oncotarget.1169. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez EA, Reinholz MM, Hillman DW, Tenner KS, Schroeder MJ, Davidson NE, Martino S, Sledge GW, Harris LN, Gralow JR, et al. HER2 and chromosome 17 effect on patient outcome in the N9831 adjuvant trastuzumab trial. J Clin Oncol. 2010;28:4307–15. doi: 10.1200/JCO.2009.26.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paik S, Kim C, Wolmark N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med. 2008;358:1409–11. doi: 10.1056/NEJMc0801440. [DOI] [PubMed] [Google Scholar]
- 26.Decensi A, Puntoni M, Pruneri G, Guerrieri-Gonzaga A, Lazzeroni M, Serrano D, Macis D, Johansson H, Pala O, Luini A, et al. Lapatinib activity in premalignant lesions and HER-2-positive cancer of the breast in a randomized, placebo-controlled presurgical trial. Cancer Prev Res (Phila) 2011;4:1181–9. doi: 10.1158/1940-6207.CAPR-10-0337. [DOI] [PubMed] [Google Scholar]
- 27.Kuerer HM, Buzdar AU, Mittendorf EA, Esteva FJ, Lucci A, Vence LM, Radvanyi L, Meric-Bernstam F, Hunt KK, Symmans WF. Biologic and immunologic effects of preoperative trastuzumab for ductal carcinoma in situ of the breast. Cancer. 2011;117:39–47. doi: 10.1002/cncr.25399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan KC, Knox WF, Gandhi A, Slamon DJ, Potten CS, Bundred NJ. Blockade of growth factor receptors in ductal carcinoma in situ inhibits epithelial proliferation. Br J Surg. 2001;88:412–8. doi: 10.1046/j.1365-2168.2001.01686.x. [DOI] [PubMed] [Google Scholar]
- 29.Untch M, Fasching PA, Konecny GE, Hasmüller S, Lebeau A, Kreienberg R, Camara O, Müller V, du Bois A, Kühn T, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29:3351–7. doi: 10.1200/JCO.2010.31.4930. [DOI] [PubMed] [Google Scholar]
- 30.Guarneri V, Frassoldati A, Piacentini F, Jovic G, Giovannelli S, Oliva C, Conte P. Preoperative chemotherapy plus lapatinib or trastuzumab or both in HER2-positive operable breast cancer (CHERLOB Trial) Clin Breast Cancer. 2008;8:192–4. doi: 10.3816/CBC.2008.n.022. [DOI] [PubMed] [Google Scholar]
- 31.Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, Zambetti M, Vazquez F, Byakhow M, Lichinitser M, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–84. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 32.Gianni L, Dafni U, Gelber RD, Azambuja E, Muehlbauer S, Goldhirsch A, Untch M, Smith I, Baselga J, Jackisch C, et al. Herceptin Adjuvant (HERA) Trial Study Team Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12:236–44. doi: 10.1016/S1470-2045(11)70033-X. [DOI] [PubMed] [Google Scholar]
- 33.Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, Pusztai L, Green MC, Arun BK, Giordano SH, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–85. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 34.Duru N, Fan M, Candas D, Menaa C, Liu HC, Nantajit D, Wen Y, Xiao K, Eldridge A, Chromy BA, et al. HER2-associated radioresistance of breast cancer stem cells isolated from HER2-negative breast cancer cells. Clin Cancer Res. 2012;18:6634–47. doi: 10.1158/1078-0432.CCR-12-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnes NL, Khavari S, Boland GP, Cramer A, Knox WF, Bundred NJ. Absence of HER4 expression predicts recurrence of ductal carcinoma in situ of the breast. Clin Cancer Res. 2005;11:2163–8. doi: 10.1158/1078-0432.CCR-04-1633. [DOI] [PubMed] [Google Scholar]