Abstract
Ataxia-telangiectasia mutated (ATM) plays crucial roles in DNA damage responses, especially with regard to DNA double-strand breaks (DSBs). However, it appears that ATM can be activated not only by DSB, but also by some changes in chromatin architecture, suggesting potential ATM function in cell cycle control. Here, we found that ATM is involved in timely degradation of Cdt1, a critical replication licensing factor, during the unperturbed S phase. At least in certain cell types, degradation of p27Kip1 was also impaired by ATM inhibition. The novel ATM function for Cdt1 regulation was dependent on its kinase activity and NBS1. Indeed, we found that ATM is moderately phosphorylated at Ser1981 during the S phase. ATM silencing induced partial reduction in levels of Skp2, a component of SCFSkp2 ubiquitin ligase that controls Cdt1 degradation. Furthermore, Skp2 silencing resulted in Cdt1 stabilization like ATM inhibition. In addition, as reported previously, ATM silencing partially prevented Akt phosphorylation at Ser473, indicative of its activation, and Akt inhibition led to modest stabilization of Cdt1. Therefore, the ATM-Akt-SCFSkp2 pathway may partly contribute to the novel ATM function. Finally, ATM inhibition rendered cells hypersensitive to induction of re-replication, indicating importance for maintenance of genome stability.
Keywords: ATM, Cdt1, control of cell cycle progression, Akt-SCFSkp2, DNA damage and repair mechanisms
Introduction
Ataxia-telangiectasia (AT) is an autosomal recessive inherited disorder with characteristic symptoms such as cerebellar ataxia, oculocutaneous telangiectasia, and cancer predisposition. AT is caused by the mutations in the gene encoding ataxia-telangiectasia mutated (ATM) kinase, a member of the phosphoinositide 3-kinase-related protein kinase family. ATM kinase plays a pivotal role in activation of checkpoint pathways in response to DNA double-strand breaks (DSBs). When DSBs occur, ATM, together with the Mre11-RAD50-NBS1 (MRN) complex, recognizes and accumulates on lesions, where it is activated to phosphorylate many downstream effector molecules, including Chk2 kinase. Activation of the checkpoint pathway eventually leads to cell cycle arrest, repair of damage, and, under certain circumstances, apoptosis.1-3 Indeed, cell lines derived from AT patients are hypersensitive to ionizing radiation (IR).
In addition to such classical pathways, several novel cascades regulated by ATM have recently been identified. For example, when cells are exposed to hypoxic conditions, ATM is activated and phosphorylates a transcription factor, hypoxia-inducible factor 1α, to downregulate mTORC1 signaling.4 In this case, NBS1 is not required, and neither detectable DSB nor phosphorylation of ATM Ser1981, a marker for ATM activation, are observed. Surprisingly, ATM appears to be activated also by oxidative stress, probably through direct oxidization of ATM.5 In addition, oxidization-induced ATM activation appears to occur in the absence of DSBs and the MRN complex. Even for the DSB-induced ATM activation, it was shown that activated ATM relocates to the cytoplasm and links DNA damage signaling to NFκB activation.6
The above elucidated functions of ATM protein may explain the pathogenesis of AT. However, severe and pleiotrophic symptoms in the affected patients suggest the possibility that ATM might function even in unperturbed cell cycling to maintain genome integrity. It should also be noted that molecular mechanisms underlying ATM activation upon DSB induction are still not fully understood. It has been demonstrated that ATM can be activated not only by DSB, but also by changes in chromatin architecture,7 further suggesting potential ATM functions in unperturbed cell cycle.
From late mitosis to the G1 phase, the sequential assembly of multiple proteins, including ORC1–6 (origin recognition complexes 1–6), Cdc6, Cdt1, and MCM2–7 (minichromosome maintenance), results in formation of a pre-replication complex (pre-RC) that is “licensed” for replication. In the late cell cycle, while the MCM helicase is activated, activity of the pre-RC components is carefully regulated so as to prohibit inappropriate reassembly of pre-RC and subsequent re-replication.8 Cdt1 strongly stimulates the licensing reaction in human cells,9-11 and its activity is tightly restricted by multiple mechanisms during the S phase, i.e., polyubiquitination-dependent proteolysis mediated by Cdk phosphorylation-dependent SCFSkp2 ubiquitin ligase and the proliferating cell nuclear antigen (PCNA)-dependent Cul4-DDB1Cdt2 ubiquitin ligase and inhibitory geminin binding.8 Overexpression of Cdt1, ORC1, or Cdc6 alone induces no detectable re-replication in normal human cells, but co-overexpression of Cdt1 plus ORC1 or Cdc6 yields a moderate level of re-replication.11 Cdt1 mutants deficient in S-phase degradation feature more re-replication than the wild type.11 In certain cancer-derived cells, Cdt1 overexpression alone can induce overt re-replication.9-11 Under such circumstances, ATM- and Rad3-related (ATR) kinase, a close relative of ATM, and the MRN complex inhibit further re-replication.12 All of these findings clearly show that degradation of Cdt1 during S phase is critical for maintenance of genomic stability. Actually, deregulation of Cdt1 is harmful to genomic stability.13
Here, we sought to clarify potential novel ATM functions during the unperturbed cell cycle and found that ATM is required for proper degradation of Cdt1 during the S phase.
Results
ATM is required for proper degradation of Cdt1 during the unperturbed S phase
We established T98G cells stably expressing shRNA targeting ATM (referred to as T98G-ATMRi2) or luciferase as a control and investigated the effects of ATM inhibition on kinetics of various cell cycle- and DNA replication-related proteins during the cell cycle (Fig. 1A). In T98G–ATMRi2 cells, expression of ATM protein was reduced by ~90% (Fig. 1A), but cell cycle progression was delayed only slightly as compared with the control (Fig. 1B). We found cell cycle-dependent regulation of Cdt1 protein to be impaired by ATM inhibition. Thus, in control cells, Cdt1 protein levels were low in G0, accumulated by 8 h after serum stimulation, and decreased remarkably after 13 h (Fig. 1A), coincident with the start of the S phase (Fig. 1B). This pattern of Cdt1 regulation is in agreement with previous findings.14 In contrast, Cdt1 levels in ATM-silenced cells remained constant (Fig. 1A) in spite of the almost unimpeded S phase progression (Fig. 1B). Interestingly, downregulation of p27Kip1 during the S phase was also impaired by ATM inhibition (Fig. 1A). The kinetics of cyclin A, Cdc6, ORC1, cyclin D1, and p21WAF1 protein levels were unaffected (Fig. 1A). In addition, phosphorylation of Cdc6 at Ser54 by cyclin E/Cdk2 and cyclin A/Cdk2 occurred with similar kinetics (Fig. 1A), suggesting both kinases were properly activated in ATM-silenced cells. Essentially the same results were obtained with another shRNA targeting ATM (ATMRi4) (Fig. S1).
Figure 1. ATM is moderately auto-phosphorylated at Ser1981 in a cell cycle-dependent manner and required for appropriate degradation of Cdt1 and p27Kip1 during the unperturbed S phase in T98G cells. (A and B) T98G cells expressing an shRNA targeting ATM (ATMRi2) or a control shRNA (LuciRi) were arrested in G0 by serum starvation and then released into the cell cycle by serum addition. Cells were harvested at the indicated times and subjected to immunoblotting analysis (A) and flow cytometry (B). The percentage of cells in G0/G1, S, and G2/M is shown by filled circle, open circle and filled triangle, respectively. (C) Cdt1 and p27Kip1 is stabilized in ATM-silenced T98G cells. Cycloheximide (50 μg/ml) was added at 10 h after serum stimulation, and then the cells were harvested at the indicated times. (D) ATM silencing does not affect Cdt1 mRNA levels. Total RNAs were prepared from cells treated as in (A), and the levels of Cdt1 mRNA were determined by quantitative RT-PCR. (E) Phosphorylation of ATM at Ser1981 during the S phase is moderately induced compared with that induced in response to DNA damage. T98G-LuciRi cells were exposed to 5 Gy IR and harvested 30 min later (IR+) and subjected to immunoblotting analysis with protein lysates shown in (A). The data were obtained by short exposure compared with the data shown in (A).
To determine whether ATM inhibition affect the stability of the Cdt1 and p27Kip1 proteins, cycloheximide (CHX) was added to the ATMRi2 and control T98G cells at 10 h after serum stimulation and the protein levels were monitored (Fig. 1C). The data revealed that ATM inhibition stabilizes Cdt1 and p27Kip1 proteins. We also found that ATM inhibition does not affect Cdt1 mRNA levels (Fig. 1D). Taken together, these results strongly indicate that ATM inhibition impedes the timely degradation of Cdt1 and p27Kip1 proteins during the S phase in T98G cells.
ATM deficiency increases production of ROS (reactive oxygen species) in several cell types.15,16 To ascertain whether Cdt1 deregulation by ATM inhibition is associated with aberrant ROS production, the levels were examined. In T98G cells, the ROS levels were not changed by ATM inhibition (Fig. S2A). In addition, treatment with antioxidant N-acetyl L-cysteine (NAC) did not prevent the inhibition of Cdt1 and p27Kip1 degradation, in spite of efficient reduction of intracellular ROS (Fig. S2). Therefore, ROS is presumably not involved in the observed phenomena.
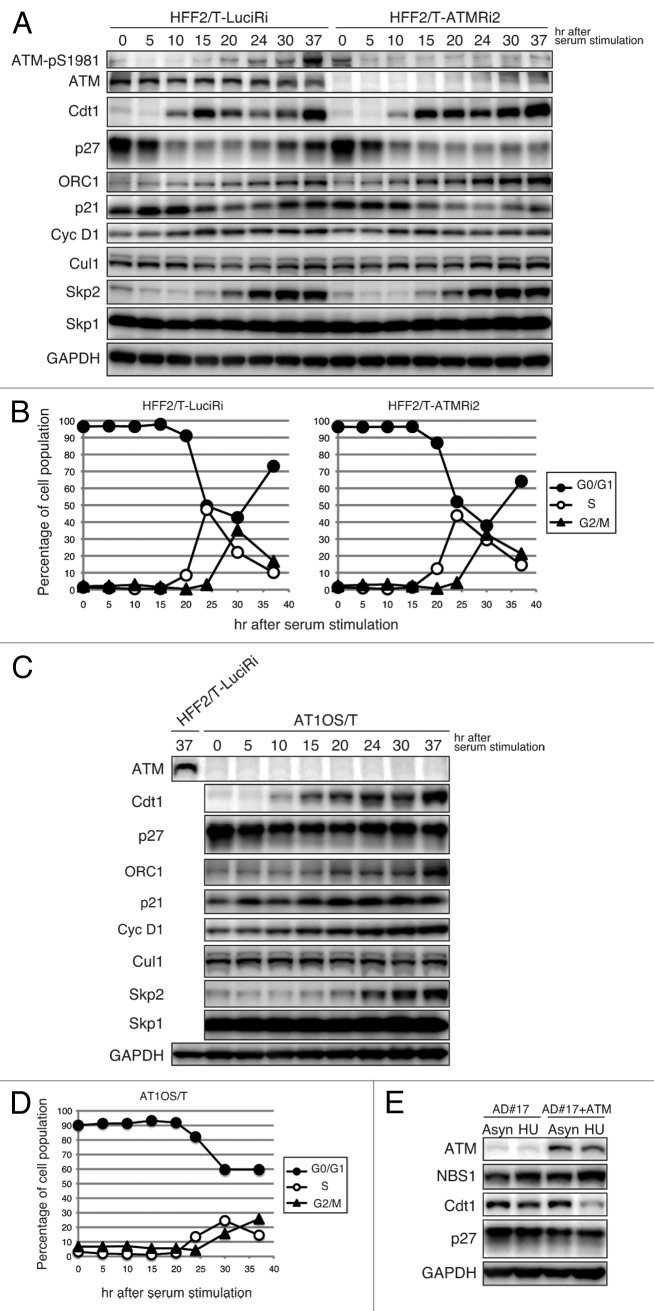
Using human foreskin fibroblasts immortalized with telomerase (HFF2/T), we further confirmed the novel function of ATM. Similar to T98G cells, whereas Cdt1 proteins were accumulated after serum stimulation and then decreased significantly upon entry into S phase in control cells, ATM silencing led to accumulation during S phase (Fig. 2A and B). On the other hand, the kinetics of p27Kip1 protein levels were almost the same in control and ATM-silenced cells (Fig. 2A and B). In addition, we also found that Cdt1 and p27Kip1 proteins remain abundant during the S phase in ATM-deficient AT1OS/T cells (Fig. 2C and D). Furthermore, we examined a set of other AT-derived fibroblasts (AD#17), and their derivatives reconstituted with ATM (AD#17+ATM) for Cdt1 and p27Kip1 protein levels upon synchronization in S phase by hydroxyurea (HU) treatment. In AD#17 cells, Cdt1 levels remained high upon HU treatment (Fig. 2E). The inappropriate accumulation of Cdt1 in S phase was abrogated by ATM add-back (Fig. 2E). HU treatment primarily blocks DNA replication fork progression and activates the ATR-Chk1 pathway. After the prolonged fork block, DSB is induced and the ATM-Chk2 pathway is activated. As mentioned below, Cdt1 is rapidly degraded upon DNA damage, which is mediated by PCNA-Cul4-DDB1Cdt2 ubiquitin ligase but independent of ATM (ref. 17; Fig. S3). Because Cdt1 was not degraded in HU-treated AD#17 cells (Fig. 2E), abundant DSB may not be induced under the experimental conditions. Thus, the results of AD#17 and AD#17+TM cells are consistent with the notion that degradation of Cdt1 during S phase is dependent on ATM. On the other hand, the protein levels of p27Kip1 did not change on HU treatment in either AD#17 or AD#17+ATM cells.
Figure 2. Involvement of ATM in Cdt1 regulation during the unperturbed S phase in human fibroblasts. (A and B) ATM is moderately auto-phosphorylated at Ser1981 in a cell cycle-dependent manner, and its silencing impairs Cdt1 downregulation during the S phase in HFF2/T cells. Cells expressing an ATMRi2 shRNA or a control shRNA (LuciRi) were synchronized and analyzed as in Figure 1A and B. (C and D) Cdt1 downregulation during the S phase is compromised in AT1OS/T, AT-derived fibroblasts. Cells were synchronized and analyzed as above. (E) Cdt1 deregulation during the S phase in AT-derived cells is alleviated by re-introduction of ATM. AD#17, another AT-derived fibroblast line, and its derivative reconstituted with ATM (AD#17+ATM), were treated with 2.5 mM hydroxyurea for synchronization in S phase (HU) or left untreated (Asyn) for 24 h and then analyzed by immunoblotting.
Taken together, these data demonstrate that ATM protein is required for the proper degradation of Cdt1 during the S phase. At least in certain cell types, it may also be involved in degradation of p27Kip1 during the S phase.
ATM kinase activity and NBS1 are required for the ATM-mediated Cdt1 regulation
We examined whether ATM could be activated during the S phase using ATM Ser1981 phosphorylation as an index,7 and found that ATM Ser1981 is significantly phosphorylated during the S phase in T98G cells (Fig. 1A; Fig. S1), although the levels were low compared with those induced by 5 Gy IR (Fig. 1E). ATM phosphorylation at Ser1981 during the S phase was also observed in HFF2/T cells (Fig. 2A).
Given that ATM is activated during the S phase, we examined whether ATM kinase activity is involved in the Cdt1 regulation. To this end, quiescent T98G cells were released into the cell cycle by serum in the presence of an ATM-specific kinase inhibitor, KU-55933. KU-55933 treatment resulted in prevention of Cdt1 downregulation during the S phase, with inhibition of ATM Ser1981 phosphorylation (Fig. 3A and B). In contrast, p27Kip1 protein was only slightly affected by the treatment. We also examined possible involvement of ATR in the Cdt1 regulation using an ATR specific kinase inhibitor, VE-821.18 However, VE-821 did not affect Cdt1 downregulation (Fig. S4).

Figure 3. ATM kinase activity and NBS1 are required for Cdt1 regulation during the unperturbed S phase. (A and B) Inhibition of ATM kinase activity with KU-55933 impairs Cdt1 downregulation during the S phase in T98G cells. Cells were serum starved, pre-treated with 10 μM KU-55933, and then reentered into the cell cycle. Cells were harvested at the indicated times and subjected to immunoblotting (A) and flow cytometry (B). (C and D) NBS1 silencing impairs Cdt1 downregulation during the S phase in T98G cells. Cells were transfected with NBS1 or control siRNAs, synchronized, and harvested at the indicated times after reentry into the cell cycle for immunoblotting (C) and flow cytometry (D). (E) Cdt1 deregulation during the S phase in NBS-derived cells is alleviated by re-introduction of NBS1. NBS1-deficient cells (GM) and the reconstituted cells with NBS1 (GM+NBS1) were treated with 2.5 mM HU for synchronization in S phase (HU) or left untreated (Asyn) for 11 h and then analyzed by immunoblotting.
The MRN complex is required for ATM activation.19 To investigate its requirement for ATM-dependent regulation of Cdt1, T98G cells were transfected with NBS1 siRNA and then synchronized. As shown in Figure 3C and D, decrease in Cdt1 protein levels during the S phase was significantly suppressed in NBS1-silenced cells. In contrast, the kinetics of p27Kip1 were similar in both control and NBS1-silenced cells. We also examined Cdt1 and p27Kip1 protein levels upon S-phase synchronization by HU treatment in NBS1-deficient cells (GM7166) and their derivatives reconstituted with NBS1 (GM7166+NBS1). Cdt1 protein levels decreased significantly with HU treatment in GM7166+NBS1 cells, whereas they were unchanged in GM7166 (Fig. 3E). In contrast, the protein levels of p27Kip1 in GM7166+NBS1 cells were very low as compared with parental GM7166 cells, irrespective of HU treatment. The reason for the unexpected response of p27Kip1 to NBS1 is not clear at present.
Taken together, these observations demonstrate that kinase activity of ATM and NBS1 are required for proper Cdt1 degradation during the S phase. On the other hand, it seems likely that the effects of ATM pathways on the regulation of p27Kip1 during the S phase are more complex and diverse, depending on the cell types.
ATM is involved in the regulation of Skp2 as a critical mediator of Cdt1 degradation in T98G cells
As mentioned above, degradation of Cdt1 during the S phase is mediated by Cdk-SCFSkp2 and PCNA-Cul4-DDB1Cdt2 ubiquitin ligases, whereas degradation of Cdt1 after DNA damage is mediated by PCNA-Cul4-DDB1Cdt2.8,17,20,21 The extent of dependence on the either degradation machinery during the S phase appears different among cell types.11,21,22
Consistent with a previous report,17 IR- and UV-mediated Cdt1 proteolysis proved independent of ATM (Fig. S3). In addition, cell cycle regulation of p21WAF1, another target of Cul4-DDB1Cdt2 ubiquitin ligase, appeared unaffected by ATM inhibition (Figs. 1A and 2A; Fig. S1). Furthermore, p27Kip1 is also regulated by SCFSkp2.23 Therefore, ATM might affect the SCFSkp2-mediated pathway. It was reported that ATM mediates full activation of Akt in response to insulin or DNA damage,24-27 and several reports have demonstrated that Akt may regulate Skp2 function at both transcriptional and posttranscriptional levels.28-31 Therefore, we investigated whether ATM inhibition might affect SCFSkp2 functions.
Levels of Cul1 and Skp1, 2 components of SCFSkp2, remained constant throughout the cell cycle in T98G cells, which were not affected by ATM inhibition (Fig. 1A; Fig. S1). The Skp2 protein level is controlled by APC/CCdh1-mediated proteolysis during the G1 phase.32,33 Consistent with this, Skp2 levels were cell cycle-regulated in T98G cells, with increase in S phase (Figs. 1A and 3A; Fig. S1). Interestingly, accumulation of Skp2 during the S phase was partially delayed and inhibited by ATM silencing in T98G cells (Fig. 4A and B, see also Fig. 1A; Fig. S1 for some of the original data used for quantification). Similar findings were also obtained with KU-55933 (Fig. 4C, see also Fig. 3A as one of the original data used for quantification). Moreover, Skp2 level during the S phase in NBS1-deficient cells was lower than that in NBS1-reconstituted cells (Fig. 3E).
Figure 4. ATM is involved in the regulation of Skp2 levels during the unperturbed S phase in T98G cells. (A–C) Either ATM depletion or inhibition of its kinase activity leads to decrease in Skp2. The signal intensities of the Skp2 proteins in cells treated as in Figure 1A, Figure S1A, or Figure 3A were quantified and normalized to the signals for GAPDH. (A) Data for T98G-ATMRi2 cells. (B) Data for T98G-ATMRi4 cells. For (A and B), the graphs show values for Skp2 relative to the level in T98G-LuciRi cells at 0 h. (C) Data for KU-55933-treated cells. The graph shows values for Skp2 relative to the level in DMSO-treated cells at 10 h after serum stimulation. The black and gray bars indicate independent experiments No. 1 and No. 2, respectively. (D and E) Skp2 depletion upregulates Cdt1 and p27Kip1 during the S phase in T98G cells. Cells were transfected with Skp2 or control siRNAs, synchronized, released into the cell cycle and analyzed as in Figure 1A and B. (F) Co-depletion of Skp2 does not further stabilize Cdt1 during the S phase in ATM-silenced T98G cells. T98G-ATMRi2 cells were transfected with Skp2 or control siRNAs, synchronized, released into the cell cycle, and analyzed as in (D). In these experiments, S-phase progression was monitored by accumulation of cyclin A.
Given that Cdt1 deregulation by ATM suppression may be attributable to, at least partly, decrease in Skp2 levels, we examined whether Skp2 silencing affects Cdt1 degradation during the S phase in T98G cells (Fig. 4D). As expected, Cdt1 protein levels remained constant during the S phase in Skp2-silenced T98G cells (Fig. 4D). Thus, although Cdt1 degradation is controlled by 2 ubiquitin ligases, SCFSkp2 and Cul4-DDB1Cdt2, the former may be predominant in T98G cells. In Skp2-silenced cells, p27Kip1 increased quite remarkably throughout the cell cycle (Fig. 4D), suggesting its strong dependence on the Skp2 protein. Therefore, in certain cell types, such as T98G, partial decrease in Skp2 levels by ATM silencing may lead to p27Kip1 accumulation during S phase. Cyclin D1 and p21WAF1 are also substrates of SCFSkp2,23 although also regulated by other ubiquitin ligases, and actually their degradation was prevented by Skp2 silencing (Fig. 4D). However, neither cyclin D1 nor p21WAF1 protein levels were changed by ATM silencing (Fig. 1A; Fig. S1). It is possible that partial decrease in Skp2 by ATM silencing may be insufficient for significant stabilization of these proteins. We also examined whether co-silencing of Skp2 further stabilizes Cdt1 during the S phase in ATM-silenced cells. Simultaneous depletion of Skp2 did not further stabilize Cdt1 in T98G-ATMRi2 cells (Fig. 4F), in line with the notion that Cdt1 stabilization by ATM inhibition is attributed to Skp2 inhibition. Finally, we investigated whether ATM silencing affects the binding of Skp2 to Skp1 and Cul1 or the intracellular localization of Skp2. However, no significant findings were obtained (Fig. S5). Taken together, these results suggest that ATM affects Cdt1 degradation through, at least partly, maintaining Skp2 levels during the S phase in T98G cells.
ATM inhibition partially affects the Akt-SCFSkp2 pathway in T98G cells
Since previous reports indicated possible interplay between ATM and Akt,24-27 we next examined whether ATM function is associated with Akt activation during the cell cycle. When quiescent T98G-LuciRi cells were stimulated with serum, phosphorylation of Akt at Ser473, indicative of Akt activation,34 was increased and then decreased gradually at 13 h after serum addition (Fig. 5A and B). When ATM was silenced with ATMRi2 or ATMRi4, such Akt phosphorylation was partially suppressed (Fig. 5A and B). Similar phenomena were also observed in KU-55933-treated cells (Fig. 5C). A previous report showed physical interaction between ATM and Akt.24 We also found that Akt can bind to ATM fragments fused to GST (data not shown).

Figure 5A–C. ATM inhibition partially affects the Akt-SCFSkp2 pathway in T98G cells. (A–C) ATM silencing and inhibition of its kinase activity reduce Akt phosphorylation at Ser473. The cell lysates prepared as in Figure 1A, Figure S1A, and Figure 3A, respectively, were subjected to immunoblotting with pan-Akt and Ser473-phosphorylated Akt antibodies. The signal intensities of total Akt and the phosphorylated Akt were quantified, and the ratio of the phosphorylated Akt to total Akt was calculated. The graphs in (A and B) show values relative to that for T98G-LuciRi cells at 10 h after serum stimulation set at 1. The graph in (C) shows values relative to that of DMSO-treated cells at 10 h after serum stimulation set at 1.
We then investigated whether Akt silencing indeed affects Cdt1 degradation during S phase. Consistent with previous findings,28-30,35 silencing of either Akt1, Akt2, or both, strongly decreased the protein level of Skp2 (Fig. 5D). In further agreement with previous reports,28,31 we also observed physical interaction between Akt and Skp2 in co-immunoprecipitation assays and Skp2 upregulation in Akt-overexpressed cells (data not shown). As expected from the Skp2 reduction, p27Kip1 levels were remarkably increased in Akt-silenced cells (Fig. 5D). Unexpectedly, however, Akt silencing only modestly stabilized Cdt1 (Fig. 5D). The reason is not clear at present but some compensatory pathway(s) might be activated upon Akt depletion. In the study using siRNA-mediated Akt silencing and subsequent cell cycle synchronization, Akt function during G1 progression is inevitably affected. To investigate the effect of Akt inhibition on Cdt1 regulation specifically in S phase, we utilized an Akt1/2 specific inhibitor, Akti-1/2.36 Treatment of Akti-1/2 resulted in prevention of Cdt1 downregulation during the S phase, with inhibition of Akt Ser473 phosphorylation (Fig. 5F). Unexpectedly, however, the protein level of Skp2 was not changed. It is possible that the function of Skp2 rather than the protein level is impaired under these experimental conditions. Moreover, we examined the effect of Akt co-inhibition on Cdt1 stabilization during the S phase in ATM-silenced T98G cells. Inhibition of Akt with Akti-1/2 did not further stabilize Cdt1 in T98G-ATMRi2 cells (Fig. 5G). Together, these observations suggest that ATM inhibition may at least partially affect the Akt-SCFSkp2–Cdt1 pathway.
Figure 5D–G. ATM inhibition partially affects the Akt-SCFSkp2 pathway in T98G cells. (D and E) Akt1 or Akt2 depletion affects Skp2-mediated Cdt1 and p27Kip1 degradation in T98G cells. Cells were transfected with siRNAs targeting Akt1, Akt2, or both, or control siRNA (si-GL2), serum starved, and then stimulated by serum. Cells were harvested at the indicated times for immunoblotting (D) and flow cytometry (E). (D) The signal intensities of the Cdt1 proteins were quantified and normalized to the signals for GAPDH. The graph shows the values relative to Cdt1 levels in control siRNA-treated cells at 10 h after serum stimulation. (F) Inhibition of Akt kinase activity with Akti-1/2 impairs Cdt1 downregulation during the S phase in T98G cells. Cells were treated with 10 μM Akti-1/2 at 9 h after cell cycle entry from G0, and then harvested at the indicated times. In these experiments, S-phase progression was monitored by accumulation of cyclin A. (G) Co-inhibition of Akt with Akti-1/2 does not further stabilize Cdt1 during the S phase in ATM-silenced T98G cells. T98G-ATMRi2 cells were treated and analyzed as above.
ATM inhibition renders cells hypersensitive to induction of re-replication
Cdt1 deregulation leads to chromosomal damage, including re-replication.9-13,37 We therefore examined whether the stabilization of Cdt1 by ATM silencing leads to re-replication. Flow cytometry analyses revealed that re-replicated population (defined by a DNA content higher than 4N) in AT cells is 1.5-fold that in ATM-complemented cells (Fig. 6A and B). If increase in re-replication in ATM-deficient cells is attributable to Cdt1 upregulation during the S phase, then it would be enhanced by co-overexpression of Cdc6.9,11 Therefore, we overexpressed Cdc6 in T98G-ATMRi2 and control T98G-LuciRi cells. Even in an asynchronous population, increase in Cdt1 levels was detectable in ATMRi2 cells, and Cdc6 overexpression was confirmed in both cells (Fig. 6C). The induction of re-replication in ATMRi2 cells was similar to that in control cells. However, Cdc6 overexpression enhanced re-replication induction only in T98G-ATMRi2 cells (Fig. 6D). Thus, ATM deficiency rendered cells hypersensitive to induction of re-replication, indicating the importance of the novel ATM function in maintenance of genome stability.
Figure 6. ATM inhibition renders cells hypersensitive to induction of re-replication. (A and B) The AT-derived AD#17, and its derivative reconstituted with ATM (AD#17+ATM), were subjected to flow cytometry. (A) In these diagrams, the x-axis is FL2-A representing whole PI signals and thus DNA content, and the y-axis is FL2-W representing duration of PI signals. Dots with higher FL2-W signals that result from aggregated cells or cell debris were excluded from measurement of rereplicated cells. (B) The means and SDs of the percentages of rereplicated cells (DNA content higher than 4N) from 3 independent experiments are shown. (C and D) T98G-LuciRi and ATM-silenced T98G-ATMRi2 cells were infected with retroviruses expressing 2HA-Cdc6 or with control retroviruses, and selected with puromycin (1 μg/ml). At 4 d after infection, cells were subjected to immunoblotting (C) and flow cytometry (D). In (D), percentages of rereplicated cells are shown.
Discussion
ATM is involved in appropriate degradation of Cdt1 during the unperturbed S phase
An ATM requirement for timely degradation of Cdt1 during the S phase was observed in several different systems, i.e., T98G, HFF2/T, and 2 different AT patient-derived cells (Figs. 1 and 2; Fig. S1). This ATM function requires its kinase activity and NBS1 (Fig. 3). Indeed, ATM activation indicated by Ser1981 phosphorylation is detected during the S phase both in T98G and HFF2/T cells (Figs. 1A and 2A; Fig. S1A). However, the mechanisms by which ATM is moderately activated during the S phase remain to be clarified. There are several non-exclusive possibilities. One is that the replication process affects the chromatin architecture, triggering ATM activation. Alternatively, replication forks accidentally encounter endogenously generated chromatin stresses, leading to transient DSB induction. At telomeres, ATM activation is generally suppressed. However, ATM may be appropriately activated during the S phase to maintain telomere structure.38-40 It was previously reported that MRN is required for suppression of re-replication induced by overexpression of Cdt1, and this mechanism is mediated by ATR.12 However, ATR is not required for Cdt1 regulation during the S phase (Fig. S4).
ATM functions to prohibit re-replication
Cdt1 deregulation induces re-replication and/or chromosomal damage, leading to chromosomal instability.9-11,13,37 In non-transformed cells, although Cdt1 overexpression induces only small amounts of re-replication, co-expression of ORC1 or Cdc6 robustly enhances re-replication.11 Therefore, it is conceivable that undesired Cdt1 upregulation in ATM-inhibited cells may cause deleterious insult. Actually, the re-replicated cell population is significantly increased in an AT-derived cell line, which could be corrected by ATM reintroduction (Fig. 6A and B). More importantly, Cdc6 overexpression enhanced re-replication in ATM-silenced T98G cells (Fig. 6C and D), indicating that Cdt1 upregulation by ATM inhibition in fact predisposes cells to re-replication. Thus, the novel function of ATM described here may have a critical role in genome maintenance.
Signaling pathways from ATM to Cdt1
Whereas it seems clear that ATM participates in Cdt1 regulation, the involved signaling pathways appear complicated and are not fully understood. It seems likely that multiple parallel pathways contribute to the novel ATM function.
At least in T98G cells, the ATM-Akt-SCFSkp2 axis may be one pathway. The evidence includes: (1) ATM inhibition, at least partially, downregulates Skp2 protein levels (Fig. 4A–C); (2) Skp2 silencing stabilizes Cdt1 during the S phase (Fig. 4D); (3) ATM inhibition moderately inhibits Akt phosphorylation (Fig. 5A–C); (4) Akt silencing decreases the Skp2 protein levels (Fig. 5D), and, conversely, Akt overexpression increases (data not shown; refs. 28–30, and 35); and (5) Akt inhibition stabilizes Cdt1 during the S phase (Fig. 5F). In addition, in agreement with previous data,24,28,31 (6) we also found ATM–Akt and Akt–Skp2 physical interactions (data not shown). This notion is also supported by the fact that co-silencing of Skp2 or co-inhibition of Akt in ATM-silenced T98G cells does not induce further stabilization of Cdt1 (Figs. 4F and 5G). However, even in T98G cells, there are some apparently contradictory findings; e.g., in spite of significant suppression of Skp2 protein levels in Akt-silenced cells, Cdt1 is only partially stabilized during the S phase (Fig. 5D), and neither cyclin D1 nor p21WAF1 protein levels, regulated by SCFSkp2,23 are changed by ATM silencing (Fig. 1A; Fig. S1). Manipulation of one pathway could activate compensatory parallel pathways in some cases. Nevertheless, these findings appears in line with previous reports showing that ATM mediates full activation of Akt in response to insulin or DNA damage,24-27 and that Akt may regulate Skp2 at both transcriptional and posttranscriptional levels.28-31 It is also reported that Mre11 promotes Akt phosphorylation in response to DNA DSBs.41 Unfortunately, to date, it remains completely unclear how ATM regulates Akt. A biological relevance of Akt-mediated Skp2 regulation has been proposed, but discussion continues.28-31,42-44 It is possible that Akt regulation of Skp2 is cell type- and/or context-dependent.
In other cell systems, the pathway by which ATM regulates Cdt1 is more obscure and remains to be determined. For instance, in HFF2/T cells, ATM silencing upregulates the Cdt1 levels during the S phase without reducing the Skp2 protein levels (Fig. 2A). In this case, ATM might affect Skp2 protein function(s) rather than the levels.
ATM involvement in p27Kip1 regulation during the unperturbed S phase
p27Kip1 is an important Cdk inhibitor that is elaborately regulated during the cell cycle and cell differentiation. This is executed at multiple levels and in a cell type- and/or context-dependent manner.45,46 For example, at least 4 ubiquitin ligases control the stability. SCFSkp2 is one representative ubiquitin ligase that controls p27Kip1 dependent on Cdk phosphorylation. We found here that, at least in T98G cells, ATM participates in regulation of p27Kip1 stability during the S phase (Fig. 1). In this context, the ATM-Akt-SCFSkp2 axis may play a role (Figs. 4, 5A–C, and 5D–G), as well as in the regulation of Cdt1, with difference of a low requirement of ATM kinase activity and NBS1. However, in other cell types, ATM does not appear to affect p27Kip1 protein levels (Fig. 2). This may be due to complexities in p27Kip1 regulations. In addition, the pathophysiological relevance of inappropriate p27Kip1 upregulation in some ATM-deficient cells remains to be elucidated in the future.
Materials and Methods
Cells
Cells used are described in Supplementary Materials and Methods. Cells were grown in Dulbecco modified Eagle medium (DMEM) with 8% fetal calf serum. T98G cells were synchronized in quiescence by serum starvation for 48 h, followed by reentry into the cell cycle by incubation in DMEM with 15% serum. Synchronization of HFF2/T and AT1OS/T cells into quiescence was achieved by contact inhibition and sequential serum starvation. The inhibitors used are detailed in Supplementary Materials and Methods.
Transfection
T98G cells (9 × 104 /well in 6-well plates) were transfected with 40 pmol of siRNA using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer’s protocol. The sequences of siRNAs are described in Supplementary Materials and Methods.
FACS
Cells were stained with propidium iodide (PI), and analyzed with a Becton Dickinson FACS Calibur.
Details of other materials and methods are described in Supplementary Materials and Methods.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Kum Kum Khanna (Queensland Institute of Medical Research, Australia) for plasmids of GST-truncated ATM. We appreciate for the technical supports from the Research Support Center, Graduate School of Medical Sciences, Kyushu University.
Financial support
Ministry of Education, Culture, Sports, Science and Technology of Japan (17080013 and 24650622) for M Fujita.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/27274
References
- 1.Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3:421–9. doi: 10.1016/S1535-6108(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 2.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–23. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 3.Nyberg KA, Michelson RJ, Putnam CW, Weinert TA. Toward maintaining the genome: DNA damage and replication checkpoints. Annu Rev Genet. 2002;36:617–56. doi: 10.1146/annurev.genet.36.060402.113540. [DOI] [PubMed] [Google Scholar]
- 4.Cam H, Easton JB, High A, Houghton PJ. mTORC1 signaling under hypoxic conditions is controlled by ATM-dependent phosphorylation of HIF-1α. Mol Cell. 2010;40:509–20. doi: 10.1016/j.molcel.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330:517–21. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 6.Hinz M, Stilmann M, Arslan SC, Khanna KK, Dittmar G, Scheidereit C. A cytoplasmic ATM-TRAF6-cIAP1 module links nuclear DNA damage signaling to ubiquitin-mediated NFκB activation. Mol Cell. 2010;40:63–74. doi: 10.1016/j.molcel.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 8.Fujita M. Cdt1 revisited: complex and tight regulation during the cell cycle and consequences of deregulation in mammalian cells. Cell Div. 2006;1:22. doi: 10.1186/1747-1028-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaziri C, Saxena S, Jeon Y, Lee C, Murata K, Machida Y, Wagle N, Hwang DS, Dutta A. A p53-dependent checkpoint pathway prevents rereplication. Mol Cell. 2003;11:997–1008. doi: 10.1016/S1097-2765(03)00099-6. [DOI] [PubMed] [Google Scholar]
- 10.Sugimoto N, Kitabayashi I, Osano S, Tatsumi Y, Yugawa T, Narisawa-Saito M, Matsukage A, Kiyono T, Fujita M. Identification of novel human Cdt1-binding proteins by a proteomics approach: proteolytic regulation by APC/CCdh1. Mol Biol Cell. 2008;19:1007–21. doi: 10.1091/mbc.E07-09-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugimoto N, Yoshida K, Tatsumi Y, Yugawa T, Narisawa-Saito M, Waga S, Kiyono T, Fujita M. Redundant and differential regulation of multiple licensing factors ensures prevention of re-replication in normal human cells. J Cell Sci. 2009;122:1184–91. doi: 10.1242/jcs.041889. [DOI] [PubMed] [Google Scholar]
- 12.Lee AY, Liu E, Wu X. The Mre11/Rad50/Nbs1 complex plays an important role in the prevention of DNA rereplication in mammalian cells. J Biol Chem. 2007;282:32243–55. doi: 10.1074/jbc.M705486200. [DOI] [PubMed] [Google Scholar]
- 13.Tatsumi Y, Sugimoto N, Yugawa T, Narisawa-Saito M, Kiyono T, Fujita M. Deregulation of Cdt1 induces chromosomal damage without rereplication and leads to chromosomal instability. J Cell Sci. 2006;119:3128–40. doi: 10.1242/jcs.03031. [DOI] [PubMed] [Google Scholar]
- 14.Nishitani H, Taraviras S, Lygerou Z, Nishimoto T. The human licensing factor for DNA replication Cdt1 accumulates in G1 and is destabilized after initiation of S-phase. J Biol Chem. 2001;276:44905–11. doi: 10.1074/jbc.M105406200. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Wong PK. Oxidative stress is linked to ERK1/2-p16 signaling-mediated growth defect in ATM-deficient astrocytes. J Biol Chem. 2009;284:14396–404. doi: 10.1074/jbc.M808116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito K, Takubo K, Arai F, Satoh H, Matsuoka S, Ohmura M, Naka K, Azuma M, Miyamoto K, Hosokawa K, et al. Regulation of reactive oxygen species by Atm is essential for proper response to DNA double-strand breaks in lymphocytes. J Immunol. 2007;178:103–10. doi: 10.4049/jimmunol.178.1.103. [DOI] [PubMed] [Google Scholar]
- 17.Higa LA, Mihaylov IS, Banks DP, Zheng J, Zhang H. Radiation-mediated proteolysis of CDT1 by CUL4-ROC1 and CSN complexes constitutes a new checkpoint. Nat Cell Biol. 2003;5:1008–15. doi: 10.1038/ncb1061. [DOI] [PubMed] [Google Scholar]
- 18.Reaper PM, Griffiths MR, Long JM, Charrier JD, Maccormick S, Charlton PA, Golec JM, Pollard JR. Selective killing of ATM- or p53-deficient cancer cells through inhibition of ATR. Nat Chem Biol. 2011;7:428–30. doi: 10.1038/nchembio.573. [DOI] [PubMed] [Google Scholar]
- 19.Uziel T, Lerenthal Y, Moyal L, Andegeko Y, Mittelman L, Shiloh Y. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J. 2003;22:5612–21. doi: 10.1093/emboj/cdg541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu J, McCall CM, Ohta T, Xiong Y. Targeted ubiquitination of CDT1 by the DDB1-CUL4A-ROC1 ligase in response to DNA damage. Nat Cell Biol. 2004;6:1003–9. doi: 10.1038/ncb1172. [DOI] [PubMed] [Google Scholar]
- 21.Nishitani H, Sugimoto N, Roukos V, Nakanishi Y, Saijo M, Obuse C, Tsurimoto T, Nakayama KI, Nakayama K, Fujita M, et al. Two E3 ubiquitin ligases, SCF-Skp2 and DDB1-Cul4, target human Cdt1 for proteolysis. EMBO J. 2006;25:1126–36. doi: 10.1038/sj.emboj.7601002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arias EE, Walter JC. PCNA functions as a molecular platform to trigger Cdt1 destruction and prevent re-replication. Nat Cell Biol. 2006;8:84–90. doi: 10.1038/ncb1346. [DOI] [PubMed] [Google Scholar]
- 23.Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer. 2008;8:438–49. doi: 10.1038/nrc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viniegra JG, Martínez N, Modirassari P, Hernández Losa J, Parada Cobo C, Sánchez-Arévalo Lobo VJ, Aceves Luquero CI, Alvarez-Vallina L, Ramón y Cajal S, Rojas JM, et al. Full activation of PKB/Akt in response to insulin or ionizing radiation is mediated through ATM. J Biol Chem. 2005;280:4029–36. doi: 10.1074/jbc.M410344200. [DOI] [PubMed] [Google Scholar]
- 25.Khalil A, Morgan RN, Adams BR, Golding SE, Dever SM, Rosenberg E, Povirk LF, Valerie K. ATM-dependent ERK signaling via AKT in response to DNA double-strand breaks. Cell Cycle. 2011;10:481–91. doi: 10.4161/cc.10.3.14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golding SE, Rosenberg E, Valerie N, Hussaini I, Frigerio M, Cockcroft XF, Chong WY, Hummersone M, Rigoreau L, Menear KA, et al. Improved ATM kinase inhibitor KU-60019 radiosensitizes glioma cells, compromises insulin, AKT and ERK prosurvival signaling, and inhibits migration and invasion. Mol Cancer Ther. 2009;8:2894–902. doi: 10.1158/1535-7163.MCT-09-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Yang DQ. The ATM inhibitor KU-55933 suppresses cell proliferation and induces apoptosis by blocking Akt in cancer cells with overactivated Akt. Mol Cancer Ther. 2010;9:113–25. doi: 10.1158/1535-7163.MCT-08-1189. [DOI] [PubMed] [Google Scholar]
- 28.Gao D, Inuzuka H, Tseng A, Chin RY, Toker A, Wei W. Phosphorylation by Akt1 promotes cytoplasmic localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction. Nat Cell Biol. 2009;11:397–408. doi: 10.1038/ncb1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reichert M, Saur D, Hamacher R, Schmid RM, Schneider G. Phosphoinositide-3-kinase signaling controls S-phase kinase-associated protein 2 transcription via E2F1 in pancreatic ductal adenocarcinoma cells. Cancer Res. 2007;67:4149–56. doi: 10.1158/0008-5472.CAN-06-4484. [DOI] [PubMed] [Google Scholar]
- 30.Barré B, Perkins ND. A cell cycle regulatory network controlling NFkappaB subunit activity and function. EMBO J. 2007;26:4841–55. doi: 10.1038/sj.emboj.7601899. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Lin HK, Wang G, Chen Z, Teruya-Feldstein J, Liu Y, Chan CH, Yang WL, Erdjument-Bromage H, Nakayama KI, Nimer S, et al. Phosphorylation-dependent regulation of cytosolic localization and oncogenic function of Skp2 by Akt/PKB. Nat Cell Biol. 2009;11:420–32. doi: 10.1038/ncb1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei W, Ayad NG, Wan Y, Zhang GJ, Kirschner MW, Kaelin WG., Jr. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature. 2004;428:194–8. doi: 10.1038/nature02381. [DOI] [PubMed] [Google Scholar]
- 33.Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M. Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature. 2004;428:190–3. doi: 10.1038/nature02330. [DOI] [PubMed] [Google Scholar]
- 34.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–95. doi: 10.1016/S0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 35.Santi SA, Lee H. Ablation of Akt2 induces autophagy through cell cycle arrest, the downregulation of p70S6K, and the deregulation of mitochondria in MDA-MB231 cells. PLoS One. 2011;6:e14614. doi: 10.1371/journal.pone.0014614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindsley CW, Zhao Z, Leister WH, Robinson RG, Barnett SF, Defeo-Jones D, Jones RE, Hartman GD, Huff JR, Huber HE, et al. Allosteric Akt (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorg Med Chem Lett. 2005;15:761–4. doi: 10.1016/j.bmcl.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 37.Saxena S, Dutta A. Geminin-Cdt1 balance is critical for genetic stability. Mutat Res. 2005;569:111–21. doi: 10.1016/j.mrfmmm.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 38.Verdun RE, Crabbe L, Haggblom C, Karlseder J. Functional human telomeres are recognized as DNA damage in G2 of the cell cycle. Mol Cell. 2005;20:551–61. doi: 10.1016/j.molcel.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 39.Wu Y, Xiao S, Zhu XD. MRE11-RAD50-NBS1 and ATM function as co-mediators of TRF1 in telomere length control. Nat Struct Mol Biol. 2007;14:832–40. doi: 10.1038/nsmb1286. [DOI] [PubMed] [Google Scholar]
- 40.O’Sullivan RJ, Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol. 2010;11:171–81. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fraser M, Harding SM, Zhao H, Coackley C, Durocher D, Bristow RG. MRE11 promotes AKT phosphorylation in direct response to DNA double-strand breaks. Cell Cycle. 2011;10:2218–32. doi: 10.4161/cc.10.13.16305. [DOI] [PubMed] [Google Scholar]
- 42.Bashir T, Pagan JK, Busino L, Pagano M. Phosphorylation of Ser72 is dispensable for Skp2 assembly into an active SCF ubiquitin ligase and its subcellular localization. Cell Cycle. 2010;9:971–4. doi: 10.4161/cc.9.5.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boutonnet C, Tanguay PL, Julien C, Rodier G, Coulombe P, Meloche S. Phosphorylation of Ser72 does not regulate the ubiquitin ligase activity and subcellular localization of Skp2. Cell Cycle. 2010;9:975–9. doi: 10.4161/cc.9.5.10915. [DOI] [PubMed] [Google Scholar]
- 44.Wang H, Cui J, Bauzon F, Zhu L. A comparison between Skp2 and FOXO1 for their cytoplasmic localization by Akt1. Cell Cycle. 2010;9:1021–2. doi: 10.4161/cc.9.5.10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–67. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- 46.Starostina NG, Kipreos ET. Multiple degradation pathways regulate versatile CIP/KIP CDK inhibitors. Trends Cell Biol. 2012;22:33–41. doi: 10.1016/j.tcb.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.