Abstract
While the peptide and protein therapeutic market has developed significantly in the past decades, delivery has limited their use. Although oral delivery is preferred, most are currently delivered intravenously or subcutaneously due to degradation and limited absorption in the gastrointestinal tract. Therefore, absorption enhancers, enzyme inhibitors, carrier systems and stability enhancers are being studied to facilitate oral peptide delivery. Additionally, transdermal peptide delivery avoids the issues of the gastrointestinal tract, but also faces absorption limitations. Due to proteases, opsonization and agglutination, free peptides are not systemically stable without modifications. This review discusses oral and transdermal peptide drug delivery, focusing on barriers and solutions to absorption and stability issues. Methods to increase systemic stability and site-specific delivery are also discussed.
Peptides and proteins have great potential as therapeutics. Currently, the market for peptide and protein drugs is estimated to be greater than US$40 billion/year, or 10% of the pharmaceutical market [1]. This market is growing much faster than that of small molecules, and will make up an even larger proportion of the market in the future [1]. At present there are over 100 approved peptide-based therapeutics on the market, with the majority being smaller than 20 amino acids [1]. Compared with the typical small-molecule drugs that currently make up the majority of the pharmaceutical market, peptides and proteins can be highly selective as they have multiple points of contact with their target [1]. Increased selectivity may also result in decreased side effects and toxicity. Peptides can be designed to target a broad range of molecules, giving them almost limitless possibilities in fields such as oncology, immunology, infectious disease and endocrinology. For an overview of some popular therapeutic peptides/proteins, see Table 1 [2–6,401–403].
Table 1.
Overview of a selection of currently available peptide/protein therapeutics.
| Generic drug name | Size | indications† | Recent sales numbers | Route delivered† | Ref. |
|---|---|---|---|---|---|
| Etanercept | 150 kDa | RA, psoriatic arthritis, plaque psoriasis, ankylosing spondylitis | US$7.9 billion (2012) | sc. | [403] |
| Insulin glargine | 53 AA, 6.1 kDa | Type 1 and 2 DM | $6.6 billion (2012) | sc. | [403] |
| Pegfilgrastim | 39 kDa | Neutropenia | $4.1 billion (2012) | sc. | [403] |
| Salmon calcitonin | 32 AA | Osteoporosis | 1,700,000 Units (2011) | im., sc., intranasal | [2] |
| Cyclosporine | Cyclic, 11 AA | Prophylaxis, solid organ rejection | $579 million (2012) | Oral, iv. | [3] |
| Octreotide | 8 AA, somatostatin analog | Acromegaly, gigantism, symptomatic relief of carcinoid syndrome | Estimated $1.5 billion (2011) | iv., sc., im. (depot) | [4] |
| Liraglutide | 31 AA, 3.8 kDa | Type 2 DM (GLP-1 agonist) | Estimated $843 million (Q3 2012–Q2 2013) | sc. | [401] |
| Bivalirudin | 2.2 kDa | Anticoagulant | $481 million (2011) | iv. | [5] |
| Desmopressin | 9 AA (8 d-AA) | Nocturnal enuresis | 610,000 Rx US (2009) | iv., im., sc., intranasal | [6] |
Data taken from [402].
AA: Amino acid; DM: Diabetes mellitus; GLP: Glucagon-like peptide; im.: Intramuscular; iv.: Intravenous; RA: Rheumatoid arthritis; sc.: Subcutaneous.
These peptide and protein therapeutics have disadvantages as well, such as low bioavailability and metabolic liability. Oral bioavailability of peptides is limited by degradation in the gastrointestinal (GI) tract as well as their inability to cross the epithelial barrier. These therapeutics tend to have high MWs, low lipophilicity and charged functional groups that hamper their absorption [7]. These characteristics lead to the low bioavailability of most orally administered peptides (<2%) and short half-lives (<30 min) [8]. Intravenous (iv.) or subcutaneous (sc.) delivery of these therapeutics overcomes the issue of absorption, but other factors limit the bio-availability of peptide and protein therapeutics including: systemic proteases; rapid metabolism; opsonization; conformational changes; dissociation of subunit proteins; non-covalent complexation with blood products; and destruction of labile side-groups [1,9].
As oral delivery improves patient compliance, there is great interest in the development of systems that allow for the oral delivery of peptide and protein therapeutics [10]. This review will summarize the barriers to various noninvasive delivery methods with a focus on oral and transdermal delivery. Additionally, current methods to overcome these delivery barriers will be discussed. The final portion of this paper will cover schemes designed to overcome the problems of therapeutic targeting and systemic stability.
Oral delivery
Barriers to oral delivery
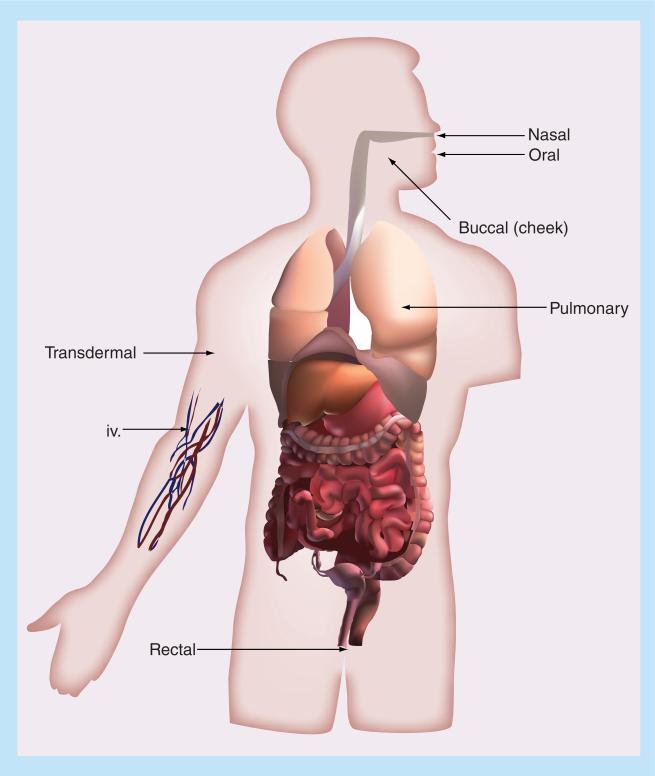
Oral delivery is the preferred route of drug administration, as the majority of patients see it as the most convenient way to take their drugs [11]. Drugs taken by the oral route have the highest level of patient compliance due to the ease and simplicity of taking medications [11,12]. Despite the large number of protein therapeutics being discovered each year, oral delivery continues to be a barrier. As a whole, protein and peptide drugs have low bioavailability when administered orally due to problematic barriers including gastrointestinal proteases, the epithelial barrier and efflux pumps. Common routes of administration for the systemic delivery of peptide and protein therapeutics are summarized in Figure 1. Table 2 provides an overview of the delivery enhancers discussed in this paper with regards to where they act.
Figure 1.
Common routes of administration for systemic delivery of peptides and proteins.
Table 2.
Overview of peptide modifications and delivery systems associated with their site of action.
| Goal of modification | Peptide |
|---|---|
| Stomach | |
| Increased Stability | PEG, d-amino acids, nanoparticles, SLN |
| Small intestine | |
| Increased Stability | Cyclization, PEG, lipidization, d-amino acids, polymer matrices, nanoparticles, esterification, N-acetylation |
| Enzyme Inhibitors | Soybean trypsin inhibitor, aprotinin, puromycin, bacitracin |
| Absorption Enhancers | Chitosans, fatty acids, lectins, ZOT, CPP, liposomes, nanoemulsions, mucoadhesive polymers, nanoparticles, SLN |
| Circulation | |
| Increased Stability | PEG, hyperglycosylation, liposomes, nanoparticles |
| Targeting | Antibody CPP |
CPP: Cell-penetrating peptide; SLN: Solid lipid nanoparticle; ZOT: Zonula occludens toxin.
Proteins are degraded via enzymes and hydrolysis in the acidic environment in the stomach and in the GI tract by a number of proteases and peptidases [13–15]. The human degradome, a complete list of proteases in human cells, consists of at least 569 proteases [16]. There are five broad classes of proteases, including serine, cysteine, threonine, aspartic and metallo proteinases [17]. These proteases play roles in DNA replication, transcription, cell proliferation, fertility, stem cell mobilization, hemostasis, inflammation, senescence, apoptosis and many other vital cellular and regulatory processes [17]. Trypsin, carboxypeptidase and chymotrypsin are secreted from the pancreas into the small intestine, mostly in the duodenum, where they are present in gram quantities. These enzymes are responsible for 20% of the enzymatic degradation of ingested proteins and peptides [13,18,19]. The causes of the remaining 80% of enzymatic degradation are discussed below.
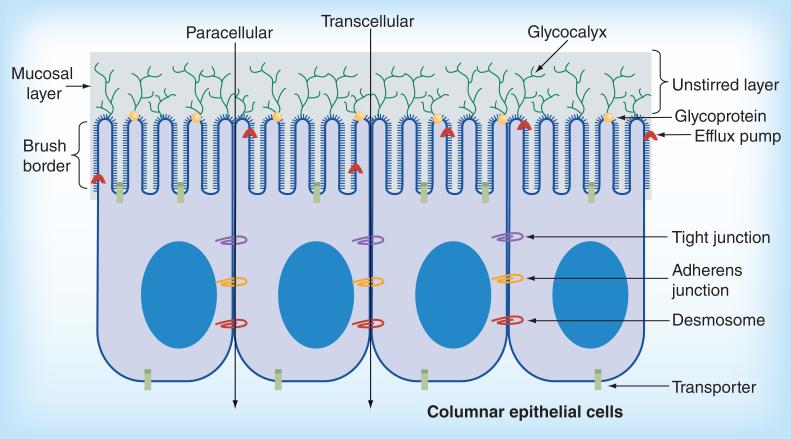
While peptide degradation is one obstacle to oral protein therapeutic delivery, the epithelial barrier of the small intestine poses an even greater challenge. This barrier consists of a single layer of columnar epithelial cells supported by lamina propria and muscularis mucosa [18]. Molecules can cross the epithelium by either transcellular or paracellular routes as depicted in Figure 2. Apical to the epithelial cell barrier is the mucosal layer, which contains glycocalyx, a layer of sulfated mucopolysaccharides [18], glyco-proteins, enzymes, electrolytes and water [18,20]. Additionally, most mucosal surfaces are coated by a hydrated gel consisting of mucins, which are high MW, heavily glycosylated proteins [21]. Bulk flow to the epithelial cells is limited, creating an unstirred layer near the epithelial surface [21]. This unstirred layer is protected from convective mixing forces, slowing the absorption of small molecules and ions. Once a molecule passes the mucosal layer, however, the unstirred layer may act as an absorption enhancer by allowing the particle more time exposed to the epithelial barrier [21].
Figure 2. Intestinal barriers to peptide delivery.
The epithelial intestinal barrier is made up of a single layer of columnar epithelial cells. The apical side of the barrier contains the mucosal layer. Drugs may penetrate the epithelial barrier either through the transcellular route (across the cell) or the paracellular route (between tight junctions). See text for other term explanations.
The brush border membrane (Figure 2) is where the majority of peptide degradation occurs [18]. The brush border is the microvilli-covered surface of cells found in the small intestine, and the microvilli play a major role in nutrient digestion and absorption [8]. Tight junctions (TJs) mediate the paracellular pathway of absorption in intact membranes (Figure 2), and are the rate limiting step in transepithelial transport [21]. Adherin junctions, which are required for the assembly of TJs, are a multiprotein complex made of trans-membrane proteins, peripheral membrane proteins and regulatory molecules including kinases [21]. Adherin junctions work with desmosomes to provide the adhesive bonds that maintain cellular proximity and intercellular communication [21]. Both adherin junctions and TJs are supported by dense perijunctional rings of actin and myosin. The most important proteins to TJ assembly and maintenance are zonula occludens-1 and -2 along with transmembrane proteins in the claudin family [21,22].
A final barrier to protein drug absorption is efflux pumps, depicted in Figure 2. These are proteins belonging to the ATP-binding cassette superfamily that sit on the apical side of mature epithelial cells and mediate multidrug resistance in humans [23]. To date, 49 ATP-binding cassette proteins have been identified, many of which are overexpressed in multi drug resistance lines [24]. One specific example of an efflux pump is P-glycoprotein I (PGP-I; also known as MDR1) [25]. After peptides are absorbed in the GI, PGP-I can pump the drug or peptide back into the GI lumen [13]. It is known that linear lipophilic and cyclic peptides (including cyclosporine) are substrates of PGP-I [13,26].
Even after the drug is absorbed, first-pass metabolism can greatly reduce the fraction of drug that reaches systemic circulation. The first-pass effect, as it is known, is the phenomenon that accounts for the decreased fraction of drug systemically available compared with the fraction of drug that is absorbed. Once a drug is absorbed after oral administration it enters the hepatic portal system. It is then carried via the portal vein to the liver prior to reaching the rest of the body. The liver then metabolizes the drug, reducing the amount of the active, parent compound that enters systemic circulation [27]. Intramuscular (im.), iv., sc., sublingual, intrarectal, transdermal and pulmonary routes of administration avoid or minimize the first-pass effect [28].
While these barriers to absorption are large, much work has been done in order to overcome them. Methods to improve the bioavailability of protein therapeutics can be broadly classified into the following categories: structural modifications, enzyme inhibitors, absorption enhancers and carrier systems.
Strategies for oral delivery of peptides
Direct structural modification
One class of structural modifications under study is cyclization. The benefits of cyclization to oral peptide/protein therapy are evidenced by cyclosporine (CSA). CSA is a fungal-derived, non-ribosomal 11-amino acid peptide with a cyclic backbone and a single d-amino acid [1]. While most naturally occurring proteins and peptides are composed of l-amino acids, d-amino acids are found in some naturally occurring non-ribosomally synthesized peptides [29]. CSA is used most frequently as an immune system modulator for the prevention of solid organ rejection [30]. This cyclic peptide is resistant to proteolytic degradation and also has higher than expected absorption after oral administration [1]. The superior oral bioavailability is thought to be due to a number of properties including decreased flexibility and hydrogen bonding characteristics. The cyclic nature of CSA incorporates seven N-methyl groups that reduce the number of hydrogen bond donors, and the remaining four hydrogens bond intramolecularly. This reduction in intermolecular bonding reduces hydrophilicity. CSA has lipophilic side chain amino acids that further raise its lipophilicity and allows it to cross the gut wall [1]. Other peptides such as somatostatin and encephalin have demonstrated similar characteristics and improved oral absorption after cyclization [31,32]. Generically, cyclization is usually carried out between side chains or ends of the peptide sequences through disulfide bonds, lanthionine, dicarba, hydrazine, or lactam bridges [1]. While cyclization is an option for some peptides, its widespread use is limited when larger peptides and proteins are needed for therapy.
PEGylation is a modification option for some peptides not amenable to cyclization. PEG is an amphipathic molecule that dissolves in organic solvents as well as in water [33]. Both PEG and its metabolites are nontoxic and US FDA approved [34,35]. PEG has been reported to be toxic at high parenteral doses, much higher than the amount of PEG a patient would be exposed to with current PEGylated therapies [36]. If PEG toxicity is seen it usually presents in the kidney, as unmodified PEG is mainly cleared through the kidneys. Interesting, even when pathological changes were seen, no functional deficits resulted [36]. Case studies exist that demonstrate high doses of PEG can induce acute tubular necrosis, and the use of PEG in colonoscopy bowel preparation is associated with an increased risk of acute renal failure in patients aged over 50 [37,38]. There is also evidence that repeat administration of PEGylated particles can lead to increased clearance rate, likely related to anti-PEG IgG and IgM antibodies [39,40]. The structure of the PEG molecules, properties of the molecule being PEGylated and method of PEGylation all play a role in determining immunogenicity [40].
Direct PEGylation confers benefits in both protein absorption and systemic stability (described later in this paper). As an example, insulin PEGylated with a 750 Da version of PEG was formulated into a mucoadhesive tablet. After oral administration, insulin activity was demonstrated by the observed drop in blood glucose levels of approximately 50% 3 h after administration. Additionally, some activity of the orally administered insulin was seen up to 30 h after administration [41]. PEGylation of another peptide, salmon calcitonin (sCT), resulted in resistance to intestinal enzymes, a nearly sixfold increase in intestinal absorptionand slowed systemic clearance compared with the unmodified version of sCT [42].
Vitamin B12 has been used to increase the oral absorption of a number of therapeutic proteins including G-CSF, erythropoietin, insulin and lutenizing hormone releasing hormone [43]. By fusing therapeutic proteins to vitamin B12, it is possible to take advantage of the binding of vitamin B12 to IF, followed by the receptor-mediated absorption of the vitamin B12–IF conjugate. However, this system is limited by the quantity of B12 that can be absorbed, GI degradation, decreased activity of the protein therapeutic due to steric hindrances, and loss of IF affinity for conjugated vitamin B12 [43]. For more information on the use of B12 to improve the oral delivery of protein and peptides, please see the review by Petrus et al. [44].
Protein lipidization is another method that increases the bioavailability of orally administered proteins. Fatty acid conjugates of polypeptides demonstrate improved transport across biological membranes, higher stability and longer plasma half-lives [45,46]. sCT was lipidized using reversible aqueous lipidization and thus it is categorized as a prodrug in this case [46]. Compared with free sCT, the reversible aqueous lipidization sCT reported increased absorption and a 19-times higher AUC value [46]. Caprates, medium-chain fatty acids, promote paracellular diffusion of Class III (highly soluble, low permeability) molecules such as peptides [47]. In addition, triglycerides can be used to evade first-pass metabolism [47]. While irreversible methods of lipidization allow for increased membrane permeability, the activity of such modified proteins may be diminished due to steric issues with the fatty acid chain [47].
Recently, stapled peptides have garnered interest due to their enhanced biochemical properties in the context of drug delivery. More specifically, these are a-helical peptides that contain a synthetic, hydrocarbon backbone linking various residues [48]. This backbone, known as the staple, locks the conformation of the peptide, increasing its helicity and stability in solution [49]. As an example, Walensky et al. have demonstrated the ability of a hydrocarbon-stapled BH3 helix to increase apoptosis in vivo [50]. The enhanced stability of these peptides, along with increased cellular penetration capabilities, makes these molecules ideal candidates for future study in peptide delivery.
A final method of peptide modification to increase oral bioavailability is the substitution of natural l-amino acids with d-amino acids. One study demonstrated that a variety of peptides cleaved by chymotrypsin, elastase, papain (a cysteine protease found in papaya), pepsin, trypsin, and carboxypeptidases are cleaved minimally or not at all by these enzymes when certain residues were replaced with d-amino acids [29,51]. Tugyi et al. investigated d-amino acid substitutions in MUC2, a mucin glycoprotein [52]. The authors noted that the substituted peptide demonstrated high resistance to proteolytic degradation in vitro in both human serum and lysosomal preparations. Their work illustrated that simultaneously modifying both N- and C-terminal regions with d-amino acids conferred the greatest stability increases [52].
The above mentioned direct modifications of peptides and proteins are key strategies that have been implemented to increase stability and oral bioavailability. Many other direct modifications have been carried out, including certain prodrug methods, an overview of which is given in Table 3 [8,31,32,41,42,44,45,52–56].
Table 3.
Direct modifications of peptides and proteins. Overview of direct modifications made to peptides and the resulting change in bioavailability.
| Method of modification | Subtype of modification | Successfully modified compound(s) | Outcome | Ref. |
|---|---|---|---|---|
| Cyclization | CSA, somatostatin, encephalin | Reduces hydrophilicity, decreases conformational flexibility, enhances membrane permeability and increases stability to proteolysis | [8,31,32] | |
| PEG | Direct, permanent modification | Insulin, salmon calcitonin | Resistance to intestinal enzymes, slowed systemic clearance, increased intestinal absorption, decreased glucose levels (insulin) | [41,42] |
| Prodrug | IFN-B-1b | Decreased aggregation, maintained activity, dimished IgG response, 100-fold increase in AUC, improved protection from enzymatic degradation | [41,53] | |
| B12 conjugation | ε position on the corrin ring | Albumin, G-CSF, EPO, LHRH and analogues, DP3, dextran nanoparticles | Increased oral absorption via vitamin B12-intrinsic factor binding | [44] |
| 5′-hydroxy on ribose unit of the α tail | IFN, insulin | Efficacy is hindered by naturally low capacity for B12 absorption | [44] | |
| Phosphate unit of the α tail | Albumin, γG-globulin | Steric hindrance limits absorption, activity of the therapeutic | [44] | |
| Lipidization | Reversible aqueous lipidization with n-palmitoyl cysteinyl 2-pyridyl disulfide | Salmon calcitonin | AUC 19-times higher than unmodified, marker for bone resorption reduced | [45] |
| N-acetylation | Salmon calcitonin | Improved oral bioavailability, marginally improved resistance to trypsin and leucine aminopeptidase, enhanced membrane permeability | [54] | |
| D-AA | 6 out of 11 l-AA substituted for d-AA | MUC2 | Resistance to proteolytic cleavage by chymotrypsin, elastase, papain, pepsin, trypsin and carboxypeptidase | [52] |
| Prodrug | Esterification | Desmopressin | Increased permeation of Caco-2 cell layer, active in plasma after cleavage | [55] |
| Perbuytrylation | Glycovir | Increased bioavailability | [56] |
AA: Amino acid; AUC: Area under the curve; CSA: Cyclosporine; DP3: Octapeptide (Glu-Ala-Ser-Ala-Ser-Tyr-Ser-Ala); EPO: Erythropoietin; LHRH:Lutenizing hormone releasing hormone.
Enzyme inhibitors
In addition to direct modifications, another method to increase oral peptide bioavailability is to coadminister with enzyme inhibitors. These enzyme inhibitors are usually more effective in the large intestine than the small intestine due to the large quantity and variety of proteases in the small intestine [57]. A leading enzyme inhibitor is soybean trypsin inhibitor, FT-448, a potent and specific inhibitor of chymotrypsin [57]. When coadministered with insulin to rats and dogs, levels of immunoreactive insulin rose proportionally to a decrease in blood glucose levels. Further, it is thought to play some role in increasing peptide absorption [57].
Aprotinin, originally branded as Trasylol™, and used to reduce bleeding during complex surgeries, is another enzyme inhibitor used [58]. When administered with insulin intraileally, blood glucose decreased by 30% over the next 3 h compared with administration of insulin alone [58]. Other enzyme inhibitors are summarized in Table 4. An alternative method to inhibit enzymes is to alter the pH at the site of action of the enzymes [59]. Most enzymes in the stomach, including pepsin, are only active at low pH (approximately 2) [60]. Therefore, if the pH in the stomach is increased, the enzymes are no longer able to degrade the peptides. Conversely, enzymes in the intestines often work at a higher pH; therefore, lowering the pH can decrease the activity of these enzymes [61,62]. These protease inhibitors do have shortcomings. First, they can disrupt the normal absorption of dietary peptides and may induce toxic shock after prolonged therapy [63,64]. It is believed this may cause the body to increase production of these proteases, which may lead to hypertrophy and hyperplasia of the pancreas [65]. The inhibitors themselves may also be toxic and damaging to the GI tract after prolonged administration [65]. Indeed, the majority of enzyme inhibitors are highly toxic. Table 4 summarizes enzyme inhibitors with some promise of therapeutic translatability [57,58,65–70].
Table 4.
Enzyme inhibitors, their targets and effects on peptide delivery. An overview of potentially clinically relevant enzyme inhibitors.
| Enzyme inhibitor | Molecules inhibited | Effect on peptide drugs | Ref. |
|---|---|---|---|
| Soybean trypsin inhibitor, FK-448 | Chymotrypsin | Enhanced intestinal absorption of insulin in rats and dogs. Suppressed digestion of insulin by pancreatic enzymes | [57] |
| Aprotinin | Serine proteases, specifically trypsin, chymotrypsin, and plasmin | Intraileally administered insulin with aprotinin led to decrease in blood glucose of 30% compared with controls | [58] |
| Puromycin | Serine and metallopeptidases | Improved stability of leucine encephalin, and stability and permeability of d-Ala2, d-Leu5 enkephalin (DADLE) | [66–68] |
| N-acetylcysteine | Inhibits aminopeptidase N and has mucolytic properties | [65,69] | |
| Bacitracin | Trypsin and pepsin, aminopeptidase N | Used to increase delivery of insulin, met-kephamid and buserelin | [65,69,70] |
Absorption enhancers
The optimal absorption enhancer should be reversible, nontoxic at the effective concentration and provide a rapid permeation enhancing effect on the intestinal cell membrane. One such compound class of absorption enhancers is chitosans. Chitosans are nontoxic, biocompatible, FDA-approved polymer derivatives of chitin that enhance the absorption of hydrophilic macromolecule drugs [71]. In addition, due to their high MW, they are minimally absorbed from the gut, limiting the possibility of systemic side effects [72]. It is thought that varying degrees of deacetylation of chitin confer different amounts of absorption enhancement, with >80% deacetylation affording the greatest promoter effect in cell culture [73]. Chitosans have been used to enhance the absorption of molecules such as atenolol, insulin and 8-R-vasopressin [72]. Further, chitosans appear to be quite safe at their effective concentration [71,74]. Chitosans work by increasing paracellular permeability. By binding tightly to the epithelium via positive charges, chitosans cause redistribution of cyto-skeletal F-actin and the zonula occludens 1 [75]. Chitosans are limited by their ability to diffuse across the mucous layer, as evidenced by their decreased activity on mucus-producing cells [76]. In vivo studies with chitosans demonstrated a threefold increase in octreotide absorption when the two were coadministered into the duodenum [72]. Another study with trimethyl chitosan chloride, a chitosan derivative, had many favorable characteristics. Trimethyl chitosan chloride was able to reversibly interact with TJs, leading to widening of the paracellular route, and at the same time did not damage cell membranes or alter the viability of intestinal epithelial cells. In vivo studies in rats demonstrated that it was able to increase the oral bioavailability of a peptide when the two were coadministered [71]. Overall, chitosans and their derivatives are a promising class of absorption enhancers.
Another class of absorption enhancers demonstrating potential includes the medium-chain fatty acids [77]. C8, C10 and C12 fatty acids (caprylate, caprate and laurate, respectively) can enhance paracellular permeability of hydrophilic compounds. First, caprate is thought to work by inducing dilation of TJs [78]. Interestingly, the lowest concentration that enhanced absorption was near the critical micelle concentration of each fatty acid [77]. The order of increased absorption in vivo is caprate>laurate>caprylate. Sodium caprate (C10) is the most studied of the medium-chain fatty acids. It is thought to increase absorption of hydrophobic molecules via the paracellular and transcellular route [79]. Unfortunately, a study reported that it can only significantly increase absorption for molecules up to 1200 g/mol, or 1.2 kDa (such as octreo-tide) [80]. At the effective dose of 13 mM, sodium caprate is nontoxic to epithelial cells [80].
Lectins are another type of absorption enhancer that have many of the characteristics of the ideal absorption enhancer. Lectins are proteins that specifically recognize and bind to sugar complexes attached to proteins and lipids [81]. Lectins are also naturally resistant to proteolytic breakdown, making inactivity before reaching their site of action unlikely [82]. They can be used to target luminal surfaces of the small intestine and trigger vesicular transport into or across epithelial cells [81]. Lectins are also mucoadhesive, which further leads to increased absorption [15].
Toxins can also be used for absorption enhancement, so long as they do not cause permanent cellular damage. Zonula occludens toxin (ZOT), is one such compound. ZOT, a 45 kDa toxin made by Vibrio cholerae, has been demonstrated to increase the permeability of small intestine mucosa by reversibly affecting the structure of TJs [83,84]. ZOT binds to ZOT receptors on the luminal surface of the intestine and causes cytoskeletal rearrangement related to changes in protein kinase C and binding to β-tubulin [84,85]. TJs can be perturbed enough to allow the transport of agents across the intestinal mucosa, although the increased bioavailability of insulin was only 20% [86]. In a study with Caco-2 cells, incubation with 4 μg/ml ZOT for 30 min increased the permeability to insulin by 6.3-fold [86]. Mediation of TJs may not be the only method by which ZOT works; a study demonstrated that a fragment of ZOT was able to increase the bioavailability of hydrophobic drugs by interacting with PGP [87]. Additional work has been done to determine the smallest portion of ZOT that maintains activity [88].
Recently, coadministration of cell-penetrating peptides (CPPs; described later in more detail) with therapeutic peptides has been attempted in order to increase absorption of the therapeutic. In one study, insulin coadministered with CPPs consisting of six to ten repeats of argi-nine led to increased GI uptake of insulin [89]. Interestingly, the study investigated both d- and l-arginine-based CPPs, and the d-based CPPs allowed for greater increases in insulin absorption, assumed to be due resistance of d-amino acids to proteases [89]. It is important to note that the CPP was not fused to insulin; rather, they were co administered. A follow-up study demonstrated that electrostatic interactions between insulin and the CPP were responsible for the enhanced absorption of insulin [90]. Another study revealed that the CPP penetratin was best able to increase ileal insulin absorption [91]. Penetratin consists of basic amino acids (lys, arg) along with some hydrophobic regions. Use of CPPs as absorption enhancers represents a relatively new area of research that has the potential to add weapons to the absorptive enhancement arsenal.
Other classes of absorption enhancers have lost favor in recent years due to irreversible epithelial damage [92]. Surfactants such as sodium dodecyl sulfate were shown to cause increased permeability of the GI tract to hydrophilic compounds, but also cause altered cell morphology and cell membrane damage [93]. Sodium dodecyl sulfate shortened microvilli of cells and produced actin disbandment, structural separation of the TJs and damage to the apical cell membrane with even limited exposure [94]. Certain in vivo rat studies support the increase in absorption and revealed the damage caused to be reversible [95]. Bile salts such as sodium cholate and deoxycho-late were originally seen as safe and effective at increasing drug absorption; however, it is now understood that these particles are damaging after long-term use [96].
Carrier systems
Many drug carrier systems are currently being developed in an attempt to increase the oral bio-availability of peptide drugs. Some of these systems contain a combination of components listed above, while others have novel mechanisms.
The first group of carrier systems consists of hydrophilic mucoadhesive polymers (polyacrylates, cellulose, chitosan), which can be altered to suit the needs of the peptide/protein being delivered [97]. While chitosan has already been discussed under the absorption enhancers category, it has also been combined with EDTA in order to create a resin that binds bivalent cations [98]. It is thought that bivalent cations are essential for the activity of proteolytic enzymes; in fact, zinc proteases, carboxypeptidases and amino peptidases were strongly inhibited by this system, but serine proteases, trypsin, α-chymotrypsin and elastase were not inhibited [98].
Thiomers, thiolated polymers, have also been used as drug carrier systems. These mucoadhesive polymers display thiol-bearing side chains; disulfide bonds form between the polymer and cysteine-rich protein domains in the mucous glycoprotein layer. These polymers are available in both cationic and anionic varieties and can increase mucoadhesive properties of gels by up to 140-fold [99]. When adhered in the small intestine, mucoadhesion allows for a steeper concentration gradient across the epithelial barrier, which may lead to increased passive drug uptake and a prolonged therapeutic effect [99].
Next, polymer matrices can be used to protect proteins from proteolysis and antibody neutralization, resulting in increased protein activity in vivo [100]. It is very important that interaction between the protein and matrix be optimized; too little attraction and the protein will not be immobilized on the gel; too great an attraction will cause the protein to remain in the gel and thus not become systemically available. A sustained release system that protects the protein in the GI tract can be developed by tuning the cross-linkage and electrostatic interactions between matrix and protein [100].
Nanoemulsions are another carrier system for oral protein therapeutics. Nanoemulsions are defined as oil-in-water (o/w) or water-in-oil (w/o) emulsions with mean droplet diameters ranging from 50 to 1000 nm. The average droplet size is usually between 100 and 500 nm [101]. Generally, these emulsions are made from surfactants approved for human consumption and are generally recognized as safe. Nano emulsions have a much higher surface area and free energy than macroemulsions, thus making them an effective transport system. Further, nano emulsions do not cream, flocculate, coalesce or sediment. One such system in development is the ‘self-nanoemulsifying drug-delivery system,’ or SNEDDS. To test the concept, fluorescein isothiocyanate-labeled β-lactamase (BLM) was loaded into SNEDDS through solid dispersion. After an o/w emulsion was formed via addition of water, the nanoemulsion was able to increase transport of fluorescein isothiocyanate–bleomycin across MDCK monolayer of cells [102]. In vivo studies demonstrated a significant increase in SNEDDS–BLM absorption compared with free BLM [102].
Hydrogels are a network of cross-linked water-soluble polymer chains that are insoluble in water but have water as their dispersion medium. The porous nature of hydrogels can be finely tuned to allow for drug loading into the hydrogel. Further, pharmacokinetic properties for release of the loaded drug can be adjusted to the requirements of individual drugs [103]. Hydrogels can designed to deliver drugs to four sites after oral ingestion – mouth, stomach, small intestine or colon [104]. Newly developed homo- and copolymeric hydrogels are capable of protecting and delivering peptides and protein therapeutics [103]. For an overview on hydrogels, see Bindu Sri et al., and for more detail on the use of hydrogels for oral peptide drug delivery, the review by Peppas et al. is helpful [103,104].
While liposome systems have potential in oral drug delivery, there is a concern with stability of the vesicles under the physiologic conditions of the GI tract [105]. Adding to the problem, mucus may act as a barrier by blocking the diffusion of liposomes to the epithelial layer [106]. Despite this, orally administered liposomes have demonstrated some successes. Calcitonin was administered in a chitosan–aprotinin coated liposome and illustrated an increased pharmacological effect compared with free calcitonin [107]. Cyclosporine has also been delivered in liposomes; the egg lectin–cremophore–lactose liposome containing CSA had nine-times the bioavailability of free CSA and four-times that of the microemulsion on the market [108]. PEG coating, enteric encapsulation and the use of archaeosomes have been proposed to decrease degradation of the liposome in the GI tract [109].
Nanoparticles (NPs) are solid particles with sizes in the range of 10–1000 nm [110]. NPs allow for the encapsulation of proteins inside a polymeric matrix, thus protecting them against hydrolysis and enzymatic degradation [110]. These systems can be tuned in order to maximize encapsulation efficiency, bioavail-ability and retention time [111]. NPs, however, have a difficult time being absorbed from the GI tract; studies have demonstrated that cells lacking mucus (including M cells and Peyer's patches in general) are best at absorbing NPs [110]. Particles of 50 and 100 nm demonstrated the greatest absorption and detection in intestinal mucosa [112]. Furthermore, NPs smaller than 100 nm demonstrate a higher extent of uptake by absorptive enterocytes while those over 500 nm will rarely be taken up by absorptive enterocytes [110]. NPs are often made from poly(lactic acid), poly(lactic-co-glyclic acid), chitosan, gelatin and poly-alkyl-acyanoacrylate, all of which are nontoxic, non-thrombogenic, non-immunogenic, non-inflammatory, stable in blood, biodegradable, avoid the reticuloendothelial system (RES), and are applicable to various biologics such as proteins, peptides and nucleotides [111]. While there are minimal scientific data on the toxicity of NPs, their size makes exposure during manufacturing almost guaranteed [113]. Impaired lung function and other respiratory symptoms have been seen in workers that were exposed to NPs [113]. iv. administration of NPs is followed by increased synthesis and release of cytokines. Furthermore, NPs passively target the liver through uptake by Kupfer cells, again followed by an inflammatory response [113]. Generally, the toxic effects of NPs are not fully understood, and care must be taken with the manufacturing and use of NPs as therapeutic agents. Table 5 provides some details regarding the various polymers used to make NPs [111,114–121].
Table 5.
Polymer carrier systems. Commonly used polymers for construction of nanoparticles, their biocompatibility and use in peptide delivery.
| Polymer | Biocompatibility† | Example of use | Ref. |
|---|---|---|---|
| PLA | Biocompatible and biodegradable | BSA loaded with 71 % efficiency, BSA was stable after release | [114] |
| PCL | Degraded by hydrolysis | Preparation of long-term implantable
device Insulin loaded with 96% efficiency, improved response to OGTT Has mucoadhesive properties AmB loaded PCL nanoparticles two- to three-times more effective than free AmB |
[115,116] |
| Chitosan | Nontoxic, biocompatible | Insulin-loaded chitosan nanoparticles enhanced intestinal absorption of insulin through a combination of insulin internalization in enterocytes and insulin-loaded particle uptake by Peyer's patches | [117] |
| Gelatin | Nontoxic, biodegradable | Encapsulated paclitaxel, oligonucleotides, chloroquine | [118–120] |
| Poly(alkyl-cyano-acrylates) | Biodegradable and biocompatible, degraded by esterases. Produce some toxic metabolites, not suitable for human use | Encapsulated doxorubicin, ampicillin, indomethacin | [111] |
| PLGA | Biodegradable, excellent toxicological profile | PLGA nanoparticles with influenza HA incorporated throughout the matrix, increased uptake via M cells | [121] |
Data taken from [111].
AmB: Amphotericin B; BSA: Bovine serum albumin; HA: Hemagglutinin; OGTT: Oral glucose tolerance test; PCL: Poly(ε-caprolactone) PLA: Poly(lactic acid); PLGA: Poly(lactic-co-glyclic acid).
NPs can be targeted to certain sites based on particle size, surface charge, surface modification and hydrophobicity [111]. Surface charge is particularly important for cell internalization, as cationic surfaces increase the rate and extent of nanoparticle internalization [111]. Carboxylated polystyrene NPs demonstrate decreased affinity to intestinal epithelia and M cells compared with neutral and positively charged polystyrene NPs [122]. While hydrophobic polymer-based NPs are better absorbed than their hydrophilic counterparts [110], in order to avoid opsonization and the mononuclear phagocytic system, the use of hydrophilic-surfaced NPs is preferred over traditional hydrophobic-surfaced NPs [111,123]. Interestingly, negatively charged, hydro-philic NPs have increased bioadhesive properties and are uptaken by absorptive enterocytes and M cells [110,124].
Surface modifications such as PEG can create a steric barrier and reduce clearance by circulating macrophages in the liver as well as by the mononuclear phagocytic system [111]. PEG coating of NPs increases blood circulation half-life as well as reducing interactions between the NPs and digestive enzymes [125]. Lectins have been conjugated to NPs, which led to increased transport across intestinal mucosa, especially via M cells of Peyer's patches [110,111]. Finally, higher MW polymers will release the peptide slower than lower MW polymers [111].
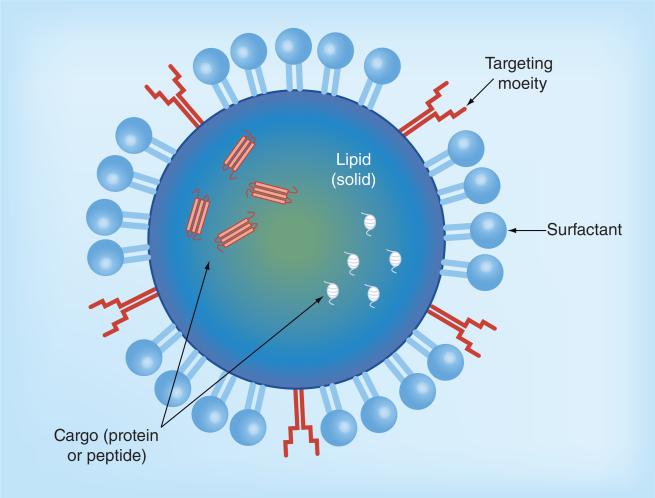
Solid lipid NPs (SLN) are solid lipids that are stabilized with an emulsifying layer in an aqueous dispersion (Figure 3). The colloidal size ranges between 50 and 1000 nm [105]. This system avoids the use of organic solvents and has the capacity to allow fast, effective, large-scale manufacturing of high-concentration suspensions. This system can be used to encapsulate peptides and proteins and thereby protect them against enzymatic degradation [126]. Another benefit of SLNs is that the drug can be incorporated into the matrix, onto the shell, or into the core of the particle [105]. A lectin-modified and insulin-coated SLN was able to deliver insulin to the system after administration to the small intestine [127]. SLNs have also been used for controlled release of sCT [128]. The systemic stability and GI absorption of SLNs and NPs as a whole make them promising protein carrier systems; research in this field continues to enhance the likelihood for oral delivery of systemically active peptides.
Figure 3. Solid lipid nanoparticle.
Solid lipid nanoparticles have a solid lipid core and is coated with surfactant. Targeting moieties may be added to decorate the surface of the solid lipid nanoparticle. Cargo for solid lipid nanoparticle are illustrated as peptides or proteins, but may also include siRNA or small-molecule drugs.
Many companies are attempting to develop carrier systems that will be able to deliver a wide variety of therapeutics with minimal modification [63]. Examples include Emisphere's Eligen™ system (NY, USA), which has the potential to deliver therapeutics from 0.5–150 kDa. The drug–carrier system known as SNAC (n-(8-[2-hydroxylbenzoyl]amino)caprylic acid) can be used to orally deliver active peptides into circulation [129,130]. The peptide/protein therapeutic is mixed with SNAC, which creates a noncovalently linked drug–carrier complex. The complex is highly lipophilic and is proposed to be able to directly cross the epithelial membrane. After absorption, the complex dissociates by simple dilution, and the therapeutic is released, unchanged and in its active conformation [63,129]. This system has demonstrated promise in both human and animal models for the oral delivery of insulin, human growth hormone, and sCT [130].
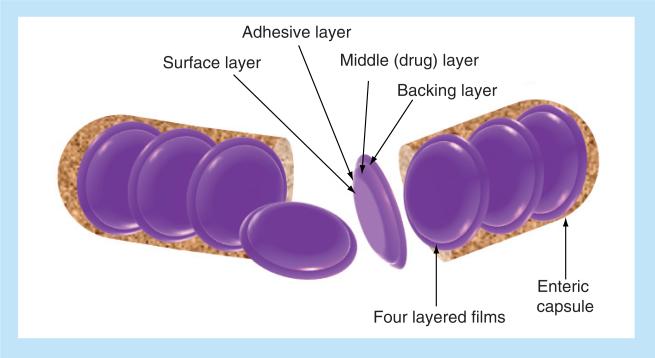
A second such system is the gastro intestional mucoadhesive patch system (GI-MAPS), depicted in Figure 4. The GI-MAPS is composed of four layers contained in an enteric capsule, which when combined result in protection of the protein in the GI tract as well as increased absorption. The backing is made of ethyl cellulose, while the surface layer is made of an enteric, pH-sensitive polymer, in this case Eudragit® L100. The middle layer is a cellulose membrane that contains both the drug and absorption enhancers. The surface layer is attached to the middle layer via an adhesive layer made of Hiviswako 103 polymer [131].
Figure 4. Gastrointestinal mucoadhesive patch system.
The system contains four layered films in an enteric capsule. Layer one is the backing layer, layer two is the middle or drug layer, layer three is the adhesive layer, and layer four is the surface layer.
When the capsule is swallowed, the enteric coating dissolves in the small intestine. Once this layer dissolves, the mucoadhesive layer of the patch is exposed. The patch therefore adsorbs to the mucus membrane of the small intestine, exposing the drug and absorption enhancer to the epithelial surface. When the patch attaches, it provides increased contact time, allowing more of the drug to be absorbed. In addition, a large concentration gradient is created across the epithelial cells, increasing the amount of drug absorbed [131]. While these are two examples of systems, Table 6 has a more complete list of other similarly functional carrier systems.
Table 6.
Multi-component carrier systems. These systems are composed of multiple parts and chemicals. They are designed to allow for the delivery of a variety of peptides and proteins.
| System | Product name | Effect on absorption | Therapeutics tested with the system |
|---|---|---|---|
| GI-MAPS | Eudragit® L100, Eudragit S100, HP-55 | Protection of peptide, increased exposure to GI tract | Granulocyte colony-stimulating factor |
| SNAC carrier molecule | Emisphere™ | Increased membrane permeability | Insulin, calcitonin, growth hormone, GLP-1 |
| Amphiphilic oligomers | HIM2 | Resists GI enzyme degradation and increases membrane permeability | Insulin, calcitonin, enkephalin, parathyroid hormone |
| Lipid-based microemulsion | Macrulin™ | Protects peptide against acidic and proteolytic degradation; increased GI absorption | Insulin, sCT |
| Protein crystallization | CLEC™ | Increased stability against proteolysis | Calcitonin, lipases, polypeptides |
GI: Gastrointestinal; GI-MAPS: Gastrointestinal mucoadhesive patch system; GLP: Glucagon like peptide; sCT: Salmon calcitonin; SNAC: n-(8-[2-hydroxylbenzoyl]amino)caprylic acid.
Adapted from [63].
The above has been a broad overview of the issues associated with oral administration of peptide and protein therapeutics. Many systems that increase stability and absorption of these therapeutics have been described. Until a more widely applicable system is developed, every protein therapeutic will require a unique system made of combinations of the above if the drug is to be orally bioavailable. While the oral route is a preferred method of administration, other routes, too, have their benefits. The next section will address the issues with transdermal peptide and protein delivery.
Transdermal delivery
Delivering peptides transdermally allows the avoidance of both GI degradation and hepatic first-pass metabolism of short half-life drugs, while still allowing administration via an easily accessible, non-invasive route. This not only diminishes the amount of potential drug–drug interactions with combined therapies, but can also lead to better patient compliance (compared with iv. injection) due to the ease of use, self-administration and less frequent dosing characterized by the prolonged, continuous and rate-controlled drug release unique to these systems [132–136].
First and foremost, the most important barrier for transdermal delivery is the skin itself [132]. Drugs that have been delivered transdermally for some time now, namely nicotine, estrogen and scopolamine, among others, are all small molecules and highly hydrophobic. Historically, it has been demonstrated that the skin tends to keep out drug molecules greater than 500 Da [137], especially those molecules of hydrophilic nature [138]. After all, the main biological function of the skin is to deny entry to foreign substances. Therefore, bypassing the skin to allow drug entry is a necessary step to successful transdermal delivery [139].
Anatomically, the skin is made up of three major layers. The outermost portion, and first line of defense to drug entry, is the stratum corneum [140]. This layer, mainly composed of dead cells (keratinocytes), is approximately 10–15 μm thick and surrounded by a lipid extra-cellular matrix. Below the stratum corneum lies the viable epidermis, which is approximately 50–100 μm thick. Taken together, these two layers are known as the full epidermis. Below the full epidermis is the first sign of vasculature, present in a layer known simply as the dermis [136]. A fibrous layer, approximately 1–2 mm thick, the dermis comprises large capillary beds that are the site of drug entry into the circulation [141].
Due to these obstacles provided by the skin, successful transdermal delivery of large, potentially hydrophilic peptides, requires some type of physical and/or chemical enhancement. Conventional enhancements in transdermal delivery generally aim to bypass the main physical barrier, the stratum corneum [134,142–146]. Direct entry into the dermis, despite being the most direct way to get the drug into circulation, is often avoided as penetration of this layer would lead to patient bleeding and possible disruption of nerve endings [144].
Because the traditional transdermal patch is used solely to deliver small, hydrophobic drugs, and not peptides, it will not be discussed in this section. Instead, many of the currently ‘in-development’ transdermal enhancement methods will be briefly described, including microneedle technology, electroporation, iontophoresis, sonophoresis, thermal ablation and chemical enhancement.
Microneedle technology
Microneedle technology involves the use of small needles that create small pores in the skin, allowing drug passage across the outermost physical barrier [134]. Because one of the overall goals of transdermal delivery is to increase efficiency while still maintaining an easy, non-invasive technique, these microneedles are designed to breach only the stratum corneum [144]. By not reaching as far as the viable dermis, both the capillaries and nerve endings are avoided, leading to a painless feeling for the patient. These needles have been created using a number of materials, including silicon, various metals, or biodegradable materials such as polymers and sugars [141].
As described by Herwadker and Banga [133], multiple microneedle designs and drug introduction routes have been tested for efficient delivery. One such method involves a two-step approach, where the needles are used to puncture the skin to create pores, followed by topical administration of the drug. Another method includes coating the microneedles themselves with drugs, allowing the drug to then enter the body after the skin is treated with the needle. A third method includes encapsulating the drug in biodegradable microneedles, slowly releasing the drug as the needles degrade. Lastly, a final method includes creating hollow needles, through which drug can be infused following puncturing of the skin. Microneedles can be introduced via physical injection on the skin or in the form of a patch. One example utilizing this technology comes from Zosano Pharma (CA, USA), who have developed a patch containing drug-coated microneedles capable of delivering a variety of drugs including peptides and vaccines [141].
Thermal ablation
Like microneedle technology, thermal ablation aims to permeabilize only the stratum corneum, avoiding a breach of the deeper capillary and nerve-containing tissue layers [146]. However, instead of using needles to perforate the skin, this technique relies on short pulses of high heat (approximately 100°C) to create small, reversible channels in the micron size range [147]. Following the short bursts of heat, drug can be applied to the treated area for entry into the circulation. Multiple systems have been designed to successfully deliver drugs via thermal ablation, including PassPort® (Nitto Denko [Osaka, Japan]) and ViaDor® (Syneron Medical Ltd [Yokneam, Israel]). While these systems have demonstrated success with smaller drugs, delivery of peptides is still under study [141].
Electroporation
Electroporation utilizes very short pulses of high voltages (between 10 and 100 V) to perforate the skin. Similar to microneedles and iontophoresis (discussed later), application of electroporation breaches only the stratum corneum, characterizing it as another non-invasive method for drug introduction [148]. Instead of simply targeting the layer of dead cells, this method targets the surrounding lipid bilayers that are spread out throughout this layer. Application of an electric current disrupts the structure of these lipids, allowing molecules to penetrate the skin. In addition, delivery of drug can be increased using this method by increasing the voltage, number of pulses and duration of pulses to levels still viewed as safe for the patient [133]. Due to the high complexity of these systems, no peptides have been FDA approved for delivery by electroporation. However, multiple DNA-based vaccines are in clinical trials, which, if successful, could pave the way for peptide-based vaccines.
Sonophoresis
Sonophoresis, also referred to as cavitational ultrasound, relies on the application of sound waves to the skin to increase its permeability. Like electroporation, sonophoresis achieves this task by targeting the lipid bilayers embedded in the stratum corneum [133]. Sound waves, generally between 20–100 kHz, are believed to cause an increase in pore sizes on the skin (increased fluidity in these lipid bilayers), thus allowing drug penetration transcellularly through the stratum corneum [147]. Though nothing is currently FDA approved, delivery of insulin for Type I diabetes using the sonophoretic U-Strip system (Transdermal Specialties, Inc. [PA, USA]) is presently in clinical trials, parts of which are expected to be completed within a year [149].
Iontophoresis
Not all methods utilized for transdermal peptide delivery require physical disruption of the skin's outer barrier. Iontophoresis is one of those methods, which instead uses principles of both electrorepulsion (for charged peptides) and electroosmosis (for uncharged peptides) to act on the drug molecules themselves rather than the skin [133]. Generally speaking, iontophoresis utilizes a device placed on the skin capable of generating an electric current, similar to a battery. When delivering charged peptides (negatively charged peptides for instance), the battery builds up a strong negative charge at the anode, which would be placed on the same portion of the skin as the drug molecules. Utilizing charge–charge repulsion, this anode will drive the negatively charged peptide into the skin [132,150]. Using this method, the rate of drug release can be controlled as the release (entry into the body) is directly proportional to the current being administered on the skin [147]. Although peptides have yet to see FDA approval for delivery via iontophoresis, the system has been fine-tuned to deliver smaller molecules such as lidocaine (LidoSite®, Vyteris [NV, USA]). In addition, iontophoretic peptide delivery, including delivery of gonadotropin releasing hormone and insulin, has reached clinical trials on multiple occasions [141].
Biochemical enhancement
A final method involves the use of biochemical molecules to enhance permeation of peptide drugs across the skin. The ultimate goal in using biochemical enhancers is to increase the permeability of the skin, which provides a path for peptide drug delivery into the circulation [147], while remaining nontoxic, non-irritating and non-allergenic [140]. One such peptide used to enhance skin permeability is magainin, a 23-amino acid peptide known to form pores in bacterial cell membranes [151,152]. While previously demonstrated to increase the permeability of small molecules, its use for peptide delivery enhancement still requires optimization [153]. In addition, recent, work by Ruan et al. demonstrated the ability of a small peptide known as TD1 to increase the transdermal penetration capability of hEGF when fused together [138,154]. This fusion system involving TD1 could have major implications in the near future for delivering hydrophilic peptides transdermally.
To summarize, all of the methods described above aim to make the drug delivery process as easy and as painless as possible. Painless, in these cases, requires avoiding a breach of the viable dermis layer of the skin, which includes vasculature and nerve endings. However, other barriers still exist to make this delivery process more efficient. Despite moderate success seen using the previously described physical and chemical enhancement methods, a recent study suggests that bypassing more than simply the stratum corneum is necessary for the most efficient transdermal delivery [136]. In addition, despite displaying low activity compared with other locations in the body, proteases do exist on the skin, adding another challenge to the transdermal delivery of peptides.
Other delivery routes
While this review has focused on delivery of peptides by oral and transdermal routes, delivery by other routes is also currently being researched. The next section of the review will give a brief overview and recommendations for readings on intranasal, buccal, pulmonary and rectal administration of peptide therapeutics. These routes of administration are illustrated in Figure 1.
The intranasal route for peptide drug delivery is an area that has already had some successes. For instance, desmopressin, calcitonin and the seasonal influenza vaccine are available via the intranasal route [155,156]. Advantages of the nasal route over injected medications include increased patient convenience and comfort, elimination of needle-stick related injuries and infections, and decreased syringe-related medical waste [156]. Disadvantages include nasal irritation, limitations on volume and milligram amount of drug that can be delivered nasally, the rapid renewal of nasal epithelium, acidic pH, endo- and exopeptidases, and large interpatient variability in absorption [156]. While the nasal route has traditionally thought to be an option only for small molecules, highly effective and non-irritating absorption enhancers have been developed [157]. For a more thorough review on intranasal peptide delivery, see Illum et al. [155].
The buccal route, administration of drug through the mucosal membranes lining the cheeks, is another option for peptide delivery [158]. Drugs delivered by the buccal route are placed in the mouth between the gums and cheek [159]. Buccal delivery has many advantages including bypassing of the GI tract and possibly first-pass metabolism, ease of use, rapid onset, large contact surface area and is generally amenable to the delivery of hydrophilic macromolecules [159,160]. There are limitations to buccal delivery and patient adherence, such as irritation of the mucosa, low permeability to peptides and the bitter taste of many buccal drugs [159]. Absorption enhancers and bioadhesive polymers are being used to resolve these problems. Oxytocin, insulin, sCT and GLP-1 have all been successfully delivered via the buccal route [159,160]. For further reading on buccal peptide delivery, see Mujoriya et al. [160].
Rectal administration of drugs, while not patients’ top choice, is sometimes necessary if other routes of administration (such as oral and iv.) are not possible. The rectum is composed of a one layer-thick epithelium complete with mucus and TJs [161]. While there are no villi, the surface area for drug absorption is approximately 200–400 cm2 [161]. Rectal administration is useful due to the minimal amount of proteases and avoidance of the first-pass effect. However, the bioavailability of peptides is low without the use of absorption enhancers [161]. Both insulin and pentagastrin have been successfully delivered via the rectal route. See Lakshmi et al. for a more in-depth discussion of rectal peptide delivery [161].
The pulmonary route can be utilized for the systemic delivery of peptide therapeutics. However, the anatomy of the lung creates many barriers to delivery including respiratory mucus, mucociliary clearance, alveolar epithelium with TJs, pulmonary enzymes, and macrophages that secrete peroxidases and proteases [162]. The alveolar epithelium and capillary endothelium have high permeability to many lipophilic substances, but passage of large hydrophilic molecules is limited [162]. Many absorption enhancers and enzyme inhibitors that have been used to increase peptide absorption have been demonstrated to be damaging to lung tissue [162]. Pulmonary delivery of insulin has been extensively studied and was FDA approved in 2006, but Pfizer (NY, USA) discontinued production in 2007 due to poor sales [162,163]. Calcitonin, human growth hormone, parathyroid hormone, and desmopressin have also been successfully delivered via inhalation [162,164]. A complete review of pulmonary peptide delivery can be found in the paper by Agu et al. [164].
Systemic peptide stability & site-specific delivery
Unfortunately, once the peptide has gained entrance to the systemic circulatory system, the task is only halfway complete. The protein must still reach its target site, and as many of the targets for protein drugs are intraceullular, this means transport through the circulation to the appropriate site, uptake by the appropriate cells and activity of the protein inside these cells. Therefore, the goals for the protein in the circulatory system include: avoidance of enzymatic degradation, opsonization and the RES, and non-selective accumulation of the protein, maintenance of protein solubility and activity, distribution to the site of action with targeting to certain cell types, cellular uptake, and release of the active protein. This portion of the paper will discuss many of the systems and methods mentioned earlier, but now focusing on issues within systemic circulation. Some of the systems discussed are not amenable to oral or transdermal delivery and would necessitate iv. delivery. Strategies discussed here include stability enhancers, drug carriers, endosomal escape and targeting moieties.
Systemic stability enhancement
Many of the stability enhancers discussed in the first portion of this review have a role in increasing the systemic stability of protein therapeutics as well. For example, fatty acid conjugation leads to extended plasma half-lives, site specific delivery and sustained release upon iv. administration [47]. As these drugs are lipophilic, they will likely be solubilized and stabilized by albumin and other serum lipoproteins [47]. Furthermore, these fatty acids can be removed from the protein via chemistry based on pH, reduction, peptidases, or esterases [47]. Non-reversible lipidization is also an option, and has been demonstrated to increase internalization and activity over non-lipidized counterparts [165].
PEGylation
PEGylation has also been used as a systemic stability enhancer. Direct PEGylation can aid in the stability of proteins for delivery, mainly leading to an increase in circulation time. PEG molecules are highly hydrated, and this increased size leads to decreased glomerular filtration [35]. Moreover, PEGylation of proteins is thought to reduce proteolysis and opsonization [166]. PEGylation also reduces uptake by the RES, decreases the formation of antibodies against the protein and decreases the apparent volume of distribution [34]. PEGylation, however, does have drawbacks. Due to the size of PEG, steric hindrance may decrease the activity of the protein. Also, increased protein aggregation after PEGylation has been noted [34]. Chronic iv. administration of PEG proteins has unintended consequences such as vacuolation of the renal cortical tubular epithelium in laboratory animals. However, these side effects were noted only after exposure to toxic, supratherapeutic doses of PEG. Newer PEGylation methods such as living radical polymerization, free radical polymerization, atom transfer radical polymerization and reversible addition fragment transfer have allowed PEGylation with greater specificity and purity while making modification with PEG a simpler task [167].
Hyperglycosylation
Hyperglycosylation has many of the same benefits as PEGylation, namely increased half-life, improved solubility and reduced immunogenicity [34]. An additional benefit is that the oligosaccharides added via glycosylation are natural and biodegradable, thus skirting the possible problem of PEG accumulation with chronic administration. The increased stability of hyper-glycosylated peptides may be due to masking hydrophobic sites on the protein surface involved in non-covalent interactions that lead to aggregation, loss of activity and/or increased immunogenicity [168]. Hyperglycosylated therapeutic proteins may, however, see decreased activity due to steric hindrance [34].
Liposomes
Liposomes demonstrate great potential as a carrier system for systemically administered protein therapeutics. If constructed from biocompatible and biodegradable materials, liposomes cause very little to no antigenic, pyrogenic, allergic or toxic reactions [169]. Furthermore, liposomes can be nonimmunogenic and have already demonstrated delivery of a variety of active protein therapies to cells in vivo [9]. Liposomes have been used to cross the blood–brain barrier to deliver an active enzyme when injected in the tail vein of a rat [9,170]. While first generation liposomes are easily cleared from the bloodstream and accumulate in Kupfer cells of the liver and macrophages in the spleen, advances have begun to reduce these problems [169]. To start, PEG-grafted liposomes have increased circulation time, reduced aggregation and decreased capture by the RES. PEGylated liposomes, or Steath™ liposomes (Johnson & Johnson, NJ, USA), have been used to deliver the anthracycline chemotherapeutic doxorubicin and were able to deliver preferentially to the tumor site, likely via the enhanced permeability and retention (EPR) effect [171].
Fusogenic modifications to liposomes
Many modifications have been made to liposomes to increase intracellular delivery of proteins. When liposomes enter the cell they are contained in an endosome. Particles smaller than 300 nm usually do not enter cells through the endosomal pathway, but particles 500–700 nm are often taken up by endocytosis [172]. If the liposome or the contents of the liposome do not escape the endosome, the endosome will deliver its contents to the lysosome, where the therapeutic peptide will be digested. One method of facilitating endosomal escape is to include a pH-sensitive element into the liposome. The pH in the endosome is approximately 5, and many systems take advantage of this relatively low pH to allow liposomes to escape the endosome [172]. Methods for endosomal escape include pore formation in the endosomal membrane, the proton sponge effect, and fusion with the endosomal membrane [172].
Pore formation is based on a pore-forming or pore-enlarging molecule binding to the rim of a pore in the endosome. Once bound, the pore-forming agent reduces tension in the membrane, which then keeps the pore radius stable [172]. Therefore, these agents act to stabilize naturally forming pores rather than to form pores de novo [173]. Pore forming compounds include penton base, cholera toxin, melittin (the major ingredient in bee venom) and Shiga toxin [174–177]. The pH buffering effect, also known as the proton sponge effect, occurs when the low pH in the endosome leads to the protonation of molecules contained inside the endosome. If the molecule has a high buffering capacity, protonation leads to an influx of H+, Cl- and H2O, resulting in osmotic swelling and eventual endosomal rupture [172]. Examples of molecules causing the proton sponge effect include gp41 with polyethyleneimine, poly (l-histidine) and chloroquine [178–181]. Fusion within the endosome requires fusogenic peptides that undergo conformational changes with the lowered pH, allowing fusion with the lipid bilayer of the endosome [172]. For example, a decrease in pH converts hemagglutinin, a protein in the capsid of the influenza virus, from an anionic hydrophilic coil to a hydrophobic helical conformation, followed by fusion of the viral membrane to the endosomal membrane. Fusogenic peptides used in liposomes include the HA-2 subunit of hemagglutinin, influenza-derived diINF-7, the major envelope protein E of the West Nile Virus, glycoprotein H from herpes simplex virus, and KALA based on the HA-2 subunit of influenza hemagglutinin [172,174,182–185]. For a complete discussion of endosomal escape pathways, please see Varkouhi et al. [172].
One more fusogenic agent worth mentioning in detail is dioleyl phosphoethanolamine, or DOPE. This peptide exhibits a conical shape due to its small and minimally hydrated head group compared with its highly lipophilic tail [169]. It can be used as a stabilizer in cationic liposomal membranes, but its major activity concerns endosomal escape [169]. As the pH drops in the endosome containing a DOPE-liposome, it is hypothesized that DOPE displays an inverted hexagonal phase, which in turn destabilizes the endosomal membrane [186]. DOPE has been used to deliver Print3G, a hydrophilic 25-amino acid antagonist of an oncoprotein involved in breast cancer. Print3G was enveloped in a Stealth™ pH-sensitive liposome and was able to deliver the peptide to the cytoplasm of cancerous cells [169]. While PEGylation reduced the pH-dependent release, it did not hinder the cytoplasmic delivery of the liposomal cargo [187].
Micelles
Due to their large size, liposomes may have difficulty reaching the desired site of action, as the liposome may be larger than the vascular cutoff size in certain tumors [188]. If this is the case, micelles may be a better alternative. A study by Weissig et al. demonstrated this by comparing micelle and liposome protein delivery side-by-side in a Lewis lung carcinoma mouse model. The PEG–micelle delivered more of the therapeutic protein at the desired site than the long-circulating PEG–liposome [189]. Micelles, however, have inherent problems that may prevent them from being used in the delivery of therapeutic proteins, including low drug loading capacity, low stability in water (especially when diluted), short half-life in biological environments and possible in vivo toxicity [190].
Nanoparticles
NPs play a role in the protection and delivery of peptides in systemic circulation as well. One example, the carbon nanotube, is well-studied and has been used to deliver proteins [191]. A 2005 study by Wong and colleagues allowed for the pro-apoptotic protein cytochrome c to spontaneously adsorb onto carbon nanotubes. The nanotubes were then incubated with a variety of cell lines and were taken up via energy-dependent endocytosis. Once in the cell, cytochrome c was released from the nanotube and caused increased apoptosis over the empty control nanotube [191]. It has been consistently reported that ‘well processed, water-soluble nanotubes exhibit no apparent cytotoxicity to all living cell lines investigated thus far, at least in the timeframe of days’ [192]. In general, carbon nanotubes have a high propensity to cross cell membranes with the apparent mechanism being passive and endocytosis independent [193,194]. A proposed mechanism for cell entry is similar to that of nanoneedles, where the NPs perforate and diffuse through the lipid bilayer without causing damage or death to the cells [194]. However, nanoparticle targeting is not optimal, and NPs often have poor tumor and tissue penetration. The EPR effect may also be overstated; thus passive targeting of NPs is not as good as once thought [195].
Functionalized NPs have been used to deliver antibodies, active proteins and epitope peptides to the immune system [194,196]. A recent study revealed new details on the mechanism of protein release from protein-loaded nanoparticle systems. The release of protein from an aliphatic polyester-based nanoparticle system was caused by bulk degradation of the nanoparticle. Moreover, intramolecular transesterification was followed by hydrolysis of the polymers, which caused the degradation [197]. Further, lyophilizing NPs led to a higher burst release of protein (40–50%) compared with nonlyophilized NPs (10–20%). The authors concluded therefore that freeze–drying forms pores in the NPs, thus facilitating burst release of encapsulated protein [197].
Many types of NPs other than carbon nano-tubes have been used in systemic drug delivery. A type of nanoparticle made of a PCL–PEO combination was able to demonstrate increased accumulation at the tumor site as well as reduced clearance by macrophages of the liver, thus increasing the possibility of the nanoparticle taking advantage of the EPR effect [198].
Cell penetrating peptides
While increased systemic circulation time and cargo stability are important factors, all of this is futile if the therapeutic is not internalized into the cells of interest. A promising and adaptable system for increased internalization is CPP. CPPs are short, water-soluble, poly basic peptides with a net positive charge at physiological pH [199]. CPPs are able to penetrate cell membranes at low micromolar concentrations without causing significant membrane damage [199]. The internalization method of these CPPs and their covalently attached cargo is still being debated; there is evidence of both energy-independent internalization and endocytosis as the mechanism of internalization. It is currently believed that endocytotic entry followed by endosomal escape is the most common entry pathway [200,201]. The endocytotic pathway is further broken down to include macro-pinocytosis and receptor-mediated endocytosis [200]. While receptor-mediated endocytosis relies on clathrin, caveolin, or both for internalization, macropinocytois may be internalized regardless of cell receptor status [200].
Targeting & membrane permeation
CPPs are a versatile tool that can be used for increased internalization of liposomes, NPs, or proteins themselves [202–204]. CPPs have been extensively researched as supplements to liposomes, and a few issues have been uncovered. First, CPPs such as TAT are susceptible to enzymatic cleavage by plasma enzymes when they are on the surface of liposomes [202]. Also, CPP-modified liposomes can cause severe toxicity and are rapidly cleared from the blood and accumulate in the kidney and liver; therefore, PEG modification is often necessary when using CPPs with liposomes [205–208]. Unfortunately, PEGylation of CPP-modified liposomes appears to decrease the effectiveness of the CPP [209]. TAT and arginine-rich CPPs have been used to target the kidney and spleen, respectively [210,211]. One study demonstrated that R8 (8 arginine repeat)-modified lipid NPs were able to efficiently deliver cargo (in this case siRNA) to the cytosol of cells in the liver [212].
CPPs have also been directly conjugated to proteins for delivery in vivo and in vitro [213–218]. One successful example of in vivo use delivered a single chain antibody Fv fragment to tumors, resulting in a decrease in tumor volume and neovascularization [216]. Targeted CPPs have also been discovered and designed. The specificity is often gained via activation of the CPP in the tumor environment or by conjugating a targeting moiety to the CPP [219,220,301]. Selectivity can also be obtained by having a homing motif in the CPP sequence; Nishimura et al. have discovered a CPP screened by phage display that selectively transduces leukemia cells [221]. The CPP consists of a lymph-node homing motif (CAY) and the CPP motif (RLRR), with the full sequence being CAYHRLRRC [221]. This CPP is currently being utilized in our laboratory to deliver a protein therapeutic for chronic myeloid leukaemia therapy. CPPs are appealing, as they may be able to increase the delivery of protein therapeutics through the cell membrane, escape from endosomes and get into the cytoplasm of the desired cells. Table 7 provides an representative example from a variety of CPP classes [217,219,221–223].
Table 7.
Sample cell-penetrating peptides and their applications in targeted and untargeted peptide delivery.
| Name/Description | Sequence | Category | Outcomes of interest | Ref. |
|---|---|---|---|---|
| TAT | CGRKKRRQRRRPPQC | Protein derived CPP from HIV-1 | Able to deliver peptides, proteins, nanoparticles and oligonucleotides intracellularly | [222] |
| Penetratin | RQIKIWFQNRRMKWKK | Protein derived CPP from Drosophila antennapedia domain | Able to deliver a variety of cargoes to many cell types | [223] |
| LS-CPP | CAYHRLRRC | Dual-motif CPP | Specific delivery to leukemia cells | [221] |
| Tumor prodrug CPP | EEEEEDDDDK/ARRRRRRRRR | Prodrug with tumor environment activation | Proteases in the tumor environment cleave the sequence, removing the acidic (negative) amino acid residues | [219] |
| Antibody–CPP conjugate | Penetratin+MAb CC49 | MAb targeted CPP | Increased tumor: normal tissue delivery ratio | [217] |
This table contains representative CPPs that represent the major classes of targeted and untargeted CPPs. TAT and Penetratin are generally untargeted, although their biodistribution may cause site-selective accumulation. LS-CPP is a leukemia-specific CPP. The tumor prodrug CPP is cleaved at the ‘/’ when in the tumor environment, thus separating the negatively charged amino acids from the positively charged CPP-cargo conjugate. Antibody-targeted CPPs can be used to selectively deliver a CPP–cargo conjugate to desired cell types.
CPP: Cell-penetrating peptide; LS-CPP: Leukemia-specific cell-penetrating peptide; MAb: Monoclonal antibody; TAT: Trans-activating transcriptional activator.
Antibodies are another modification strategy that has been implemented to increase the targeting ability of liposomes and NPs. One study by Kirpotin and colleagues demonstrated that monoclonal antibodies (MAb) directed against Her2/neu increased the cytoplasmic delivery of the liposomes contents [224]. Interestingly, in this study the MAb did not alter the biodistribution of the liposome, but rather increased MAb-mediated endocytosis, which increased drug delivery to the cytoplasm. Similar methods have been used with PLGA NPs [225]. Researchers were able to demonstrate in vitro selectivity and increased internalization of the MAb-adsorbed NPs [224]. As both liposomes and NPs can be loaded and/or coated with therapeutic peptides, targeting via antibodies can lead to increased peptide delivery to a specific site or cell type. The conjugation and adsorption of antibodies to liposomes and NPs is a promising field; further research will likely produce translatable results that will aid in the targeting of therapeutics.
Future perspective
While proteins and peptides have immense therapeutic potential, delivery and systemic stability currently limit their clinical use. The oral route is appealing, as it is a simple and often inexpensive route of delivery. Couple this with reduced consumption of the supplies needed for invasive delivery (iv., intramuscular, sc. and so on), and it is easy to see why patient compliance is highest for orally delivered drugs. Peptides and proteins are readily metabolized in the GI tract, and the hydrophilic nature of most natural peptides restricts movement across the epithelial barrier. Advances in the oral delivery of proteins and peptides have been made by the use of absorption enhancers, enzyme inhibitors and direct structural modification of the therapeutic. Muco-adhesive polymers, nanoemulsions and NPs have been utilized to increase the stability of peptides as well to increase their absorption. However, as of yet, no generalizable strategy for the delivery of peptide and protein therapeutics has been found; many of the strategies in this paper were customized to the peptide being delivered, as the complex nature and variety of peptides and proteins makes this difficult. Work on generalizable peptide delivery systems is ongoing; both the GI-MAPS and SNAC systems demonstrate encouraging data and appear to be well-suited to deliver a large range of peptides. With all of these systems, however, safety and efficacy questions loom large. Long-term safety has not been studied, and safety issues have arisen even with the short-term use of some of the compounds discussed in this review.
By delivering drugs transdermally, issues with GI stability can be avoided, but absorption still poses problems. Systems developed to overcome the barriers posed by the skin include micro-needle technology, thermal ablation, electro-poration, sonophoresis and iontophoresis. Each of these systems was designed with patient comfort and ease of use in mind. Despite some successes with transdermal delivery, most of the systems above have not been used to deliver therapeutic peptides or proteins to humans; further development and study in this area is necessary. While peptide delivery by other non-invasive routes (pulmonary, intranasal, buccal, rectal) is being studied, other reviews provide a more complete coverage of these topics.
After a peptide therapeutic enters the systemic circulation, it must remain active and reach the correct site/cell type in the body. Direct peptide modifications, liposomes, and NPs are used to increase stability, while the addition of antibodies and CPPs are used for targeting and to supplement cellular delivery. Endosomal escape, which threatens to inactivate the therapeutic peptide at the last stage, has also had some promising advances. It is important to note that some of the methods that increase systemic stability are not currently amenable to oral delivery (e.g., liposomes).
Peptide and protein therapeutics, with their high target specificity and broad applicability, have the potential to revolutionize medical therapy. Clearly, there are still challenges to overcome in each of the areas discussed. Optimally, in the future, there will be a system that can be used for the oral delivery and systemic stability of a variety of peptides and proteins. As delivery and systemic stability are two overarching issues with protein and peptide therapeutics, overcoming these would likely lead to even further development of peptide and protein therapeutics with great therapeutic potential.
Key Term.
Opsonization: The process by which an antibody recognizes and binds to foreign particles. After binding, the antibody–particle combination is phagocytosed or directly destroyed by via antibody–dependent cellular toxicity.
Key Terms.
P-glycoprotein: 170 kDa transmembrane ATP-dependent drug efflux pump, expressed in various compartments throughout the body. Also known as MDR1, the main function of P-glycoprotein is the transport of xenobiotics across membranes, actively pushing them out of a cell.
Direct PEGylation: The covalent attachment of PEG to the protein or peptide therapeutic. This is accomplished by incubating functionalized PEG molecules with the protein. The linkage is often on the side groups of basic, acidic or polar amino acids.
Key Term.
Hyperglycosylation: The addition of extra sugar molecules to the peptide to achieve many of the same benefits as PEGylation. This can be carried out by either specific chemical reactions in situ or by site-directed mutagenesis to create additional glycosylation sites into the sequence of the peptide.
Executive summary.
Oral delivery of peptide therapeutics
■ Barriers to oral delivery:
■ Gastrointestinal proteases, tight junctions, efflux pumps and the intestinal epithelial layer limit the oral delivery of peptides.
■ Methods to overcome the barriers:
■ A variety of absorption enhancers, direct peptide modifications, enzyme inhibitors and carrier molecules have been used to increase the bioavailability of orally delivered peptides;
■ Current modifications must be customized to the peptide being delivered, but versatile carrier systems are being developed.
Transdermal delivery
■ Barriers to transdermal delivery:
■ The skin itself physically acts as the major barrier to transdermal delivery, generally prohibiting passive entry of hydrophilic molecules and hydrophobic molecules greater than 500 D.
■ Methods to overcome the barriers:
■ Microneedle technology, thermal ablation, electroporation, sonophoresis, iontophoresis and biochemical enhancers are techniques and methods that are currently under study as a means to overcome transdermal peptide delivery barriers;
■ Systemic stability.
Systemic inactivation of peptide & protein drugs
■ After peptides enter systemic circulation they are susceptible to enzymatic degradation, opsonization, agglutination and poor solubility, all of which can lead to decreased protein activity.
■ Methods to increase systemic stability and site-specific targeting:
■ Combinations of PEGylation, hyperglycosylation, liposomes and nanoparticles can be used to increase peptide stability;
■ Cell-penetrating peptides and antibodies can be used for targeting and increased uptake of the therapeutic at the site of action.
Acknowledgements
The authors would like to thank N Cheatham for assistance with figure illustration.
This work was funded by NIH R01-CA129528, NIH R01-CA151847, and by an AFPE Pre-Doctoral Fellowship (BJ Bruno).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
References/Patent/Websites
- 1.Craik DJ, Fairlie DP, Liras S, Price D. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013;81(1):136–147. doi: 10.1111/cbdd.12055. [DOI] [PubMed] [Google Scholar]
- 2.Department of health and human services . US FDA. Background document for meeting of advisory committee for reproductive health drugs and drug safety and risk management advisory committee. Vol. 113. MD, USA: 2013. [Google Scholar]
- 3.Novartis . Novartis Annual Report. Basel, Switzerland: 2013. [Google Scholar]
- 4.Zacks Investment Research; Novartis AG . Brokerage Research Digest. IL, USA: 2013. [Google Scholar]
- 5.Mitchell M. The medicines company reports full year and fourth quarter 2011 financial results. NJ, USA: 2012. [Google Scholar]
- 6.Department of health and human services US FDA; Gill R. Desmopressin acetate drug use review in pediatric population. 2010 [Google Scholar]
- 7.Aungst B, Saitoh H, Burcham D, Huand S, Mousa S, Hussain M. Enhancement of the intestinal absorption of peptides and nonpeptides. J. Control. Release. 1996;41(1):19–31. [Google Scholar]
- 8.Borchardt T, Jeffrey A, Siahaan TJ, Gangwar S, Pauletti GM. Improvement of oral peptide bioavailability: peptidomimetics and prodrug strategies. Adv. Drug Deliv. Rev. 1997;27(2–3):235–256. doi: 10.1016/s0169-409x(97)00045-8. [DOI] [PubMed] [Google Scholar]
- 9.Torchilin V. Intracellular delivery of protein and peptide therapeutics. Drug Discov. Today Technol. 2009;5(2–3):e95–e103. doi: 10.1016/j.ddtec.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Maher S, Brayden D. Overcoming poor permeability: translating permeation enhancers for oral peptide delivery. Drug Discov. Today Technol. 2012;9(2):e113–e119. doi: 10.1016/j.ddtec.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Hamman JH, Enslin GM, Kotze AF. Oral delivery of peptide drugs: barriers and developments. BioDrugs. 2005;19(3):165–177. doi: 10.2165/00063030-200519030-00003. [DOI] [PubMed] [Google Scholar]
- 12.Shaji J, Patole V. Protein and Peptide drug delivery: oral approaches. Indian J. Pharm. Sci. 2008;70(3):269–277. doi: 10.4103/0250-474X.42967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahato RI, Narang AS, Thoma L, Miller DD. Emerging trends in oral delivery of peptide and protein drugs. Crit. Rev. Ther. Drug Carrier Syst. 2003;20(2–3):153–214. doi: 10.1615/critrevtherdrugcarriersyst.v20.i23.30. [DOI] [PubMed] [Google Scholar]
- 14.Fruton JS. A history of pepsin and related enzymes. Q. Rev. Biol. 2002;77(2):127–147. doi: 10.1086/340729. [DOI] [PubMed] [Google Scholar]
- 15.Yamanaka YJ, Leong KW. Engineering strategies to enhance nanoparticle-mediated oral delivery. J. Biomater. Sci. Polym. Ed. 2008;19(12):1549–1570. doi: 10.1163/156856208786440479. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Otin C, Palavalli LH, Samuels Y. Protective roles of matrix metalloproteinases: from mouse models to human cancer. Cell Cycle. 2009;8(22):3657–3662. doi: 10.4161/cc.8.22.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi KY, Swierczewska M, Lee S, Chen X. Protease-activated drug development. Theranostics. 2012;2(2):156–178. doi: 10.7150/thno.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carino GP, Mathiowitz E. Oral insulin delivery. Adv. Drug Deliv. Rev. 1999;35(2–3):249–257. doi: 10.1016/s0169-409x(98)00075-1. [DOI] [PubMed] [Google Scholar]
- 19.Pauletti G. Structural requirements for intestinal absorption of peptide drugs. J. Control. Release. 1996;41:3–17. [Google Scholar]
- 20.Khafagy El S, Morishita M. Oral biodrug delivery using cell-penetrating peptide. Adv. Drug Deliv. Rev. 2012;64(6):531–539. doi: 10.1016/j.addr.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Turner JR. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009;9(11):799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 22.Schulzke JD, Fromm M. Tight junctions: molecular structure meets function. Ann. N. Y. Acad. Sci. 2009;1165:1–6. doi: 10.1111/j.1749-6632.2009.04925.x. [DOI] [PubMed] [Google Scholar]
- 23.Eckford PD, Sharom FJ. ABC efflux pump-based resistance to chemotherapy drugs. Chem. Rev. 2009;109(7):2989–3011. doi: 10.1021/cr9000226. [DOI] [PubMed] [Google Scholar]
- 24.Kourtesi C, Ball AR, Huang YY, et al. Microbial efflux systems and inhibitors: approaches to drug discovery and the challenge of clinical implementation. Open Microbiol. J. 2013;7:34–52. doi: 10.2174/1874285801307010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou SF. Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. Xenobiotica. 2008;38(7–8):802–832. doi: 10.1080/00498250701867889. [DOI] [PubMed] [Google Scholar]
- 26.Sharom FJ, Didiodato G, Yu X, Ashbourne KJ. Interaction of the P-glycoprotein multidrug transporter with peptides and ionophores. J. Biol. Chem. 1995;270(17):10334–10341. doi: 10.1074/jbc.270.17.10334. [DOI] [PubMed] [Google Scholar]
- 27.Pond SM, Tozer TN. First-pass elimination. Basic concepts and clinical consequences. Clin. Pharmacokinet. 1984;9(1):1–25. doi: 10.2165/00003088-198409010-00001. [DOI] [PubMed] [Google Scholar]
- 28.Buxton I. Pharmacokinetics and pharmacodynamics: the dynamics of drug absorption, distribution, action, and elimination. In: Brunton L, Lazo J, Parker K, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. McGraw-Hill Medical Publishing Division; NY, USA: 2006. pp. 1–40. [Google Scholar]
- 29.Mahalakshmi R, Balaram P. The use of d-amino acids in peptide design. In: Konno R, Brückner H, D'Aniello A, Fisher GH, Fujii N, Homma H, editors. d-Amino Acids: A New Frontier in Amino Acid and Protein Research – Practical Methods and Protocols. Nova Biomedica Books; Hauppauge: 2007. pp. 415–430. [Google Scholar]
- 30.Kaminski HJ. Myasthenia Gravis and Related Disorders. Vol. 393. Blackwell Publishing; NJ, USA: 2008. Cyclosporine is derived from a fungus and is a cyclic undecapeptide with actions directed exclusively on T cells. [Google Scholar]
- 31.Mcmartin C, Hutchinson LE, Hyde R, Peters GE. Analysis of structural requirements for the absorption of drugs and macromolecules from the nasal cavity. J. Pharm. Sci. 1987;76(7):535–540. doi: 10.1002/jps.2600760709. [DOI] [PubMed] [Google Scholar]
- 32.Vlieghe P, Lisowski V, Martinez J, Khrestchatisky M. Synthetic therapeutic peptides: science and market. Drug Discov. Today. 2010;15(1–2):40–56. doi: 10.1016/j.drudis.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Greenwald RB, Choe YH, Mcguire J, Conover CD. Effective drug delivery by PEGylated drug conjugates. Adv. Drug Deliv. Rev. 2003;55(2):217–250. doi: 10.1016/s0169-409x(02)00180-1. [DOI] [PubMed] [Google Scholar]
- 34.Pisal DS, Kosloski MP, Balu-Iyer SV. Delivery of therapeutic proteins. J. Pharm. Sci. 2010;99(6):2557–2575. doi: 10.1002/jps.22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veronese FM, Harris JM. Introduction and overview of peptide and protein pegylation. Adv. Drug Deliv. Rev. 2002;54(4):453–456. doi: 10.1016/s0169-409x(02)00020-0. [DOI] [PubMed] [Google Scholar]
- 36.Webster R, Elliot V, Park B, Walker D, Hankin M, Taupin P. PEG and PEG conjugates toxicity: towards an understanding of the toxicity of PEG and its relevance to PEGylated biologicals. In: Veronese F, editor. PEGylated Protein Drugs: Basic Science and Clinical Applications. Birkhauser; Basel, Switzerland: 2009. pp. 127–146. [Google Scholar]
- 37.Laine GA, Hossain SM, Solis RT, Adams SC. Polyethylene glycol nephrotoxicity secondary to prolonged high-dose intravenous lorazepam. Ann. Pharmacother. 1995;29(11):1110–1114. doi: 10.1177/106002809502901107. [DOI] [PubMed] [Google Scholar]
- 38.Choi N, Lee J, Chang Y, et al. A population-based case-crossover study of polyethylene glycol use and acute renal failure risk in the elderly. World J. Gastroenterol. 2011;17(5):651–656. doi: 10.3748/wjg.v17.i5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishida T, Ichihara M, Wang X, Kiwada H. Spleen plays an important role in the induction of accelerated blood clearance of PEGylated liposomes. J. Control. Release. 2006;115(3):243–250. doi: 10.1016/j.jconrel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Park K. To PEGylate or not to PEGylate, that is not the question. J. Control. Release. 2010;142(2):147–148. doi: 10.1016/j.jconrel.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 41.Calceti P, Salmaso S, Walker G, Bernkop-Schnurch A. Development and in vivo evaluation of an oral insulin-PEG delivery system. Eur. J. Pharm. Sci. 2004;22(4):315–323. doi: 10.1016/j.ejps.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 42.Youn YS, Jung JY, Oh SH, Yoo SD, Lee KC. Improved intestinal delivery of salmon calcitonin by Lys18-amine specific PEGylation: stability, permeability, pharmacokinetic behavior and in vivo hypocalcemic efficacy. J. Control. Release. 2006;114(3):334–342. doi: 10.1016/j.jconrel.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Chalasani KB, Russell-Jones GJ, Yandrapu SK, Diwan PV, Jain SK. A novel vitamin B12-nanosphere conjugate carrier system for peroral delivery of insulin. J. Control. Release. 2007;117(3):421–429. doi: 10.1016/j.jconrel.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Petrus AK, Fairchild TJ, Doyle RP. Traveling the vitamin B12 pathway: oral delivery of protein and peptide drugs. Angew. Chem. Int. Ed. Engl. 2009;48(6):1022–1028. doi: 10.1002/anie.200800865. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Wu D, Shen WC. Structure-activity relationship of reversibly lipidized peptides: studies of fatty acid-desmopressin conjugates. Pharm. Res. 2002;19(5):609–614. doi: 10.1023/a:1015397811161. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Chow D, Heiati H, Shen WC. Reversible lipidization for the oral delivery of salmon calcitonin. J. Control. Release. 2003;88(3):369–380. doi: 10.1016/s0168-3659(03)00008-7. [DOI] [PubMed] [Google Scholar]
- 47.Hackett MJ, Zaro JL, Shen WC, Guley PC, Cho MJ. Fatty acids as therapeutic auxiliaries for oral and parenteral formulations. Adv. Drug Deliv. Rev. 2012 doi: 10.1016/j.addr.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verdine GL, Hilinski GJ. Stapled peptides for intracellular drug targets. Methods Enzymol. 2012;503:3–33. doi: 10.1016/B978-0-12-396962-0.00001-X. [DOI] [PubMed] [Google Scholar]
- 49.Schafmeister CE, Po J, Verdine GL. An allhydrocarbon cross-linking system for enhancing the helicity and metabolic stability of peptides. J. Am. Chem. Soc. 2000;122(24):5891–5892. [Google Scholar]
- 50.Walensky LD, Kung AL, Escher I, et al. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science. 2004;305(5689):1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller S, Reyna J, Ng S, Zuckermann R, Kerr J, Moos W. Comparison of the proteolytic susceptibilities of homologous l-amino acid, d-amino acid, and N-substituted glycine peptide and peptoid oligomers. Drug Dev. Res. 2004;35(1):20–32. [Google Scholar]
- 52.Tugyi R, Uray K, Ivan D, Fellinger E, Perkins A, Hudecz F. Partial D-amino acid substitution: improved enzymatic stability and preserved Ab recognition of a MUC2 epitope peptide. Proc. Natl Acad. Sci. USA. 2005;102(2):413–418. doi: 10.1073/pnas.0407677102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basu A, Yang K, Wang M, et al. Structure-function engineering of interferon-beta-1b for improving stability, solubility, potency, immunogenicity, and pharmacokinetic properties by site-selective mono-PEGylation. Bioconjug. Chem. 2006;17(3):618–630. doi: 10.1021/bc050322y. [DOI] [PubMed] [Google Scholar]
- 54.Leone-Bay A, Santiago N, Achan D, et al. N-acylated a-amino acids as novel oral delivery agents for proteins. J. Med. Chem. 1995;38(21):4263–4269. doi: 10.1021/jm00021a015. [DOI] [PubMed] [Google Scholar]
- 55.Kahns AH, Buur A, Bundgaard H. Prodrugs of peptides. 18. Synthesis and evaluation of various esters of desmopressin (dDAVP). Pharm. Res. 1993;10(1):68–74. doi: 10.1023/a:1018973029651. [DOI] [PubMed] [Google Scholar]
- 56.Cook CS, Karabatsos PJ, Schoenhard GL, Karim A. Species dependent esterase activities for hydrolysis of an anti-HIV prodrug glycovir and bioavailability of active SC-48334. Pharm. Res. 1995;12(8):1158–1164. doi: 10.1023/a:1016259826037. [DOI] [PubMed] [Google Scholar]
- 57.Fujii S, Yokoyama T, Ikegaya K, Sato F, Yokoo N. Promoting effect of the new chymotrypsin inhibitor FK-448 on the intestinal absorption of insulin in rats and dogs. J. Pharm. Pharmacol. 1985;37(8):545–549. doi: 10.1111/j.2042-7158.1985.tb03064.x. [DOI] [PubMed] [Google Scholar]
- 58.Ziv E, Lior O, Kidron M. Absorption of protein via the intestinal wall. A quantitative model. Biochem. Pharmacol. 1987;36(7):1035–1039. doi: 10.1016/0006-2952(87)90411-4. [DOI] [PubMed] [Google Scholar]
- 59.Bass T, Thacker P. Impact of gastric pH on dietary enzyme activity and survivability in swine fed b-glucanase supplemented diets. Can. J. Animal Sci. 1996;76(2):245–252. [Google Scholar]
- 60.Piper DW, Fenton BH. pH stability and activity curves of pepsin with special reference to their clinical importance. Gut. 1965;6(5):506–508. doi: 10.1136/gut.6.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knarreborg A, Jensen SK, Engberg RM. Pancreatic lipase activity as influenced by unconjugated bile acids and pH, measured in vitro and in vivo. J. Nutr. Biochem. 2003;14(5):259–265. doi: 10.1016/s0955-2863(03)00008-1. [DOI] [PubMed] [Google Scholar]
- 62.Hyun HH, Zeikus JG. General biochemical characterization of thermostable extracellular beta-amylase from clostridium thermosulfurogenes. Appl. Environ. Microbiol. 1985;49(5):1162–1167. doi: 10.1128/aem.49.5.1162-1167.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park K, Kwon I, Park KI. Oral protein delivery: current status and future prospects. Reactive Funct. Polymers. 2011;71(3):280–287. [Google Scholar]
- 64.Renukuntla J, Vadlapudi AD, Patel A, Boddu SH, Mitra AK. Approaches for enhancing oral bioavailability of peptides and proteins. Int. J. Pharm. 2013;447(1–2):75–93. doi: 10.1016/j.ijpharm.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bernkop-Schnurch A. The use of inhibitory agents to overcome the enzymatic barrier to perorally administered therapeutic peptides and proteins. J. Control. Release. 1998;52(1–2):1–16. doi: 10.1016/s0168-3659(97)00204-6. [DOI] [PubMed] [Google Scholar]
- 66.Uchiyama T, Kotani A, Kishida T, et al. Effects of various protease inhibitors on the stability and permeability of [d-Ala2,d-Leu5] enkephalin in the rat intestine: comparison with leucine enkephalin. J. Pharm. Sci. 1998;87(4):448–452. doi: 10.1021/js970357+. [DOI] [PubMed] [Google Scholar]
- 67.Chen JM, Dando PM, Rawlings ND, et al. Cloning, isolation, and characterization of mammalian legumain, an asparaginyl endopeptidase. J. Biol. Chem. 1997;272(12):8090–8098. doi: 10.1074/jbc.272.12.8090. [DOI] [PubMed] [Google Scholar]
- 68.Mcdonald JK, Reilly TJ, Zeitman BB, Ellis S. Dipeptidyl arylamidase II of the pituitary. Properties of lysylalanyl-beta-naphthylamide hydrolysis: inhibition by cations, distribution in tissues, and subcellular localization. J. Biol. Chem. 1968;243(8):2028–2037. [PubMed] [Google Scholar]
- 69.Bernkop-Schnurch A, Marschutz MK. Development and in vitro evaluation of systems to protect peptide drugs from aminopeptidase. N. Pharm. Res. 1997;14(2):181–185. doi: 10.1023/a:1012096510125. [DOI] [PubMed] [Google Scholar]
- 70.Hickey RJ. Bacitracin, its manufacture and uses. Prog. Ind. Microbiol. 1964;5:93–150. [PubMed] [Google Scholar]
- 71.Thanou M, Verhoef JC, Junginger HE. Chitosan and its derivatives as intestinal absorption enhancers. Adv. Drug Deliv. Rev. 2001;50(Suppl. 1):S91–S101. doi: 10.1016/s0169-409x(01)00180-6. [DOI] [PubMed] [Google Scholar]
- 72.Cano-Cebrian M, Zornoza T, Granero L, Polache A. Intestinal absorption enhancement via the paracellular route by fatty acids, chitosans, and others: a target for drug delivery. Curr. Drug Deliv. 2005;2(1):9–22. doi: 10.2174/1567201052772834. [DOI] [PubMed] [Google Scholar]
- 73.Smith J, Wood E, Dornish M. Effect of chitosan on epithelial cell tight junctions. Pharm. Res. 2004;21(1):43–49. doi: 10.1023/b:pham.0000012150.60180.e3. [DOI] [PubMed] [Google Scholar]
- 74.Bernkop-Schnurch A. Chitosan and its derivatives: potential excipients for peroral peptide delivery systems. Int. J. Pharm. 2000;194(1):1–13. doi: 10.1016/s0378-5173(99)00365-8. [DOI] [PubMed] [Google Scholar]
- 75.Schipper NG, Varum KM, Artursson P. Chitosans as absorption enhancers for poorly absorbable drugs. 1: Influence of molecular weight and degree of acetylation on drug transport across human intestinal epithelial (Caco-2) cells. Pharm. Res. 1996;13(11):1686–1692. doi: 10.1023/a:1016444808000. [DOI] [PubMed] [Google Scholar]
- 76.Schipper NG, Varum KM, Stenberg P, Ocklind G, Lennernas H, Artursson P. Chitosans as absorption enhancers of poorly absorbable drugs. 3: Influence of mucus on absorption enhancement. Eur. J. Pharm. Sci. 1999;8(4):335–343. doi: 10.1016/s0928-0987(99)00032-9. [DOI] [PubMed] [Google Scholar]
- 77.Lindmark T, Nikkila T, Artursson P. Mechanisms of absorption enhancement by medium chain fatty acids in intestinal epithelial Caco-2 cell monolayers. J. Pharmacol. Exp. Ther. 1995;275(2):958–964. [PubMed] [Google Scholar]
- 78.Sawada T, Ogawa T, Tomita M, Hayashi M, Awazu S. Role of paracellular pathway in nonelectrolyte permeation across rat colon epithelium enhanced by sodium caprate and sodium caprylate. Pharm. Res. 1991;8(11):1365–1371. doi: 10.1023/a:1015840921203. [DOI] [PubMed] [Google Scholar]
- 79.Sakai M, Imai T, Ohtake H, Azuma H, Otagiri M. Effects of absorption enhancers on the transport of model compounds in Caco-2 cell monolayers: assessment by confocal laser scanning microscopy. J. Pharm. Sci. 1997;86(7):779–785. doi: 10.1021/js960529n. [DOI] [PubMed] [Google Scholar]
- 80.Artursson P, Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991;175(3):880–885. doi: 10.1016/0006-291x(91)91647-u. [DOI] [PubMed] [Google Scholar]
- 81.Bies C, Lehr CM, Woodley JF. Lectin-mediated drug targeting: history and applications. Adv. Drug Deliv. Rev. 2004;56(4):425–435. doi: 10.1016/j.addr.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 82.Haas J, Lehr CM. Developments in the area of bioadhesive drug delivery systems. Expert Opin. Biol. Ther. 2002;2(3):287–298. doi: 10.1517/14712598.2.3.287. [DOI] [PubMed] [Google Scholar]
- 83.Fasano A, Baudry B, Pumplin DW, et al. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc. Natl Acad. Sci. USA. 1991;88(12):5242–5246. doi: 10.1073/pnas.88.12.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fasano A, Fiorentini C, Donelli G, et al. Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J. Clin. Invest. 1995;96(2):710–720. doi: 10.1172/JCI118114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang WL, Lu RL, Dipierro M, Fasano A. Zonula occludin toxin, a microtubule binding protein. World J. Gastroenterol. 2000;6(3):330–334. doi: 10.3748/wjg.v6.i3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fasano A, Uzzau S. Modulation of intestinal tight junctions by zonula occludens toxin permits enteral administration of insulin and other macromolecules in an animal model. J. Clin. Invest. 1997;99(6):1158–1164. doi: 10.1172/JCI119271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salama NN, Fasano A, Thakar M, Eddington ND. The impact of DeltaG on the oral bioavailability of low bioavailable therapeutic agents. J. Pharmacol. Exp. Ther. 2005;312(1):199–205. doi: 10.1124/jpet.104.073205. [DOI] [PubMed] [Google Scholar]
- 88.Song KH, Fasano A, Eddington ND. Effect of the six-mer synthetic peptide (AT1002) fragment of zonula occludens toxin on the intestinal absorption of cyclosporin A. Int. J. Pharm. 2008;351(1–2):8–14. doi: 10.1016/j.ijpharm.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 89.Morishita M, Kamei N, Ehara J, Isowa K, Takayama K. A novel approach using functional peptides for efficient intestinal absorption of insulin. J. Control. Release. 2007;118(2):177–184. doi: 10.1016/j.jconrel.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 90.Kamei N, Morishita M, Takayama K. Importance of intermolecular interaction on the improvement of intestinal therapeutic peptide/protein absorption using cell-penetrating peptides. J. Control. Release. 2009;136(3):179–186. doi: 10.1016/j.jconrel.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 91.Kamei N, Morishita M, Eda Y, Ida N, Nishio R, Takayama K. Usefulness of cell-penetrating peptides to improve intestinal insulin absorption. J. Control. Release. 2008;132(1):21–25. doi: 10.1016/j.jconrel.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 92.Hochman J, Artursson P. Mechanisms of absorption enhancement and tight junction regulation. J. Control. Release. 1994;1(29):253–257. [Google Scholar]
- 93.Anderberg EK, Artursson P. Epithelial transport of drugs in cell culture. VIII: effects of sodium dodecyl sulfate on cell membrane and tight junction permeability in human intestinal epithelial (Caco-2) cells. J. Pharm. Sci. 1993;82(4):392–398. doi: 10.1002/jps.2600820412. [DOI] [PubMed] [Google Scholar]
- 94.Anderberg EK, Nystrom C, Artursson P. Epithelial transport of drugs in cell culture. VII: Effects of pharmaceutical surfactant excipients and bile acids on transepithelial permeability in monolayers of human intestinal epithelial (Caco-2) cells. J. Pharm. Sci. 1992;81(9):879–887. doi: 10.1002/jps.2600810908. [DOI] [PubMed] [Google Scholar]
- 95.Swenson ES, Milisen WB, Curatolo W. Intestinal permeability enhancement: efficacy, acute local toxicity, and reversibility. Pharm. Res. 1994;11(8):1132–1142. doi: 10.1023/a:1018984731584. [DOI] [PubMed] [Google Scholar]
- 96.Davis SS, Illum L. Absorption enhancers for nasal drug delivery. Clin. Pharmacokinet. 2003;42(13):1107–1128. doi: 10.2165/00003088-200342130-00003. [DOI] [PubMed] [Google Scholar]
- 97.Asane GS, Nirmal SA, Rasal KB, Naik AA, Mahadik MS, Rao YM. Polymers for mucoadhesive drug delivery system: a current status. Drug Dev. Ind. Pharm. 2008;34(11):1246–1266. doi: 10.1080/03639040802026012. [DOI] [PubMed] [Google Scholar]
- 98.Bernkop-Schnurch A, Krajicek ME. Mucoadhesive polymers as platforms for peroral peptide delivery and absorption: synthesis and evaluation of different chitosan-EDTA conjugates. J. Control. Release. 1998;50(1–3):215–223. doi: 10.1016/s0168-3659(97)00136-3. [DOI] [PubMed] [Google Scholar]
- 99.Bernkop-Schnurch A. Thiomers: a new generation of mucoadhesive polymers. Adv. Drug Deliv. Rev. 2005;57(11):1569–1582. doi: 10.1016/j.addr.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 100.Lee K, Yuk S. Polymeric protein delivery systems. Prog. Polymer Sci. 2007;1(32):669–697. [Google Scholar]
- 101.Shah P, Bhalodia D, Shelat P. Nanoemulsion: a pharmaceutical review. Syst. Rev. Pharm. 2010;1(1):24–32. [Google Scholar]
- 102.Rao SV, Agarwal P, Shao J. Self-nanoemulsifying drug delivery systems (SNEDDS) for oral delivery of protein drugs: II. In vitro transport study. Int. J. Pharm. 2008;362(1–2):10–15. doi: 10.1016/j.ijpharm.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 103.Bindu Sri M, Ashok V, Arkendu C. As a review on hydrogels as drug delivery in the pharmaceutical field. Int. J. Pharm. Chem. Sci. 2012;1(2):642–661. [Google Scholar]
- 104.Peppas NA, Wood KM, Blanchette JO. Hydrogels for oral delivery of therapeutic proteins. Expert Opin. Biol. Ther. 2004;4(6):881–887. doi: 10.1517/14712598.4.6.881. [DOI] [PubMed] [Google Scholar]
- 105.Fricker G, Kromp T, Wendel A, et al. Phospholipids and lipid-based formulations in oral drug delivery. Pharm. Res. 2010;27(8):1469–1486. doi: 10.1007/s11095-010-0130-x. [DOI] [PubMed] [Google Scholar]
- 106.Chen Y, Ping Q, Guo J, Lv W, Gao J. The absorption behavior of cyclosporin A lecithin vesicles in rat intestinal tissue. Int. J. Pharm. 2003;261(1–2):21–26. doi: 10.1016/s0378-5173(03)00274-6. [DOI] [PubMed] [Google Scholar]
- 107.Werle M, Takeuchi H. Chitosan-aprotinin coated liposomes for oral peptide delivery: development, characterisation and in vivo evaluation. Int. J. Pharm. 2009;370(1–2):26–32. doi: 10.1016/j.ijpharm.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 108.Shah NM, Parikh J, Namdeo A, Subramanian N, Bhowmick S. Preparation, characterization and in vivo studies of proliposomes containing Cyclosporine A. J. Nanosci. Nanotechnol. 2006;6(9–10):2967–2973. doi: 10.1166/jnn.2006.403. [DOI] [PubMed] [Google Scholar]
- 109.Iwanaga K, Ono S, Narioka K, et al. Application of surface-coated liposomes for oral delivery of peptide: effects of coating the liposome's surface on the GI transit of insulin. J. Pharm. Sci. 1999;88(2):248–252. doi: 10.1021/js980235x. [DOI] [PubMed] [Google Scholar]
- 110.Jung T, Kamm W, Breitenbach A, Kaiserling E, Xiao JX, Kissel T. Biodegradable nanoparticles for oral delivery of peptides: is there a role for polymers to affect mucosal uptake? Eur. J. Pharm. Biopharm. 2000;50(1):147–160. doi: 10.1016/s0939-6411(00)00084-9. [DOI] [PubMed] [Google Scholar]
- 111.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces. 2010;75(1):1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 112.Jani P, Halbert GW, Langridge J, Florence AT. Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J. Pharm. Pharmacol. 1990;42(12):821–826. doi: 10.1111/j.2042-7158.1990.tb07033.x. [DOI] [PubMed] [Google Scholar]
- 113.Yah CS, Simate GS, Iyuke SE. Nanoparticles toxicity and their routes of exposures. Pak. J. Pharm. Sci. 2012;25(2):477–491. [PubMed] [Google Scholar]
- 114.Gao H, Wang YN, Fan YG, Ma JB. Synthesis of a biodegradable tadpole-shaped polymer via the coupling reaction of polylactide onto mono(6-(2-aminoethyl)amino-6-deoxy)-beta-cyclodextrin and its properties as the new carrier of protein delivery system. J. Control. Release. 2005;107(1):158–173. doi: 10.1016/j.jconrel.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 115.Damge C, Maincent P, Ubrich N. Oral delivery of insulin associated to polymeric nanoparticles in diabetic rats. J. Control. Release. 2007;117(2):163–170. doi: 10.1016/j.jconrel.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 116.Espuelas MS, Legrand P, Loiseau PM, Bories C, Barratt G, Irache JM. In vitro antileishmanial activity of amphotericin B loaded in poly(epsilon-caprolactone) nanospheres. J. Drug Target. 2002;10(8):593–599. doi: 10.1080/1061186021000060738. [DOI] [PubMed] [Google Scholar]
- 117.Sarmento B, Ribeiro A, Veiga F, Sampaio P, Neufeld R, Ferreira D. Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm. Res. 2007;24(12):2198–2206. doi: 10.1007/s11095-007-9367-4. [DOI] [PubMed] [Google Scholar]
- 118.Lu Z, Yeh TK, Tsai M, Au JL, Wientjes MG. Paclitaxel-loaded gelatin nanoparticles for intravesical bladder cancer therapy. Clin. Cancer Res. 2004;10(22):7677–7684. doi: 10.1158/1078-0432.CCR-04-1443. [DOI] [PubMed] [Google Scholar]
- 119.Zillies J, Coester C. Evaluating gelatin based nanoparticles as a carrier system for double stranded oligonucleotides. J. Pharm. Pharm. Sci. 2005;7(4):17–21. [PubMed] [Google Scholar]
- 120.Bajpai AK, Choubey J. Design of gelatin nanoparticles as swelling controlled delivery system for chloroquine phosphate. J. Mater. Sci. Mater. Med. 2006;17(4):345–358. doi: 10.1007/s10856-006-8235-9. [DOI] [PubMed] [Google Scholar]
- 121.Rajapaksa TE, Stover-Hamer M, Fernandez X, Eckelhoefer HA, Lo DD. Claudin 4-targeted protein incorporated into PLGA nanoparticles can mediate M cell targeted delivery. J. Control. Release. 2010;142(2):196–205. doi: 10.1016/j.jconrel.2009.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jani P, Halbert GW, Langridge J, Florence AT. The uptake and translocation of latex nanospheres and microspheres after oral administration to rats. J. Pharm. Pharmacol. 1989;41(12):809–812. doi: 10.1111/j.2042-7158.1989.tb06377.x. [DOI] [PubMed] [Google Scholar]
- 123.Storma G, Belliota SO, Lasicc T. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv. Drug Deliv. Rev. 1995;1(17):31. [Google Scholar]
- 124.Mathiowitz E, Jacob JS, Jong YS, et al. Biologically erodable microspheres as potential oral drug delivery systems. Nature. 1997;386(6623):410–414. doi: 10.1038/386410a0. [DOI] [PubMed] [Google Scholar]
- 125.Tobio M, Sanchez A, Vila A, et al. The role of PEG on the stability in digestive fluids and in vivo fate of PEG-PLA nanoparticles following oral administration. Colloids Surf. B Biointerfaces. 2000;18(3–4):315–323. doi: 10.1016/s0927-7765(99)00157-5. [DOI] [PubMed] [Google Scholar]
- 126.Morishita M, Peppas NA. Is the oral route possible for peptide and protein drug delivery? Drug Discov. Today. 2006;11(19–20):905–910. doi: 10.1016/j.drudis.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 127.Zhang N, Ping Q, Huang G, Han X, Cheng Y, Xu W. Transport characteristics of wheat germ agglutinin-modified insulin-liposomes and solid lipid nanoparticles in a perfused rat intestinal model. J. Nanosci. Nanotechnol. 2006;6(9–10):2959–2966. doi: 10.1166/jnn.2006.425. [DOI] [PubMed] [Google Scholar]
- 128.Garcia-Fuentes M, Torres D, Alonso MJ. New surface-modified lipid nanoparticles as delivery vehicles for salmon calcitonin. Int. J. Pharm. 2005;296(1–2):122–132. doi: 10.1016/j.ijpharm.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 129.Steinert RE, Poller B, Castelli MC, et al. Orally administered glucagon-like peptide-1 affects glucose homeostasis following an oral glucose tolerance test in healthy male subjects. Clin. Pharmacol. Ther. 2009;86(6):644–650. doi: 10.1038/clpt.2009.159. [DOI] [PubMed] [Google Scholar]
- 130.Chin J, Mahmud K, Kim S, Park K, Byun Y. Insight of current technologies for oral delivery of proteins and peptides. Drug Discov. Today Technol. 2012;9(2):e105–e112. doi: 10.1016/j.ddtec.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 131.Eiamtrakarn S, Itoh Y, Kishimoto J, et al. Gastrointestinal mucoadhesive patch system (GI-MAPS) for oral administration of G-CSF, a model protein. Biomaterials. 2002;23(1):145–152. doi: 10.1016/s0142-9612(01)00089-8. [DOI] [PubMed] [Google Scholar]
- 132.Kalluri H, Banga AK. Transdermal delivery of proteins. AAPS PharmSciTech. 2011;12(1):431–441. doi: 10.1208/s12249-011-9601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Herwadkar A, Banga AK. Peptide and protein transdermal drug delivery. Drug Discov. Today Technol. 2012;9(2):e147–e154. doi: 10.1016/j.ddtec.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 134.Arora A, Prausnitz MR, Mitragotri S. Micro-scale devices for transdermal drug delivery. Int. J. Pharm. 2008;364(2):227–236. doi: 10.1016/j.ijpharm.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Saroha K, Sharma B, Yadav B. Sonophoresis: an advanced tool in transdermal drug delivery system. Int. J. Curr. Pharm. Res. 2011;3(3):89–97. [Google Scholar]
- 136.Andrews SN, Jeong E, Prausnitz MR. Transdermal delivery of molecules is limited by full epidermis, not just stratum corneum. Pharm. Res. 2013;30(4):1099–1109. doi: 10.1007/s11095-012-0946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000;9(3):165–169. doi: 10.1034/j.1600-0625.2000.009003165.x. [DOI] [PubMed] [Google Scholar]
- 138.Ruan RQ, Wang SS, Wang CL, et al. Transdermal delivery of human epidermal growth factor facilitated by a peptide chaperon. Eur. J. Med. Chem. 2013;62:405–409. doi: 10.1016/j.ejmech.2012.12.054. [DOI] [PubMed] [Google Scholar]
- 139.Jitendra Sharma PK, Bansal S, Banik A. Noninvasive routes of proteins and peptides drug delivery. Indian J. Pharm. Sci. 2011;73(4):367–375. doi: 10.4103/0250-474X.95608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pathan IB, Setty CM. Chemical penetration enhancers for transdermal drug delivery systems. Trop. J. Pharm. Res. 2009;8(2):173–179. [Google Scholar]
- 141.Wilson EJ. Three Generations: The Past, Present, and Future of Transdermal Drug Delivery Systems. Pharmcon; SC, USA: 2011. [Google Scholar]
- 142.Badkar AV, Smith AM, Eppstein JA, Banga AK. Transdermal delivery of interferon a-2B using microporation and iontophoresis in hairless rats. Pharm. Res. 2007;24(7):1389–1395. doi: 10.1007/s11095-007-9308-2. [DOI] [PubMed] [Google Scholar]
- 143.Bloom BS, Brauer JA, Geronemus RG. Ablative fractional resurfacing in topical drug delivery: an update and outlook. Dermatol. Surg. 2013;39(6):839–848. doi: 10.1111/dsu.12111. [DOI] [PubMed] [Google Scholar]
- 144.Garland MJ, Caffarel-Salvador E, Migalska K, Woolfson AD, Donnelly RF. Dissolving polymeric microneedle arrays for electrically assisted transdermal drug delivery. J. Control. Release. 2012;159(1):52–59. doi: 10.1016/j.jconrel.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gratieri T, Alberti I, Lapteva M, Kalia YN. Next generation intra- and transdermal therapeutic systems: using non- and minimally-invasive technologies to increase drug delivery into and across the skin. Eur. J. Pharm. Sci. 2013;50(5):609–622. doi: 10.1016/j.ejps.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 146.Lee JW, Gadiraju P, Park JH, Allen MG, Prausnitz MR. Microsecond thermal ablation of skin for transdermal drug delivery. J. Control. Release. 2011;154(1):58–68. doi: 10.1016/j.jconrel.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Prausnitz MR, Langer R. Transdermal drug delivery. Nat. Biotechnol. 2008;26(11):1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Denet AR, Vanbever R, Preat V. Skin electroporation for transdermal and topical delivery. Adv. Drug Deliv. Rev. 2004;56(5):659–674. doi: 10.1016/j.addr.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 149.Transdermal Specialties Inc. U-strip Insulin Program: HPT-7 Clinical Trial Scheduled for 2013. Transdermal Specialties, Inc.; PA, USA: 2012. [Google Scholar]
- 150.Ghosh B, Iyer D, Nair AB, Sree HN. Prospects of iontophoresis in cardiovascular drug delivery. J. Basic Clin. Pharm. 2013;4(1):25–30. doi: 10.4103/0976-0105.109407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim YC, Late S, Banga AK, Ludovice PJ, Prausnitz MR. Biochemical enhancement of transdermal delivery with magainin peptide: modification of electrostatic interactions by changing pH. Int. J. Pharm. 2008;362(1–2):20–28. doi: 10.1016/j.ijpharm.2008.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim YC, Ludovice PJ, Prausnitz MR. Transdermal delivery enhanced by magainin pore-forming peptide. J. Control. Release. 2007;122(3):375–383. doi: 10.1016/j.jconrel.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kim YC, Ludovice PJ, Prausnitz MR. Optimization of transdermal delivery using magainin pore-forming peptide. J. Phys. Chem. Solids. 2008;69(5–6):1560–1563. doi: 10.1016/j.jpcs.2007.10.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen Y, Shen Y, Guo X, et al. Transdermal protein delivery by a coadministered peptide identified via phage display. Nat. Biotechnol. 2006;24(4):455–460. doi: 10.1038/nbt1193. [DOI] [PubMed] [Google Scholar]
- 155.Illum L. Nasal drug delivery: new developments and strategies. Drug Discov. Today. 2002;7(23):1184–1189. doi: 10.1016/s1359-6446(02)02529-1. [DOI] [PubMed] [Google Scholar]
- 156.Maggio ET. Intravail: highly effective intranasal delivery of peptide and protein drugs. Expert Opin. Drug. Deliv. 2006;3(4):529–539. doi: 10.1517/17425247.3.4.529. [DOI] [PubMed] [Google Scholar]
- 157.Pillion DJ, Hosmer S, Meezan E. Dodecylmaltoside-mediated nasal and ocular absorption of lyspro-insulin: independence of surfactant action from multimer dissociation. Pharm. Res. 1998;15(10):1637–1639. doi: 10.1023/a:1011975721569. [DOI] [PubMed] [Google Scholar]
- 158.Shojaei AH. Buccal mucosa as a route for systemic drug delivery: a review. J. Pharm. Pharm. Sci. 1998;1(1):15–30. [PubMed] [Google Scholar]
- 159.Gandhi P, Patel K. A review article on mucoadhesive buccal drug delivery system. Int. J. Pharm. Res. 2011;3(5):159–173. [Google Scholar]
- 160.Mujoriya R, Dhamande K, Wankhede U, Angure S. A review on study of buccal drug delivery system. Innovative Syst. Design Eng. 2011;2(3) [Google Scholar]
- 161.Lakshmi P, Deepthi B, Rama N. Rectal drug delivery: a promising route for enhancing drug absorption. Asian J. Res. Pharm. Sci. 2012;2(4):143–149. [Google Scholar]
- 162.Niven RW. Delivery of biotherapeutics by inhalation aerosol. Crit. Rev. Ther. Drug Carrier Syst. 1995;12(2–3):151–231. doi: 10.1615/critrevtherdrugcarriersyst.v12.i2-3.20. [DOI] [PubMed] [Google Scholar]
- 163.Bailey C. Why is Exubera being withdrawn? BMJ. 2007;335(7630):1156. [Google Scholar]
- 164.Agu R, Ugwoke M, Armand M, Kinget R. The lung as a route for systemic delivery of therapeutic proteins and peptides. Respir. Res. 2001;2(4):198–209. doi: 10.1186/rr58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kocevar N, Obermajer N, Strukelj B, Kos J, Kreft S. Improved acylation method enables efficient delivery of functional palmitoylated cystatin into epithelial cells. Chem. Biol. Drug Des. 2007;69(2):124–131. doi: 10.1111/j.1747-0285.2007.00477.x. [DOI] [PubMed] [Google Scholar]
- 166.Bailon P BW. Polyethylene glycol-conjugated pharmaceutical proteins. Pharm. Sci. Technol. Today. 1998;1:352–356. [Google Scholar]
- 167.Magnusson JS, Saeed AO, Fernández-Trillo F, Alexander C. Synthetic polymers for biopharmaceutical delivery. Polym. Chem. 2011;2:48–59. [Google Scholar]
- 168.Rajan RS, Li T, Aras M, et al. Modulation of protein aggregation by polyethylene glycol conjugation: GCSF as a case study. Protein Sci. 2006;15(5):1063–1075. doi: 10.1110/ps.052004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ducat E, Deprez J, Gillet A, et al. Nuclear delivery of a therapeutic peptide by long circulating pH-sensitive liposomes: benefits over classical vesicles. Int. J. Pharm. 2011;420(2):319–332. doi: 10.1016/j.ijpharm.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 170.Naoi M, Yagi K. Incorporation of enzyme through blood-brain barrier into the brain by means of liposomes. Biochem. Int. 1980;1:591–596. [Google Scholar]
- 171.Gabizon A, Martin F. Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Rationale for use in solid tumours. Drugs. 1997;54(Suppl. 4):15–21. doi: 10.2165/00003495-199700544-00005. [DOI] [PubMed] [Google Scholar]
- 172.Varkouhi AK, Scholte M, Storm G, Haisma HJ. Endosomal escape pathways for delivery of biologicals. J. Control. Release. 2011;151(3):220–228. doi: 10.1016/j.jconrel.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 173.Fuertes G, Gimenez D, Esteban-Martin S, Sanchez-Munoz OL, Salgado J. A lipocentric view of peptide-induced pores. Eur. Biophys. J. 2011;40(4):399–415. doi: 10.1007/s00249-011-0693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Prchla E, Plank C, Wagner E, Blaas D, Fuchs R. Virus-mediated release of endosomal content in vitro: different behavior of adenovirus and rhinovirus serotype 2. J. Cell. Biol. 1995;131(1):111–123. doi: 10.1083/jcb.131.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sun J, Pohl EE, Krylova OO, et al. Membrane destabilization by ricin. Eur. Biophys. J. 2004;33(7):572–579. doi: 10.1007/s00249-004-0400-9. [DOI] [PubMed] [Google Scholar]
- 176.Ogris M, Carlisle RC, Bettinger T, Seymour LW. Melittin enables efficient vesicular escape and enhanced nuclear access of nonviral gene delivery vectors. J. Biol. Chem. 2001;276(50):47550–47555. doi: 10.1074/jbc.M108331200. [DOI] [PubMed] [Google Scholar]
- 177.Sandvig K, Spilsberg B, Lauvrak SU, Torgersen ML, Iversen TG, Van Deurs B. Pathways followed by protein toxins into cells. Int. J. Med. Microbiol. 2004;293(7–8):483–490. doi: 10.1078/1438-4221-00294. [DOI] [PubMed] [Google Scholar]
- 178.Kwon EJ, Bergen JM, Pun SH. Application of an HIV gp41-derived peptide for enhanced intracellular trafficking of synthetic gene and siRNA delivery vehicles. Bioconjug. Chem. 2008;19(4):920–927. doi: 10.1021/bc700448h. [DOI] [PubMed] [Google Scholar]
- 179.Singh RS, Goncalves C, Sandrin P, Pichon C, Midoux P, Chaudhuri A. On the gene delivery efficacies of pH-sensitive cationic lipids via endosomal protonation: a chemical biology investigation. Chem. Biol. 2004;11(5):713–723. doi: 10.1016/j.chembiol.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 180.Boussif O, Lezoualc'h F, Zanta MA, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl Acad. Sci. USA. 1995;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu. Rev. Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- 182.Kimura T, Ohyama A. Association between the pH-dependent conformational change of West Nile flavivirus E protein and virus-mediated membrane fusion. J. Gen. Virol. 1988;69(Pt 6):1247–1254. doi: 10.1099/0022-1317-69-6-1247. [DOI] [PubMed] [Google Scholar]
- 183.Mastrobattista E, Koning GA, Van Bloois L, Filipe AC, Jiskoot W, Storm G. Functional characterization of an endosome-disruptive peptide and its application in cytosolic delivery of immunoliposome-entrapped proteins. J. Biol. Chem. 2002;277(30):27135–27143. doi: 10.1074/jbc.M200429200. [DOI] [PubMed] [Google Scholar]
- 184.Tu Y, Kim JS. A fusogenic segment of glycoprotein H from herpes simplex virus enhances transfection efficiency of cationic liposomes. J. Gene Med. 2008;10(6):646–654. doi: 10.1002/jgm.1184. [DOI] [PubMed] [Google Scholar]
- 185.Wyman TB, Nicol F, Zelphati O, Scaria PV, Plank C, Szoka FC. Design, synthesis, and characterization of a cationic peptide that binds to nucleic acids and permeabilizes bilayers. Biochemistry. 1997;36(10):3008–3017. doi: 10.1021/bi9618474. [DOI] [PubMed] [Google Scholar]
- 186.Lam JKW, Liang W. Endosomal escape pathways for non-viral nucleic acid delivery systems. In: Ceresa DB, editor. Molecular Regulation of Endocytosis. Vol. 465. InTech; Rijeka, Croatia: 2012. [Google Scholar]
- 187.Simoes S, Moreira JN, Fonseca C, Duzgunes N, De Lima MC. On the formulation of pH-sensitive liposomes with long circulation times. Adv. Drug Deliv. Rev. 2004;56(7):947–965. doi: 10.1016/j.addr.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 188.Torchilin VP, Lukyanov AN. Peptide and protein drug delivery to and into tumors: challenges and solutions. Drug Discov. Today. 2003;8(6):259–266. doi: 10.1016/s1359-6446(03)02623-0. [DOI] [PubMed] [Google Scholar]
- 189.Weissig V, Whiteman KR, Torchilin VP. Accumulation of protein-loaded long-circulating micelles and liposomes in subcutaneous Lewis lung carcinoma in mice. Pharmaceut. Res. 1998;15(10):1552–1556. doi: 10.1023/a:1011951016118. [DOI] [PubMed] [Google Scholar]
- 190.Kim S, Shi Y, Kim JY, Park K, Cheng JX. Overcoming the barriers in micellar drug delivery: loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin. Drug Deliv. 2010;7(1):49–62. doi: 10.1517/17425240903380446. [DOI] [PubMed] [Google Scholar]
- 191.Kam NW, Dai H. Carbon nanotubes as intracellular protein transporters: generality and biological functionality. J. Am. Chem. Soc. 2005;127:6021–6026. doi: 10.1021/ja050062v. [DOI] [PubMed] [Google Scholar]
- 192.Caocci G, Maioli MA, Atzeni S, Piras R, Carboni N, La Nasa G. Absence of histological myopathy in chronic myeloid leukemia patients complaining of muscle spasms and myalgia during treatment with nilotinib. Leuk. Res. 2012;36(9):e206–e208. doi: 10.1016/j.leukres.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 193.Pantarotto D, Briand JP, Prato M, Bianco A. Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem. Commun. (Camb.) 2004;1:16–17. doi: 10.1039/b311254c. [DOI] [PubMed] [Google Scholar]
- 194.Bianco A, Kostarelos K, Prato M. Applications of carbon nanotubes in drug delivery. Curr. Opin. Chem. Biol. 2005;9(6):674–679. doi: 10.1016/j.cbpa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 195.Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J. Control. Release. 2012;161(2):175–187. doi: 10.1016/j.jconrel.2011.09.063. [DOI] [PubMed] [Google Scholar]
- 196.Bale SS, Kwon SJ, Shah DA, Banerjee A, Dordick JS, Kane RS. Nanoparticle-mediated cytoplasmic delivery of proteins to target cellular machinery. ACS Nano. 2010;4(3):1493–1500. doi: 10.1021/nn901586e. [DOI] [PubMed] [Google Scholar]
- 197.Samadi N, Van Nostrum CF, Vermonden T, Amidi M, Hennink WE. Mechanistic studies on the degradation and protein release characteristics of poly(lactic-co-glycolic-cohydroxymethylglycolic acid) nanospheres. Biomacromolecules. 2013;14(4):1044–1053. doi: 10.1021/bm301900t. [DOI] [PubMed] [Google Scholar]
- 198.Shenoy DB, Amiji MM. Poly(ethylene oxide)-modified poly(epsilon-caprolactone) nanoparticles for targeted delivery of tamoxifen in breast cancer. Int. J. Pharm. 2005;293(1–2):261–270. doi: 10.1016/j.ijpharm.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 199.Jarver P, Langel U. Cell-penetrating peptides – a brief introduction. Biochim. Biophys. Acta. 2006;1758(3):260–263. doi: 10.1016/j.bbamem.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 200.Madani F, Lindberg S, Langel U, Futaki S, Graslund A. Mechanisms of cellular uptake of cell-penetrating peptides. J. Biophys. 2011;2011:414729. doi: 10.1155/2011/414729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.El-Andaloussi S, Holm T, Langel U. Cell-penetrating peptides: mechanisms and applications. Curr. Pharm. Des. 2005;11(28):3597–3611. doi: 10.2174/138161205774580796. [DOI] [PubMed] [Google Scholar]
- 202.Koren E, Apte A, Sawant RR, Grunwald J, Torchilin VP. Cell-penetrating TAT peptide in drug delivery systems: proteolytic stability requirements. Drug Deliv. 2011;18(5):377–384. doi: 10.3109/10717544.2011.567310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Torchilin VP, Levchenko TS, Rammohan R, Volodina N, Papahadjopoulos-Sternberg B, D'Souza GG. Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes. Proc. Natl Acad. Sci. USA. 2003;100(4):1972–1977. doi: 10.1073/pnas.0435906100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ziegler A, Nervi P, Durrenberger M, Seelig J. The cationic cell-penetrating peptide CPP (TAT) derived from the HIV-1 protein TAT is rapidly transported into living fibroblasts: optical, biophysical, and metabolic evidence. Biochemistry. 2005;44(1):138–148. doi: 10.1021/bi0491604. [DOI] [PubMed] [Google Scholar]
- 205.Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24(7):1121–1131. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- 206.Tseng WC, Haselton FR, Giorgio TD. Mitosis enhances transgene expression of plasmid delivered by cationic liposomes. Biochim. Biophys. Acta. 1999;1445(1):53–64. doi: 10.1016/s0167-4781(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 207.Koren E, Apte A, Jani A, Torchilin VP. Multifunctional PEGylated 2C5-immunoliposomes containing pH-sensitive bonds and TAT peptide for enhanced tumor cell internalization and cytotoxicity. J. Control. Release. 2012;160(2):264–273. doi: 10.1016/j.jconrel.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sarko D, Beijer B, Garcia Boy R, et al. The pharmacokinetics of cell-penetrating peptides. Mol. Pharm. 2010;7(6):2224–2231. doi: 10.1021/mp100223d. [DOI] [PubMed] [Google Scholar]
- 209.Torchilin VP, Rammohan R, Weissig V, Levchenko TS. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc. Natl Acad. Sci. USA. 2001;98(15):8786–8791. doi: 10.1073/pnas.151247498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nakamura Y, Kogure K, Futaki S, Harashima H. Octaarginine-modified multifunctional envelope-type nano device for siRNA. J. Control. Release. 2007;119(3):360–367. doi: 10.1016/j.jconrel.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 211.Akita H, Kogure K, Moriguchi R, et al. Nanoparticles for ex vivo siRNA delivery to dendritic cells for cancer vaccines: programmed endosomal escape and dissociation. J. Control. Release. 2010;143(3):311–317. doi: 10.1016/j.jconrel.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 212.Hayashi Y, Yamauchi J, Khalil IA, Kajimoto K, Akita H, Harashima H. Cell penetrating peptide-mediated systemic siRNA delivery to the liver. Int. J. Pharm. 2011;419(1–2):308–313. doi: 10.1016/j.ijpharm.2011.07.038. [DOI] [PubMed] [Google Scholar]
- 213.Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat. Med. 2004;10(3):310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 214.Shokolenko IN, Alexeyev MF, Ledoux SP, Wilson GL. TAT-mediated protein transduction and targeted delivery of fusion proteins into mitochondria of breast cancer cells. DNA Repair (Amst.) 2005;4(4):511–518. doi: 10.1016/j.dnarep.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 215.Jo D, Liu D, Yao S, Collins RD, Hawiger J. Intracellular protein therapy with SOCS3 inhibits inflammation and apoptosis. Nat. Med. 2005;11(8):892–898. doi: 10.1038/nm1269. [DOI] [PubMed] [Google Scholar]
- 216.Shin I, Edl J, Biswas S, Lin PC, Mernaugh R, Arteaga CL. Proapoptotic activity of cell-permeable anti-Akt single-chain antibodies. Cancer Res. 2005;65(7):2815–2824. doi: 10.1158/0008-5472.CAN-04-2898. [DOI] [PubMed] [Google Scholar]
- 217.Jain M, Chauhan SC, Singh AP, Venkatraman G, Colcher D, Batra SK. Penetratin improves tumor retention of single-chain antibodies: a novel step toward optimization of radioimmunotherapy of solid tumors. Cancer Res. 2005;65(17):7840–7846. doi: 10.1158/0008-5472.CAN-05-0662. [DOI] [PubMed] [Google Scholar]
- 218.Mae M, Langel U. Cell-penetrating peptides as vectors for peptide, protein and oligonucleotide delivery. Curr. Opin. Pharmacol. 2006;6(5):509–514. doi: 10.1016/j.coph.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 219.Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc. Natl Acad. Sci. USA. 2004;101(51):17867–17872. doi: 10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.D'ursi AM, Giusti L, Albrizio S, et al. A membrane-permeable peptide containing the last 21 residues of the G a(s) carboxyl terminus inhibits G(s)-coupled receptor signaling in intact cells: correlations between peptide structure and biological activity. Mol. Pharmacol. 2006;69(3):727–736. doi: 10.1124/mol.105.017715. [DOI] [PubMed] [Google Scholar]
- 221.Nishimura S, Takahashi S, Kamikatahira H, et al. Combinatorial targeting of the macropinocytotic pathway in leukemia and lymphoma cells. J. Biol. Chem. 2008;283(17):11752–11762. doi: 10.1074/jbc.M708849200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Silhol M, Tyagi M, Giacca M, Lebleu B, Vives E. Different mechanisms for cellular internalization of the HIV-1 Tat-derived cell penetrating peptide and recombinant proteins fused to Tat. Eur. J. Biochem. 2002;269(2):494–501. doi: 10.1046/j.0014-2956.2001.02671.x. [DOI] [PubMed] [Google Scholar]
- 223.Zorko M, Langel U. Cell-penetrating peptides: mechanism and kinetics of cargo delivery. Adv. Drug Deliv. Rev. 2005;57(4):529–545. doi: 10.1016/j.addr.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 224.Kirpotin DB, Drummond DC, Shao Y, et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66(13):6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 225.Kocbek P, Obermajer N, Cegnar M, Kos J, Kristl J. Targeting cancer cells using PLGA nanoparticles surface modified with monoclonal antibody. J. Control. Release. 2007;120(1–2):18–26. doi: 10.1016/j.jconrel.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 301.Hällbrink M, Pooga M, Metsis M, et al. 2003 WO2003106491.
- 401.Victoza Sales Data. www.drugs.com/stats/victoza.
- 402.UpToDate® clinical decision support resource. www.uptodate.com. [Google Scholar]
- 403.Genetic Engineering and Biotechnology News. Top 20 Best-Selling Drugs of 2012. www.genengnews.com/insight-and-intelligence/top-20-best-selling-drugs-of-2012/77899775.