Abstract
Background
Blue-gray ovoids (B-GOs), a critical dermoscopic structure for basal cell carcinoma (BCC), offer an opportunity for automatic detection of BCC. Due to variation in size and color, B-GOs can be easily mistaken for similar structures in benign lesions. Analysis of these structures could afford accurate characterization and automatic recognition of B-GOs, furthering the goal of automatic BCC detection. This study utilizes a novel segmentation method to discriminate B-GOs from their benign mimics.
Methods
Contact dermoscopy images of 68 confirmed BCCs with B-GOs were obtained. Another set of 131 contact dermoscopic images of benign lesions possessing B-GO mimics provided a benign competitive set. A total of 22 B-GO features were analyzed for all structures: 21 color features and one size feature. Regarding segmentation, this study utilized a novel sector-based, non-recursive segmentation method to expand the masks applied to the B-GOs and mimicking structures.
Results
Logistic regression analysis determined that blue chromaticity was the best feature for discriminating true B-GOs in BCC from benign, mimicking structures. Discrimination of malignant structures was optimal when the final B-GO border was approximated by a best-fit ellipse. Using this optimal configuration, logistic regression analysis discriminated the expanded and fitted malignant structures from similar benign structures with a classification rate as high as 96.5%.
Conclusions
Experimental results show that color features allow accurate expansion and localization of structures from seed areas. Modeling these structures as ellipses allows high discrimination of B-GOs in BCCs from similar structures in benign images.
Keywords: Blue-Gray Ovoids, Image Analysis, Basal Cell Carcinoma, Skin Lesion, Dermoscopy, Region Growing, Computational Intelligence
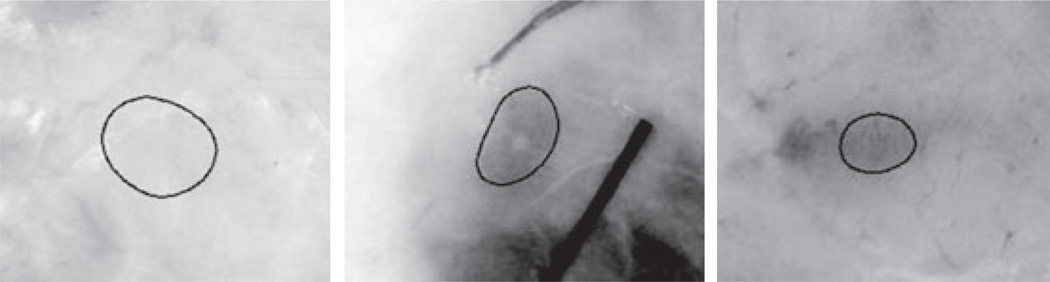
With an estimated 3.5 million new cases annually in the US, Basal cell carcinoma (BCC) is the most commonly occurring cancer. (1). Dermoscopy aids physicians in the early detection of BCC, allowing visualization of structures which cannot normally be seen without instrumentation. The classic BCC structures visible with the dermatoscope (3Gen LLC, San Juan Capistrano, CA; Heine Optotechnik, Herrsching, Germany) are called the BASAL features: Blue-gray ovoids and globules (B-GOs), Arborizing telangiectasia, Semitranslucency/Spoke wheel structures, Atraumatic ulcerations, and Leaf-like structures (2, 3). B-GOs vary in color from light blue-gray to dark blue-gray (Fig. 1).
Fig. 1.
Dermoscopy images of blue-gray ovoids in BCCs. Hue, brightness, shape, and size vary significantly.
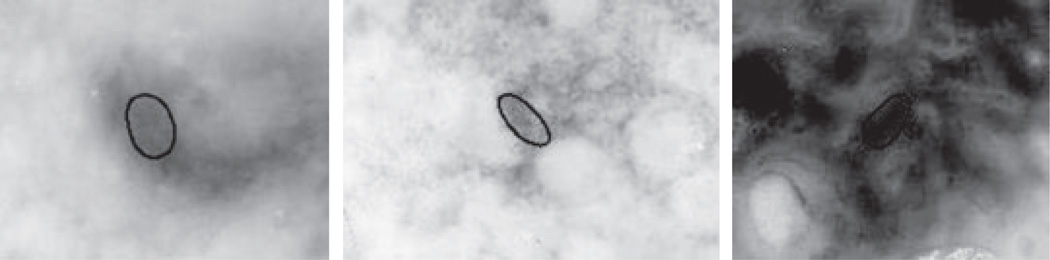
These objects are the dermoscopic equivalent of the clusters of pigmented basal cell tumor islands, which are the histopathologic hallmark of this skin malignancy (4). Because of the variation in color and size of these objects, B-GOs can be mimicked by any structure lying deeper in the skin (Fig. 2), creating a challenge for automatic detection of these structures. This article focuses on semi-automatic discrimination of these structures from mimics, using sector-based structure expansion and statistical modeling by logistic regression. The remainder of this article includes II. Materials and Methods, III. Experiments and Results, and IV. Discussion.
Fig. 2.
Examples of similar blue-gray structures found in various benign lesions. Note that hue, brightness, shape, size and indistinct borders can resemble those same features in blue-gray ovoids.
Materials and Methods
Data sets used
In this study, 199 contact, non-polarized dermoscopic images, having full color and 1024 × 768 resolution, were collected for the study SBIR R44 CA-101639-02A2 of the National Institutes of Health (NIH) and approved by the Phelps County Regional Medical Center Institutional Review Board, Rolla, MO, under the guidelines of the Belmont Report. Of these 199 images, 68 were basal cell carcinoma confirmed by histopathology. The competitive set consisted of 131 images of benign dermoscopy lesions on the head and neck that exhibited features which mimicked B-GO structures. To use the relative color method of Cheng et al. (5), borders were drawn by a dermatologist for all 199 lesions within the images using a second-order spline function. Outlines were drawn around the B-GO structures and their mimics using the spline function, shown in Figs 1 and 2. These images were converted to binary image masks to assist in computation of the color and texture features.
Sector-based expansion
The centroid of each B-GO structure was determined. An optimized median filter was applied for pre-processing. Fast sector-based expansion, following the methods of (Guvenc et al., unpublished data), was used. This method starts with an 8 × 8 block which is expanded by sectors, using criteria determined by logistic regression, as illustrated in Fig. 3.
Fig. 3.
12-step sector expansion with first three sectors shown expanded from original 8 × 8 block.
Using logistic regression, blue chromaticity and red variance located at B-GO boundaries, were found to be the two most significant features that accurately segment the B-GO from surrounding regions. These two features were used as the criteria for expansion with the bounds, natural process or upper and lower control limits (UCL and LCL) defined as μ ± 3σ (6) Eq. (1). Inclusion of a sector within the B-GO area required that both the blue chromaticity and red variance values in that sector lie within the upper and lower control limits. Because the UCL and LCL are calculated based solely on the original 8 × 8 block, no re-calculations between expansions need occur, consequently improving the efficiency of the expansion algorithms.
| (1) |
μBlockBlueChrom denotes the average blue chromaticity within the initial 8 × 8 seed block; the other variables are similarly calculated.
Applying the best-fit ellipse on the expanded B-GO masks
Analysis of the initial 199 border masks generated for the B-GOs revealed irregular, jagged edges (Fig. 4). Very jagged outlines were particularly evident in the B-GO mimics that populate benign lesions. The least-square ellipse fitting method (7) was used to create the best-fit ellipse that circumscribes the irregular spot masks Eq. (2).
| (2) |
Fig. 4.
Result of 12-sector expansion of a B-GO.
In Eq. (2), u represents a vector of points. Here, the points are the white pixels on the edge of the binary, irregular, spot-mask images. The Canny edge detection algorithm was applied to the binary mask to extract the boundary points, thereby creating the vector used in applying the best-fit ellipse, shown in Fig. 5.
Fig. 5.
(a) Canny edge detection algorithm applied to irregular spot mask. (b) Best-fit ellipse created from irregular spot mask. (c) Overlap of the two masks. (d) Overlap superposed with original blue-gray ovoid image.
Color features obtained from B-GO masks—applying the best-fit ellipse on the expanded B-GO masks
Using the final B-GO mask synthesized from the original B-GO mask and best-fit ellipse, 21 color features were determined to be the most effective features for characterizing B-GOs (see Table 1).
TABLE 1.
Color feature equations.
| Color Features | Equations |
|---|---|
| Absolute RGB | Absolute R = Red Average over mask |
| Absolute G = Green Average over maskE* | |
| Absolute B = Blue Average over maskE*, # | |
| Relative RGB | Relative R = Absolute R - Skin R |
| Relative G = Absolute G - Skin GE* | |
| Relative B = Absolute G - Skin B | |
| RGB Chromaticity | R Chromaticity = R/(R+G+B)# |
| G Chromaticity = G/(R+G+B) | |
| B Chromaticity = B/(R+G+B) | |
| Relative RGB Chromaticity | Relative R Chromaticity = Relative R/Relative(R+G+B) |
| Relative G Chromaticity = Relative G/Relative(R+G+B)# | |
| Relative B Chromaticity = Relative B/Relative(R+G+B) | |
| RGB Variance | R Variance = Σ(R- total_R)2 |
| G Variance = Σ(G- total_G)2 | |
| B Variance = Σ(B- total_B)2 E*, # | |
| Relative Color Ratios | Relative B/Relative R |
| Relative B/Relative G | |
| Relative G/Relative R | |
| Converting RGB Color Plane to XYZ Color Plane | R to X = (R*0.49 + G*0.31 + B*0.20)/0.17697E* |
| G to Y = (R*0.17697 + G*0.81240 + B*0.01063)/0.17697 | |
| B to Z = (R*0.0 + G*0.01 + B*0.99)/0.17697# |
Features in the final logistic regression model for BCC identification are marked by an emboldened “E*”, indicating their retention within the best-fit ellipse circumscribing the B-GO.
“#” indicates the final model of the expanded B-GO mask
Experiments and Results
This study employed a logistic regression model (SAS PROC LOGIT, SAS Inc., Cary, NC, USA) to determine the success rate for discriminating expanded B-GOs from benign mimics. The two sets of images used for the model included 68 BCC lesions, and 131 benign lesions containing B-GO mimics (as described in Section IIA—Data Sets). Twenty-one color features and the final, expanded B-GO size comprised the data set of the model.
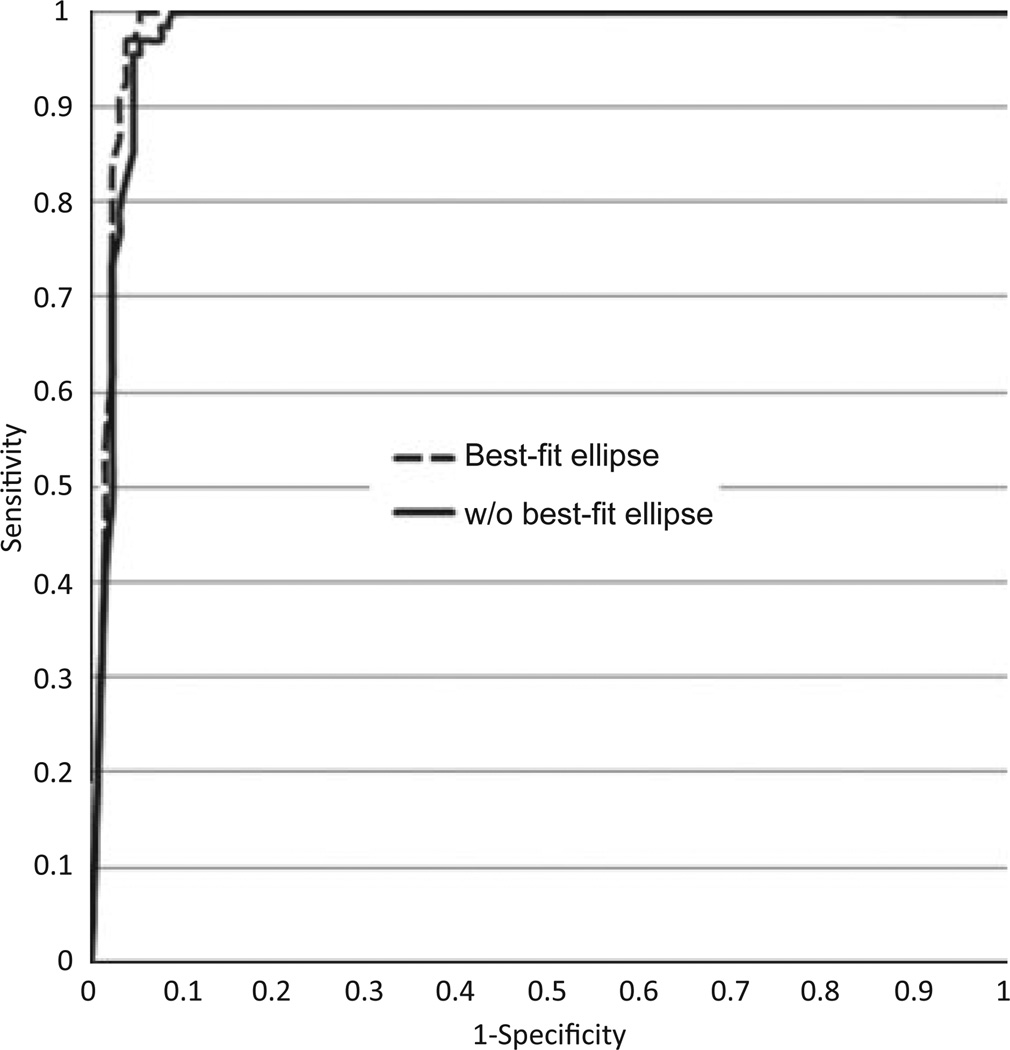
Two methods were used to discriminate BCC from benign lesions. The first method applied logistic regression model (SAS PROC LOGIT) which included 21 color features and the final size of the expanded B-GO generated by the expanded mask, resulted in a correct diagnosis in 95.5% of cases. This first logistic regression model retained six features: size of the final expanded B-GO (p < 0.001, χ2 = 89.27); and five color features, which in order include: blue variance, absolute green, red in XYZ plane, absolute blue, and relative green. All five color features had p > 0.05. The identification success rate of this model was calculated using the receiver operating characteristic (ROC) curve (shown in Fig. 6, solid line). Calculating the area under the curve resulted in a success rate of 0.986(given by the c variable in SAS PROC LOGIT).
Fig. 6.
The receiver operating characteristic (ROC) curve comparing sensitivity and 1-specificity of the obtained SAS logistic regression results using 21 color features and one feature for the size of the B-GOs with and without ellipse fitting.
The second method applied the best-fit ellipse for the expanded B-GO mask (Fig. 5). The second logistic regression model for the B-GO detected using the best-fit ellipse resulted in a correct diagnosis in 96.5% of the cases. The second logistic regression model also retained six features: size of the final expanded B-GO (p < 0.001, χ2 = 89.18); and five color features, which in order include: blue variance, absolute red chromaticity, relative green chromaticity, blue in XYZ plane, and absolute blue. Blue variance had a P value of 0.022, with no other color feature having P < 0.05. The receiver operating characteristic (ROC) curve for this model is shown in Fig. 6 (dashed line). The estimate for this model’s success rate via calculation of the area under the curve was 0.988 (given by the c variable in SAS PROC LOGIT).
Discussion
This study is the first to focus on the differentiation of B-GO structures in BCCs from similar structures in benign mimics. The study determined that analysis of B-GOs will differentiate BCC from similar benign lesions with high accuracy. The methods employed in differentiating BCCs from benign lesions included the analysis of spot samples within the lesion, a method first employed by Celebi (8) to describe blue-veil structures in melanoma. The Celebi spot-analysis method was extended by analyzing spot features to automatically detect B-GO borders.
B-GOs can be difficult to distinguish from similar benign structures. Accurate differentiation of B-GOs from mimics could aid in the automatic detection of BCCs at an earlier stage, thus reducing the cost and complexity of surgery incurred in treating this form of skin cancer, in later stages.
The sector-based expansion employed to find the periphery of B-GOS is non-recursive, i.e., the decision to stop expansion is automatically made without re-calculating properties of areas already added to the body of the B-GO. Automatic cessation of expansion was accomplished using the control limits equation Eq. (2). Using 8 × 8 seed blocks at the B-GOs centroids as points from which B-GO analysis and expansion would originate was theoretically, intuitively, and experimentally determined by the principal author. The expansion method can be effectively employed to determine the borders of any structure whose features effectively characterize the structure, but whose borders are fuzzy.
The ellipse-fitting process operates as follows: Guided by sector feature analysis which characterized B-GO areas, the borders of B-GO areas were found and demarcated by an elliptical mask that automatically circumscribed and fitted the B-GOs. We have used ellipse-fitting to better characterize the automatically determined borders. The higher-level knowledge of the limits of B-GO areas helped foster the constitution of a model that optimally characterizes B-GOs, thereby improving the differentiation of these structures from close mimics. Evaluation of the discrimination success rate using the area under the ROC curve, shows that there is slightly more (better) area under the curve when B-GOs are modeled as elliptical shapes. For both B-GO models, the size of the B-GO is the most significant feature in differentiating B-GOs in BCC from B-GO mimics in benign lesions. Color features were also useful in the final version of the model, because the variance of over the B-GO area is the most useful color measure in both models.
Acknowledgements
This publication was made possible by SBIR GrantsR43 CA153927-01 and CA101639-02A2 of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB, Coldiron BM. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 2.Stoecker WV, Stolz W. Dermoscopy and the diagnostic challenge of amelanotic and hypomelanotic melanoma. Arch Dermatol. 2010;144:1120–1127. doi: 10.1001/archderm.144.9.1207. [DOI] [PubMed] [Google Scholar]
- 3.Altamura D, Menzies SW, Argenziano G, Zalaudek I, Soyer HP, Sera F, Avramidis M, DeAmbrosis K, Fargnoli MC, Peris K. Dermatoscopy of basal cell carcinoma: morphologic variability of global and local features and accuracy of diagnosis. J Am Acad Dermatol. 2010;62:67–75. doi: 10.1016/j.jaad.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 4.Agero AL, Busam KJ, Benvenuto-Andrade C, Scope A, Gill M, Marghoob AA, González S, Halpern AC. Reflectance confocal microscopy of pigmented basal cell carcinoma. J Am Acad Dermatol. 2006;53:638–643. doi: 10.1016/j.jaad.2005.11.1096. [DOI] [PubMed] [Google Scholar]
- 5.Cheng Y, Swamisai R, Umbaugh SE, Moss RH, Stoecker WV, Teegala S, Srinivasan SK. Skin Lesion Classification Using Relative Color Features. Skin Res Technol. 2008;14:53–64. doi: 10.1111/j.1600-0846.2007.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute of Technical Standards (NIST) Department of Commerce. [accessed February 23, 2012]; http://www.itl.nist.gov/div898/handbook/pmc/section3/pmc31.htm.
- 7.Gander W, Golub GH, Strebel R. Least-Squares Fitting of Circles and Ellipses. BIT. 1994;34:558–578. [Google Scholar]
- 8.Celebi ME, Iyatomi H, Stoecker WV, Moss RH, Rabinovitz HS, Argenziano G, Soyer HP. Automatic detection of blue-white veil and related structures in dermoscopy images. Comput Med Imaging Graph. 2008;32:670–677. doi: 10.1016/j.compmedimag.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]