Abstract
Smoking-related lung diseases are among the leading causes of death worldwide, underscoring the need to understand their pathogenesis and develop new effective therapies. We have shown that CD1a+ antigen-presenting cells (APCs) from lungs of patients with emphysema can induce autoreactive T helper 1 (TH1) and TH17 cells. Similarly, the canonical cytokines interferon-γ (IFN-γ) and interleukin-17A (IL-17A) are specifically linked to lung destruction in smokers, but how smoke activates APCs to mediate emphysema remains unknown. Here, we show that, in addition to increasing IFN-γ expression, cigarette smoke increased the expression of IL-17A in both CD4+ and γδ T cells from mouse lung. IL-17A deficiency resulted in attenuation of, whereas lack of γδ T cells exacerbated, smoke-induced emphysema in mice. Adoptive transfer of lung APCs isolated from mice with emphysema revealed that this cell population was capable of transferring disease even in the absence of active smoke exposure, a process that was dependent on IL-17A expression. Spp1 (the gene for osteopontin) was highly expressed in the pathogenic lung APCs of smoke-exposed mice and was required for the TH17 responses and emphysema in vivo, in part through its inhibition of the expression of the transcription factor Irf7. Thus, the Spp1-Irf7 axis is critical for induction of pathological TH17 responses, revealing a major mechanism by which smoke activates lung APCs to induce emphysema and identifying a pathway that could be targeted for therapeutic purposes.
INTRODUCTION
The global burden of smoking-induced chronic obstructive pulmonary disease (COPD), encompassing chronic bronchitis and emphysema, exacts a large and rapidly increasing toll on human health and society. Over the next decade, COPD is expected to become the fifth leading cause of death worldwide, and for the foreseeable future, lung cancer, the incidence of which is increased in emphysema, will continue to kill more smokers than all other cancers combined (1–3). Yet, despite the massive impact of smoking on health, the pathophysiology of emphysema in particular remains poorly understood.
The transient nature of innate immunity fails to account for the notoriously progressive course of emphysema, which can occur long after smoking cessation (4), suggesting that an adaptive immune component drives the chronic and unremitting forms of this disease in a subtype of smokers. We and others have previously demonstrated that the presence of T helper type 1 (TH1)– and TH17-biased CD4+ T cells in the emphysematous lung correlated with disease severity (5–7). Further, recall TH1 and TH17 responses can be demonstrated from peripheral blood of smokers with emphysema by stimulation with lung-derived elastin fragments, suggesting a role for autoimmune mechanisms in disease pathogenesis (8, 9). Although these human studies suggest a critical role for TH1 and TH17 cells in emphysema, the mechanism for their development and rigorous evidence of a pathogenic role are lacking.
TH1 and TH17 cells mediate tissue damage in several chronic auto-immune diseases, such as rheumatoid arthritis, multiple sclerosis, and colitis (10–13). Although the exact processes by which different subsets of TH cells cause damage in various organs remain poorly understood, direct cytotoxic effects of autoreactive T cells and chronic release of proteinases in response to cytokines are among plausible mechanisms (14, 15). For example, interferon-γ (IFN-γ) expressed by TH1 cells increases expression of CXCL10 (IP-10) in diverse cells, causing enhanced expression of the potent elastase MMP12 (matrix metalloproteinase 12) (5, 16). Moreover, interleukin-17A (IL-17A), the canonical TH17 cytokine, also increases expression of MMP12, and its overexpression in the lung results in spontaneous inflammation in aging mice (9, 17). Even though overexpression of these cytokines in the lungs may recapitulate some pathophysiology of smoke-induced lung disease (18), the upstream molecular events leading to induction of lung TH1 and TH17 cells and formal evidence of a causal role of IL-17A in smoke-induced emphysema remain speculative.
Dendritic cells (DCs) present antigen and provide cytokines and costimulatory molecules that are necessary for activation of CD4 TH cells (19, 20). Specifically, DCs isolated from patients with psoriasis, an autoimmune skin disease, direct CD4+ T cell differentiation into TH17 cells in vitro (21). Similarly, CD1a+-expressing lung myeloid DCs (mDCs) from emphysematous lung are sufficient to induce TH1 and TH17 responses in a cell-cell contact–dependent manner (9). However, the mechanism(s) by which smoke transforms lung DCs to promote TH1 and TH17 differentiation in response to cigarette smoke has not been defined.
Using an experimental model of smoke exposure combined with complementary analyses of human lung cells, we investigated the immune mechanisms underlying TH1 and TH17 cell induction in emphysema and their contribution to disease expression.
RESULTS
Cigarette smoke induces TH1 and TH17 responses in mice
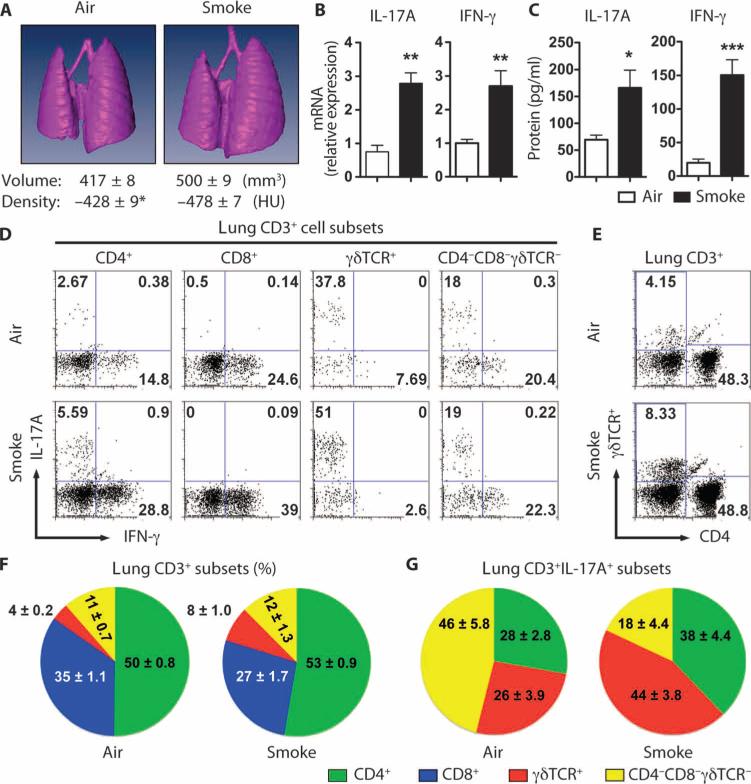
We developed an active smoke exposure chamber in which mice are exposed to smoke from commercially available cigarettes in a manner mimicking natural human smoking habits (fig. S1). Four months of daily smoke exposure significantly increased the number of lung macrophages, DCs, and neutrophils when compared with air-exposed mice (fig. S2). Moreover, smoke-exposed mice showed significant increases in lung volume and decreased lung density, the essential hallmarks of human emphysema (fig. S3 and Fig. 1A). Smoke-exposed mice also showed higher levels of inflammatory cytokines and chemokines in their bronchoalveolar lavage fluid (BALF) (fig. S4), as well as increased expression of IL-17A and IFN-γ mRNA and protein (Fig. 1, B and C). Further analysis of CD3+ T cells from lung parenchyma revealed baseline (air) expression of IL-17A in predominantly CD4+, γδ, and CD4/CD8/γδ TCR (T cell receptor) triple-negative T cells, but not in CD8+ T cells (Fig. 1D). We did not find a significant change in the overall abundance of CD4+ T cells with smoke exposure, but the number of lung γδ T cells doubled in smoke-exposed mice (Fig. 1, E and F). Thus, both CD4+ T cells and γδ T cells, but not CD8+ T cells, contribute to the up-regulation of IL-17A production in the lungs of smoke-exposed mice (Fig. 1G).
Fig. 1.
Cigarette smoke induces emphysema and increases lung IFN-γ and IL-17A production in mice. (A) Representative three-dimensional images of lungs from air- or cigarette smoke–exposed mice. Average lung volume and density are shown below. n = 10 per group. *P < 0.01. HU, Hounsfield unit. (B) Il17a and IFN-γ mRNA expression from total lung mRNA of air- or cigarette smoke–exposed mice. Data are representative of two independent studies. n = 5 per group per study. **P < 0.01. (C) IL-17A and IFN-γ protein expression from total lung mRNA of air- or cigarette smoke–exposed mice. Lung CD11c-depleted cells were stimulated overnight with PMA and ionomycin, after which supernatant protein concentrations were measured. n = 5 per group. *P < 0.05; ***P < 0.001. (D) Representative intracellular staining for IL-17A and IFN-γ from the indicated T cell subsets isolated from lungs of air- and smoke-exposed mice. Data are representative of three independent studies. n = 5 per group per study. (E) Representative flow cytometry analyses of lung γδ T cells and CD4+ T cells from total lung CD3+ lymphocytes in (D). (F and G) Cumulative pie chart data depicting the relative abundance of defined lung CD3+ (F) and IL-17A–producing, CD3+ lung T cell subsets (G) from air- and smoke-exposed mice. Data represent three independent studies. n = 5 per group per study.
IL-17A is required for cigarette smoke–induced emphysema
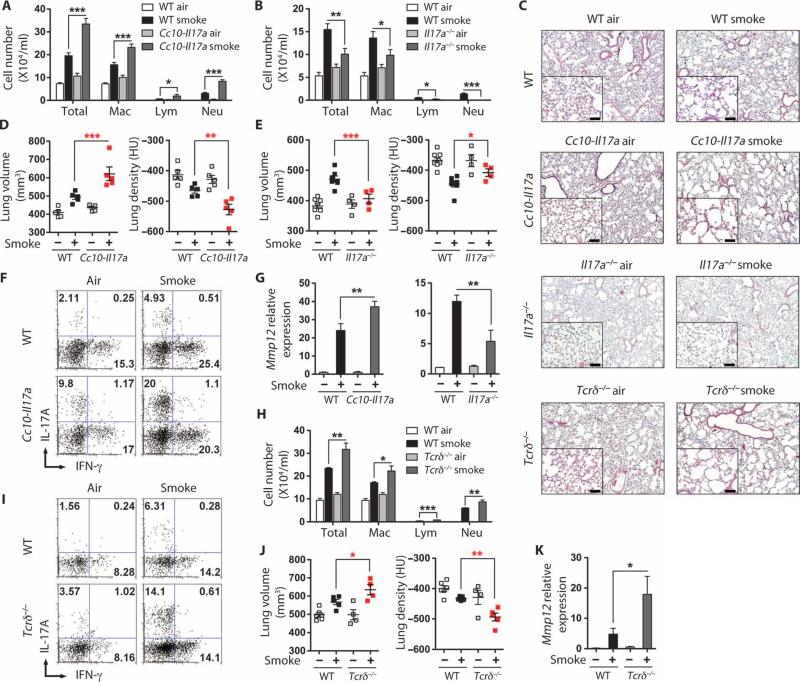
Given the prominent expression of IL-17A in this model, we next elucidated its role in cigarette smoke–induced emphysema. Lung-specific IL-17A transgenic (Cc10-Il17a) and Il-17a−/− mice are born morphologically normal, but the former develop spontaneous lung inflammation after 10 months of age (17). Therefore, we exposed 2-month-old otherwise syngeneic Cc10-Il17a, Il-17a−/−, and wild-type mice to air or smoke for 4 months. The constitutive overexpression of IL-17A in the lungs of the Cc10-Il17a mice exposed to smoke resulted in increased total numbers of lung inflammatory cells when compared to wild-type mice treated identically (Fig. 2A). Multinucleate giant cells were more numerous in the BALF of smoke-exposed Cc10-Il17a mice, indicating a more activated phenotype, whereas, relative to wild-type, Il-17a−/− macrophages appeared smaller (fig. S5). In contrast, the number of inflammatory cells was attenuated in BALF of Il-17a−/− mice when compared to that of wild-type mice (Fig. 2B). Furthermore, smoke-induced emphysema was exaggerated in Cc10-Il17a mice and attenuated in Il-17a−/− mice when compared to wild-type mice, as assessed by light microscopy (Fig. 2C) and confirmed by quantitative microcomputed tomography (μCT) (Fig. 2, D and E). Intracellular cytokine analysis of lung leukocytes further showed that IL-17A–expressing T cells were more abundant in Cc10-Il17a than in wild-type mice (Fig. 2F).
Fig. 2.
IL-17A is required for smoke-induced emphysema in mice. (A) Numbers of total macrophages (Mac), lymphocytes (Lym), and neutrophils (Neu) in BALF of air- and cigarette smoke–exposed wild-type (WT) and Cc10- Il17a mice (n = 5 per group). (B) Numbers of cells as in (A) in BALF from WT and Il17a−/− mice (n = 4 to 8 per group). *P < 0.05; **P < 0.01; ***P < 0.001. (C) Representative H&E staining of lung sections from air- or cigarette smoke–exposed mice as indicated on each panel; inset represents ×200 magnification. Scale bars, 100 μm. (D and E) μCT quantification of total lung volume and lung density from air (–)– and cigarette smoke (+)–exposed WT and Cc10-Il17a mice (D) or WT and Il17a−/− mice (E) (n = 4 to 8 per group). *P < 0.05; **P < 0.01; ***P < 0.001. Data represent at least three independent studies. (F) Intracellular cytokine staining of IL-17A and IFN-γ from lung CD4+ T cells of air- or smoke-exposed WT and Cc10-Il17a mice. Data represent at least three independent studies with five mice in each group. (G) Mmp12 mRNA expression from total BALF cells of airand smoke-exposed mice (n = 4 to 8 per group). **P < 0.01. (H) Numbers of cells as in (A) in BALF from WT and Tcrδ−/− mice (n = 4 to 5 per group). *P < 0.05; **P < 0.01; ***P < 0.001. (I) Intracellular cytokine staining of IL-17A and IFN-γ from lung CD4+ T cells of air- or smoke-exposed WT and Tcrδ−/− mice. Data represent at least three independent studies with four to five mice in each group. (J) μCT quantification of total lung volume and lung density from air- and cigarette smoke–exposed WT and Tcrδ−/− mice (n = 4 to 5 per group). *P < 0.05; **P < 0.01. (K) Mmp12 mRNA expression from total BALF cells of air- and smoke-exposed WT and Tcrδ−/− mice (n = 4 to 8 per group). *P < 0.01.
In agreement with previous observations of the effect of IL-17A treatment of human lung macrophages (9), IL-17A transgenic mice exposed to smoke showed enhanced expression of MMP9 and MMP12, whereas Il-17a−/− mice showed attenuated expression (Fig. 2G and fig. S6). Together, these data suggest that TH17 cells and IL-17A promote smoke-induced emphysema by up-regulating elastolytic MMPs.
Because we observed that lung γδ T cells doubled in abundance in smoke-exposed mice simultaneously with an increase in the relative abundance of IL-17A–expressing lung γδ T cells, we next determined the contribution of this cell population to smoke-induced emphysema. Unexpectedly, we found that Tcrδ−/− mice (lacking functional γδ T cells) exposed to smoke showed an increase in total numbers of lung inflammatory cells when compared to wild-type mice treated the same way (Fig. 2H). Consistently, we found an increase in TH17, but not in TH1 cells, in these mice, suggesting that lack of γδ T cells specifically resulted in an increase in TH17 inflammatory cell in response to smoke. Further, Tcrδ−/− mice exposed to smoke showed increases in Mmp12 gene expression and in lung volume as shown by μCT analysis of lung parenchyma (Fig. 2, C and I to K, and fig. S6). These findings underscore the importance of TH17 cells as mediators of smoke-induced lung inflammation and emphysema and suggest that IL-17A–secreting lung γδ T cells act to inhibit pathological lung TH17 responses in this context.
Lung antigen-presenting cells from smoke-exposed mice induce TH17 cells
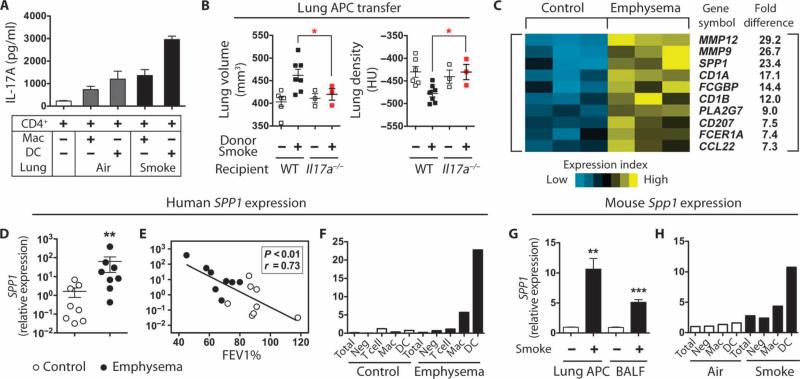
Because human emphysematous lung DCs induce TH17 (9), we next examined the capacity of cigarette smoke–exposed mouse lung DCs to cause differentiation of syngeneic, naïve CD4+ T cells in vitro. Splenic CD4+ T cells cultured with lung DCs (CD11c+CD11b+) isolated from mice exposed to smoke for 4 months were more potent in inducing TH17 differentiation (as determined by IL-17A production) than were alveolar macrophages (CD11c+CD11blow) or air-exposed DCs (Fig. 3A).
Fig. 3.
Mouse lung DCs induce TH17 differentiation. (A) Effect of CD4 T cells on IL-17A production in cells from air- or smoke-exposed mice. Splenic CD4 T cells were cultured with mouse lung macrophages (Mac) or DCs for 3 days with soluble anti-CD3 antibody (1 μg/ml), after which IL-17A was quantified from culture supernatants (n = 3). Data represent at least three independent studies. (B) μCT quantification of total lung volume and lung density from mice that received lung APCs from mice with the indicated phenotype (n = 3 to 7 per group). *P < 0.05. Data represent two independent studies. (C) Heat map depicting top 10 significantly up-regulated genes as determined by gene microarray analysis of human lung DCs from healthy control (n = 3) and emphysema (n = 3) subjects. (D) SPP1 mRNA expression in control lung DCs (n = 8) and emphysema lung DCs (n = 8). **P < 0.01. (E) Correlation of lung DC SPP1 mRNA expression and disease severity (FEV1%) (control, n = 8; emphysema, n = 8). (F) qPCR analysis of SPP1 mRNA expression in different lung cell population. Mac, macrophages; Neg, DC, macrophage, T cell–depleted cells. Data are representative of four independent experiments. (G) Spp1 mRNA expression in lung CD11c+ APCs and BALF cells from air-treated (n = 3) and cigarette smoking–treated (n = 3) mice. **P < 0.01; ***P < 0.001. (H) qPCR analysis of Spp1 mRNA expression in different lung cell population. Mac, macrophages; Neg, DC, macrophage-depleted cells. Data represent three independent studies with five mice pooled for each group.
To further examine the pathogenic role of lung antigen-presenting cells (APCs) and the requirement for IL-17A in the pathogenesis of emphysema, we transferred lung APCs isolated from control or emphysematous lungs of wild-type mice into wild-type and Il17a−/− mice. Lung APCs from mice with emphysema but not cells from control mice induced emphysema and increased inflammation in mice after 12 weeks in the absence of smoke; this process was partly dependent on the function of IL-17A, because Il17a−/− recipient mice failed to develop emphysema (Fig. 3B and fig. S7). After as little as 2 months of smoke exposure, we found evidence for TH1 and TH17 cell accumulation in the lungs. Further, APCs isolated from these recipient mice could induce TH1 and TH17 cell differentiation in coculture experiments, showing that even before disease development, the APCs are conditioned to induce characteristic pathological TH cell responses (fig. S8).
We next sought to elucidate the mechanism by which lung DCs selectively induce TH17 differentiation by conducting a gene expression analysis of human lung DCs from control and emphysema patients. Using strict criteria for significance (more than twofold increase with P < 0.004 relative to control), we identified 116 up- and 62 down-regulated genes in emphysema lung DCs (GSE26296). Among the genes showing highest expression was SPP1 (encoding osteopontin) (Fig. 3C), a pleiotropic, cytokine-like molecule that has previously been linked to TH1 and TH17 responses in several autoimmune diseases (22–24).
We confirmed by reverse transcription–quantitative polymerase chain reaction (RT-qPCR) that SPP1 was expressed to a significantly greater degree in human DCs (Fig. 3D) and macrophages (fig. S9A) from emphysema subjects than from control subjects. SPP1 expression correlated positively with obstructive lung disease severity as assessed by forced expiratory volume in 1 s (FEV1), consistent with a pathogenic role in lung function (Fig. 3E and fig. S9B). Detailed analysis of distinct cell populations from human lungs confirmed that SPP1 was most abundantly expressed in DCs and lung macrophages from emphysema subjects (Fig. 3F). Similarly, expression of Spp1 was enhanced in total lung APCs (CD11c+ cells), as well as total BALF cells, of smoke-exposed emphysematous mice (Fig. 3G). Detailed analyses of mouse lung single-cell populations confirmed that Spp1 expression was enhanced selectively in alveolar macrophages and, especially, DCs (Fig. 3H).
Osteopontin is required for TH1 and TH17 differentiation through mDCs in vitro
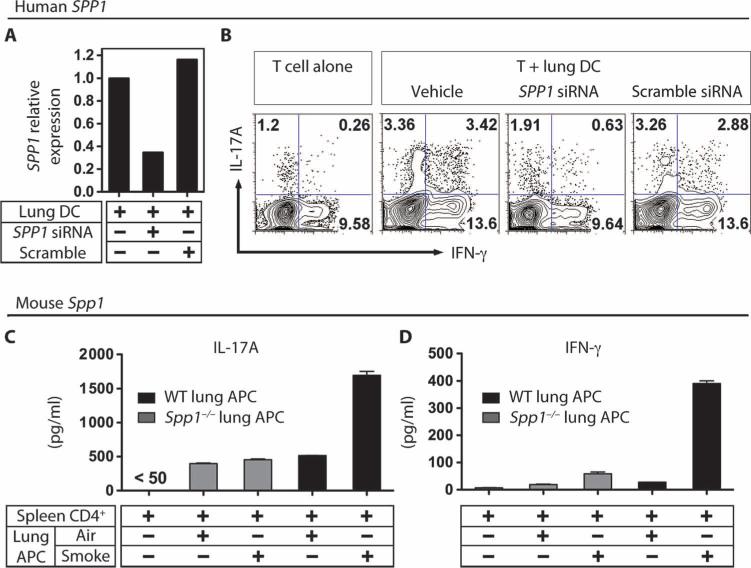
We next determined whether lung mDCs require osteopontin production for TH1 and TH17 cell differentiation. Transfection of human lung DCs with anti-osteopontin small interfering RNA (siRNA), but not scrambled siRNA, reduced SPP1 mRNA (Fig. 4A) and decreased the ability of the DCs to promote TH1 and TH17 differentiation in vitro (Fig. 4B and fig. S10A). A similar effect was also observed in identically treated human macrophages isolated from emphysematous lung (fig. S10B).
Fig. 4.
Spp1 expression is required for induction of TH1 and TH17 cells by lung DCs. (A) Effect of Spp1 siRNA on SPP1 mRNA. Human emphysematous lung DCs were transfected with 10 ng of Spp1 or scrambled siRNA, after which SPP1 mRNA was quantitated by PCR. (B) Human lung DCs require Spp1 to differentiate T cells. Similarly treated human lung DCs were cultured with allogeneic human PBMC-derived CD4 T cells in the presence of anti-CD3 antibody (1 μg/ml) for 3 days, followed by intracellular cytokine staining for IL-17A and IFN-γ. Data are representative of at least five independent experiments. (C and D) IL-17A (C) and IFN-γ (D) were quantitated from 3-day cultures of lung APCs isolated from smoke-exposed WT and Spp1−/− mice that were incubated with spleen-derived CD4 T cells in the presence of soluble anti-CD3 antibody (1 μg/ml) (n = 3 independent experiments with triplicate replicates in each experiment).
We next examined the role of osteopontin in the mouse model of emphysema. Similar to results from human DCs, we found that relative to air-exposed controls, incubation of syngeneic wild-type mouse CD4 T cells with APCs isolated from emphysematous lung of wild-type mice exposed to smoke resulted in a 3- to 10-fold enhancement of IL-17A and IFN-γ secretion, respectively (Fig. 4, C and D, black bars). Further, lung APCs isolated from lungs of osteopontin-deficient (Spp1−/−) mice exposed to smoke consistently failed to induce significant increase of IL-17A and IFN-γ in CD4 T cells (Fig. 4, C and D, gray bars).
Osteopontin is required for IL-17A production and emphysema in vivo
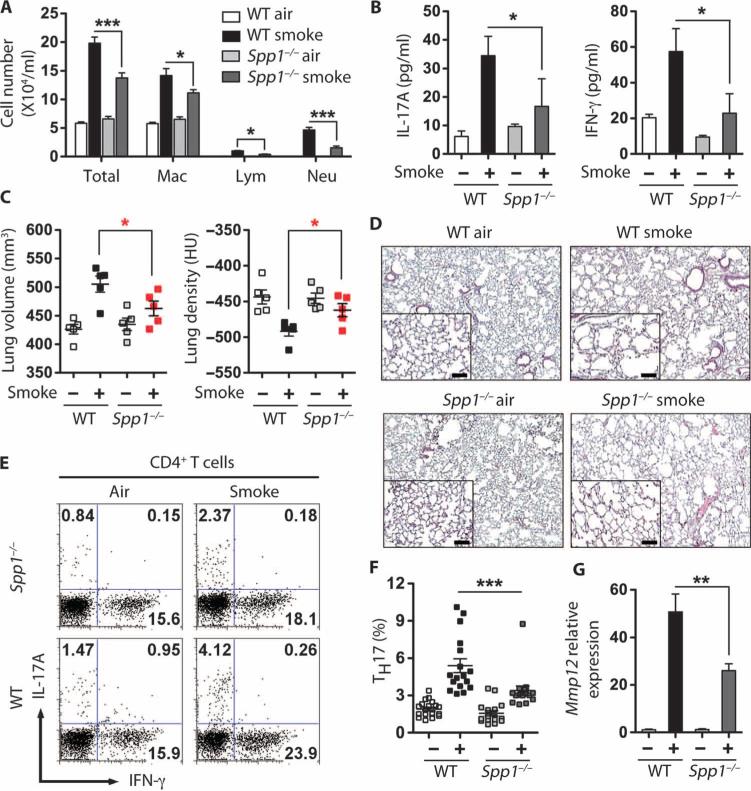
We next determined immune and physiological endpoints from tobacco smoke–exposed wild-type and Spp1−/− mice. Spp1−/− mice exposed to 4 months of smoke showed significantly reduced total and individual inflammatory cells in BALF and concomitant reductions in secreted IL-17A and IFN-γ from whole lung when compared to smoke-exposed wild-type mice (Fig. 5, A and B). Consistent with these findings, μCT and histological analyses of lungs confirmed that Spp1−/− mice showed significantly less smoke-induced emphysema than wild-type mice, indicating reduced lung parenchymal damage (Fig. 5, C and D). In tracellular cytokine analysis of whole lung cells confirmed that smoke exposure resulted in the generation of fewer lung TH1 and TH17 cells in Spp1−/− than in wild-type mice (Fig. 5, E and F), and Spp1−/− mice showed reduced Mmp12 mRNA expression in BALF cells (Fig. 5G). Together, these findings demonstrate that osteopontin is required in mice for the induction of robust lung IL-17A responses and emphysema after exposure to cigarette smoke.
Fig. 5.
Spp1-deficient mice are protected from cigarette smoke–induced emphysema. (A) Number of cells in BALF of air- and cigarette smoke–exposed mice. Total cells, macrophages (Mac), lymphocytes (Lym), and neutrophils (Neu) (n = 5 per group). *P < 0.05; ***P < 0.001. (B) IL-17A and IFN-γ levels in cells from airand cigarette smoke–exposed Spp1−/− mice. CD11c-depleted cells from whole lung homogenates were stimulated overnight with PMA and ionomycin, and supernatant protein concentrations were measured. n = 3 to 5 per group. *P < 0.05. (C) μCT quantification of total lung volume and lung density from air (–)– and cigarette smoke (+)–exposed WT and Spp1−/− mice (n = 5 per group). *P < 0.05. Data represent at least three independent studies. (D) Representative H&E staining of lung sections from WT and Spp1−/− mice exposed to air or smoke for 4 months; insets represent ×200 magnification. Scale bars, 100 μm. (E and F) Representative (E) and cumulative (F) intracellular cytokine analyses of CD4 T cells derived from lungs of air- and smoke-exposed WT and Spp1−/− mice (n = 16 to 20). ***P < 0.001 by one-way ANOVA test. (G) Mmp12 mRNA expression in total BALF cells from air- and smoke-exposed mice (n = 5 per group). **P < 0.01.
Irf7 negatively regulates activation of APCs
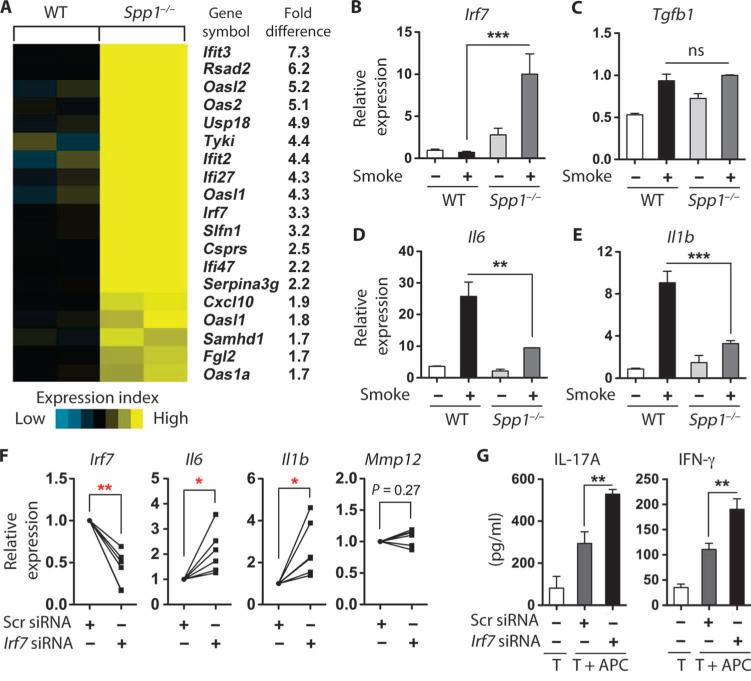
Because Spp1−/− mice were protected against cigarette smoke–induced disease and their lung APCs failed to induce TH1 or TH17 cell differentiation in vitro, we next sought to determine the mechanism by which osteopontin mediated these pathological changes. We performed gene expression analysis in lung APCs isolated from mice exposed to 4 months of smoke. Using strict criteria for significance (>1.5-fold increase with adjusted P < 0.05), we found that 19 of the 83 selected IFN-stimulated genes (GSE30906) are up-regulated in Spp1−/− when compared with wild-type mice (Fig. 6A). Among the newly identified candidate genes, we chose to examine IFN regulatory factor 7 (Irf7) because it is an inducible transcription factor with diverse immunological functions, and we verified that it is up-regulated in Spp1−/− lungs and is further increased in response to smoke (Fig. 6B). Further, although Il1b and Il6 were significantly decreased, we detected no significant differences in Tgfb1 expression between wild-type and Spp1−/− lung APCs exposed to smoke (Fig. 6, C to E), showing differential regulation of pro-inflammatory genes by Spp1. Next, we examined the functional significance of the Irf7 in lung APCs isolated from Spp1−/− mice exposed to smoke. Reduction in Irf7 expression with a specific siRNA in Spp1−/− lung APCs from mice exposed to smoke (Fig. 6F) resulted in up-regulation of genes for the proinflammatory cytokines Il1b and Il6, whereas Mmp12 gene expression was not affected (Fig. 6F). In agreement with these findings, Irf7-inhibited, Spp1−/− APCs isolated from mice exposed to smoke induced increased IL-17A and IFN-γ in cocultured TH cells (Fig. 6G).
Fig. 6.
Irf7 regulates the function of Spp1−/− lung APCs. (A) Heat map depicting significantly up-regulated type I IFN–stimulated genes (fold change > 1.5) from gene microarray analysis of mouse lung APCs of cigarette smoke–exposed WT (n = 2) and Spp1−/− (n = 2). (B) Irf7 mRNA expression in total BALF cells from air- and smoke-exposed WT and Spp1−/− mice (n = 5 per group). ***P < 0.001. (C to E) Il6, Il1b, and Tgfb1 mRNA expression in lung APCs from air- and smoke-exposed WT and Spp1−/− mice (n = 3 per group). **P < 0.01; ***P < 0.001. ns, nonsignificant. (F) Irf7, Il6, Il1b, and Mmp12 mRNA expression in lung APCs from smoke-exposed Spp1−/− mice and transfected with scrambled or Irf7 siRNA (n = 6 per group). *P < 0.05; **P < 0.01. (G) IL-17A and IFN-γ in spleen-derived CD4+ T cells cocultured with lung APCs as described in (F) for 3 days. Protein production was measured by Milliplex kit (n = 5 independent experiments with triplicate replicates in each experiment). **P < 0.01.
DISCUSSION
Using an experimental mouse model of emphysema, we have demonstrated that tobacco smoke exposure faithfully recapitulates the predominant lung TH1 and TH17 responses that we have previously demonstrated are characteristic of human emphysema. Active tobacco smoke also increased the number of IL-17A–expressing γδ T cells in lungs and induced emphysema that was enhanced by over-expression of IL-17A. In addition to elastases that are induced by TH1 and TH17 cytokines and are associated with emphysema pathogenesis, mouse and human lung DCs from emphysematous subjects showed increased expression of osteopontin, a cytokine previously linked to autoimmune disease. Osteopontin was required for differentiation of TH17 cells from both mouse and human naïve precursor T cells and was essential for robust induction of emphysema in mice, in part through the regulation of the transcription factor Irf7. These findings confirm the critical importance of the osteopontin–IL-17A pathway in emphysema and suggest that it may provide a therapeutic target for smoking-related lung disease.
The immune response of the lung to chronic tobacco smoke exposure, dominated by IFN-γ and IL-17A secretion, is consistent between humans and, as shown here, mice. Although respiratory tract infections are common in human emphysema and potentially drive life-threatening disease exacerbations (25), the experimental emphysema model used here involved no respiratory tract infections of our specific pathogen-free animals. Thus, type 1– and type 17–inducing in fections may be important for exacerbating extant disease, but are unlikely to be the primary smoke-related event that initiates lung destruction.
We have previously shown that human emphysema involves a prominent autoimmune component marked by anti-elastin TH1 and TH17 responses (8, 9). We have not identified the specific endogenous antigen(s), if any, that induces TH1 and TH17 cells in the lungs of mice exposed to chronic smoke; nonetheless, our studies do not rule out the possibility that chronic smoke inhalation in mice is sufficient to elicit autoimmune responses to lung antigens that are capable of perpetuating disease after cessation of exposure (26).
IL-17A is produced by numerous leukocytes, including CD4+ and CD8+ T cells, γδ T cells, natural killer T cells, neutrophils, and lymphoid tissue inducer cells (27–30). Here, we show that in the naïve lung, IL-17A is constitutively expressed by CD4+ T cells, γδ T cells, and a γδ TCR−CD4−CD8− T cell subset. IL-17A– and IL-17 receptor–deficient mice are morphologically normal (31), suggesting that IL-17A is not required in development. Therefore, it is likely that, unique among canonical TH cytokines, constitutive IL-17A expression is important for baseline lung immune surveillance, which may in turn contribute to autoimmunity in the setting of pathological exposures such as tobacco smoke. Indeed, the lung Il-17a transgene used in our study is driven by the Clara cell promoter, and functional IL-17A protein secreted in the lung likely establishes an autocrine loop that further induces TH17 differentiation, thereby exacerbating the effect of smoke-induced TH1 and TH17 inflammation in the lungs. This hypothesis is supported by the finding that IL-17A induces two critical TH17 regulatory proteins, IL-6 and IL-1β, in APCs (32).
Although γδ T cells represent only a small fraction of all lung T cells, we found that after exposure to smoke, they account for about 40% of all IL-17A–expressing cells. The next most abundant IL-17A–expressing subset was TH17 cells, which have been previously described to be associated with human emphysema (6, 9). Perhaps most surprising was the virtually complete lack of IL-17A production by CD8+ T cells, which have also been linked to human and experimental emphysema (33, 34). These findings suggest that CD8+ T cells contribute to emphysema through a non–IL-17A–related mechanism. We found that TCRδ−/− mice exposed to cigarette smoke displayed exaggerated TH17 responses in the lungs and increased emphysema. These findings suggest that the increase in γδ T cells in the lungs of mice exposed to cigarette smoke may represent a regulatory response to inhibit TH17 cells. These data further support our original hypothesis that TH17 cells are critical effectors of lung inflammation in response to cigarette smoke, but that γδ T cells with a similar immune profile exert a negative regulatory function in vivo.
Osteopontin was first described as a product of activated T cells (35) and differs from most cytokines in having both secreted and intracellular forms, each with distinct functional properties (36, 37). This uniquely pleiotropic cytokine has been widely described in association with diverse chronic inflammatory conditions, including autoimmune syndromes and cancer (22, 38, 39). Serving as a secreted autocrine factor, osteopontin promotes recruitment and activation of human epidermal mDCs through increased expression of human leukocyte antigen (HLA)–DR and costimulatory molecules (40). It also promotes TH17 responses and autoimmunity in distinct experimental systems, an effect that is antagonized by ligation of the type I IFN receptor (24). These studies suggest that osteopontin influences TH17 responses through its intracellular form.
Using gene microarrays to examine global transcriptome changes in lung DCs from wild-type and Spp1−/− mice in response to cigarette smoke, we discovered a link between Spp1 and activation of type I IFN–stimulated genes. Specifically, genes such as IFN-induced protein with tetratricopeptide repeat 3 (Ifit3), Ifit2, and Irf7 were significantly up-regulated in Spp1−/− APCs. When we compared data obtained from human lung mDCs using micro-arrays (GSE26296), we found that many of the same type I IFN genes, in particular IFIT3, IFI27, OASL, CXCL10, and OAS2, were significantly down-regulated in emphysema when compared to controls. Consistent with findings from human lung mDCs, inhibition of Irf7, an inducible transcription factor with multiple immune-modulating functions (41), was critical in dampening production of the proinflammatory cytokines IL-6 and IL-1β in lung APCs. Reduction of Irf7 expression restored the function of the Spp1−/− lung DCs required for TH17 cell differentiation in our model. Therefore, in addition to the findings that type I IFN–stimulated genes regulate Spp1 and DC function, our data reveal a mechanism by which Irf7 expression inhibits expression of proinflammatory cytokines by lung DCs in the setting of smoke exposure.
Emphysema is a destructive lung disease that is most frequently linked to tobacco smoking but is also seen in nonsmoking subjects exposed to coal and biofuel combustion products in poorly ventilated areas (42, 43). Despite the global reach and rising importance of emphysema, medical management remains primitive, with no diagnostic assays capable of predicting disease emergence in smoke-exposed, at-risk subjects, no reliable prognostic indices, and no specific therapy aside from lung transplantation. In addition to the contribution of TH1 responses, we have shown here that emphysema is characterized by a specific immune pathogenesis involving the cooperative interplay between osteopontin-producing lung macrophages and DCs and IL-17A–secreting T cells. These discoveries provide a foundation for developing diagnostic, prognostic, and therapeutic strategies that are critically needed for emphysema and other smoking-related diseases.
MATERIALS AND METHODS
Mice
Wild-type, Spp1−/−, and Tcrδ−/− mice (C57BL/6 background) were purchased from the Jackson Laboratory. Cc10-Il17a and Il17a−/− mice backcrossed six to eight generations with C57BL/6 were provided by C. Dong from the University of Texas M. D. Anderson Cancer Center. All mice were bred in the transgenic animal facility at Baylor College of Medicine. All experimental protocols used in this study were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine and followed the National Research Council Guide for the Care and Use of Laboratory Animals.
Human study subjects
A total of 26 non-atopic current or former smokers were serially entered into the study (table S1); all smoker subjects had significant (>20 pack-years) history of smoking and had quit smoking for an average of 13 ± 6 (SEM) and 5 ± 3 years in control and COPD/emphysema groups, respectively (table S1). COPD was diagnosed according to the criteria recommended by the National Institutes of Health–World Health Organization workshop summary (44). Smoking one pack of cigarettes per day each year is defined as one “pack-year.” Subjects were recruited from the chest or surgical clinics at Methodist and Michael E. DeBakey Houston Veterans Affairs Medical Center hospitals. Studies were approved by the Institutional Review Board at Baylor College of Medicine, and informed consents were obtained from all patients.
Cigarette smoke exposure
Six- to 8-week-old mice were exposed to active smoke from commercial cigarettes (Marlboro 100's). Exposure to four cigarettes (about 4 to 5 min per cigarette) per day, 5 days a week was carried by intermittently forcing air (4 liters/min) through the burning cigarette. Intermittent cycles were designed to mimic puffing cycles of actual human smokers and to prevent CO2-induced asphyxiation. Puffing cycles consisted of 5 s of active cigarette smoke followed by 25 s of forced air as controlled by a timer-controlled two-way valve (Humphrey). Mice were given 10 min of rest in between each cycle of cigarette smoke exposure. In total, mice were given four cigarettes each day (1 hour), 5 days each week for 4 months.
Quantification of experimental model of emphysema
The severity of mouse emphysema was determined by CT methods originally developed for humans (45) with modifications for μCT imaging in mice (46). Mice were anesthetized with etomidate (30 mg/kg) and placed in an animal CT scanner (Gamma Medica), and completed images of the chest were obtained by the Animal Phenotyping Core in Baylor College of Medicine. Amira 3.1.1 software was used to process the images and quantification of emphysema in three dimensions.
Analysis of experimental model of emphysema
Collection of BALF and lung tissue is as described (47). After mice were anesthetized with etomidate, BAL was collected by instilling and withdrawing 0.8 ml of sterile phosphate-buffered saline twice through the trachea. Total and differential cell count in the BALF were determined with the standard hemocytometer and HEMA3 staining (Protocol) of 200 μl of BALF cytospin slide preparation. Cytokine and chemokine concentrations in the BALF were measured by Milliplex kit. In some experiments, mouse lungs were dissected to prepare single-cell suspensions; alternatively, lungs were fixed with instillation of 4% paraformaldehyde solution via a tracheal cannula at 25-cm H2O pressure followed by paraffin embedding and were sectioned for histopathological studies. Hematoxylin and eosin (H&E) staining was performed as described (47).
Immune cell preparation and isolation
Mouse lung and spleen single-cell suspensions were prepared as described (47). Red blood cell (RBC)–free single-cell suspensions were labeled with bead-conjugated anti-CD11c (Miltenyi Biotec) to isolate lung APCs using autoMACS. Alternatively, lung DCs and alveolar macrophages were isolated from single-cell suspensions of total lung homogenates by fluorescence-activated cell sorting (FACSAria, BD Biosciences) for CD3−B220−CD11c+/CD11b+ (lung DCs) or CD3−B220−CD11c+/CD11blow (alveolar macrophages). Mouse splenic CD4 T cells were selected from peripheral splenocytes by labeling with bead-conjugated anti-CD4 (Miltenyi Biotec) followed by autoMACS separation. Human lung DCs, macrophages, and peripheral blood mononuclear cell (PBMC) CD4 T cells were prepared as described (5).
Adoptive transfer of lung APCs
Wild-type donor mice were exposed to cigarette smoke for at least 12 weeks. A total of 8 × 105 lung APCs were isolated with CD11c beads as described above and were intraperitoneally injected into 6- to 8-week-old naïve recipient mice. Mice were then exposed to room air for another 12 weeks before lungs were analyzed by μCT and cytokine expression.
Intracellular cytokine staining
Mouse lung RBC-free single-cell suspensions were stimulated with phorbol 12-myristate 13-acetate (PMA) (10 ng/ml) (Sigma) and ionomycin (1 μg/ml) (Sigma) supplemented with brefeldin A (10 μg/ml) (Sigma) for 6 hours. Cells were stained for surface markers with anti-CD3, anti-CD4, anti-CD8, and anti–δδ TCR antibodies and then fixed with 1% paraformaldehyde, permeabilized with 0.5% saponin (Sigma), and stained with anti–IFN-γ and anti–IL-17A antibodies for analysis of intracellular cytokine production by flow cytometry.
mRNA isolation and qPCR
Cell pellets were treated with TRIzol (Invitrogen) and mRNA was extracted for qPCR as described (47). All probes, Il17a (Mm00439619_m1), Ifng (Mm01168134_m1), Mmp9 (Mm00600164_g1), Mmp12 (Mm00500554_m1), Spp1 (Mm00436767_m1), SPP1 (Hs00167093_m1), Irf7 (Mm00516788_m1), Il6 (Mm00446190_m1), Il1b (Mm013361891_m1), and Tgfb1 (Mm00441724_m1), were purchased from Applied Biosystems. All data were normalized to 18S ribosomal RNA (Hs99999901_s1) expression.
In vitro T cell coculture and cytokine measurements
Human PBMC CD4 T cells were cocultured with lung APCs (10:1 ratio) for 3 days in the presence of soluble anti-human CD3 (BD, 1 μg/ml). Alternatively, cells were stimulated with PMA (10 ng/ml) (Sigma) and ionomycin (200 ng/ml) supplemented with monensin (10 ng/ml) (Sigma) for 3 hours, followed by intracellular cytokine staining. Similarly, mouse spleen CD4 T cells were cocultured with lung APCs (10:1 ratio) for 3 days with the presence of soluble anti-mouse CD3 (BD, 1 μg/ml). Milliplex kit (Millipore) was used to measure concentrations of IL-17 and IFN-γ according to the manufacturer's instructions.
Microarray analysis of lung DC gene expression
Human lung DC and mouse lung APC microarray analysis was performed by Genomics and Proteomics Core Laboratory, Texas Children's Hospital, Baylor College of Medicine, with an Illumina Human WG-6 V3.0 and Illumina Mouse WG-6 v 2.0 chip, respectively. Data were analyzed by Bioconductor. Briefly, data were analyzed with variance-stabilizing transform and quantile normalization; linear models were used to identify differentially expressed transcripts. Nominal P values were adjusted to yield false discovery rates with the empirical Bayes method.
siRNA transfection
Human SPP1 siRNA, mouse Irf7 siRNA, and scrambled siRNA were purchased from Applied Biosystems. Human lung DCs were transfected with human DC Nucleofection kit (Lonza) according to the manufacturer's instructions. Mouse lung APCs were transfected with mouse DC Nucleofection kit (Lonza) according to the manufacturer's instructions. Lung DCs treated with siRNA were incubated overnight and washed with medium before further experiments were performed.
Flow cytometry and antibodies
Flow cytometry was performed with BD LSRII (BD Biosciences), and data were analyzed with FlowJo (TreeStar). Mouse-specific antibodies Pacific Blue–CD3 (500A2), allophycocyanin (APC)–Cy7–CD8 (53-6.7), phycoerythrin (PE)–IL-17A (TC11-18H10), APC–IFN-γ (XMG1.2), PECy5-CD4 (PM4-5), and APC-Cy7-Gr1 (RB6-8C5) were purchased from BD Pharmingen. Fluorescein isothiocyanate (FITC)–γδ TCR (eBioGL3), eFluro450-B220 (RA3-6B2), PE-CD11b (M1/70), and APC-CD11c (N418) were purchased from eBioscience. Human-specific antibodies APC-CD19 (SJ25C1), APC-CD3 (SK7), Pacific Blue–CD3 (UCHT1), APC–IFN-γ (B27), APC-CD1a (HI149), FITC-CD1a (HI149), PE-CD11c (B-ly6), and FITC-CD14 (M5E2) were purchased from BD Pharmingen. PE–IL-17A (eBio64DEC17) was purchased from eBioscience.
Statistical analysis
For the comparison of cytokine production and gene expression from air- and smoke-exposed mice, we used the Student's t test or one-way analysis of variance (ANOVA) test. For the comparison of human lung DC gene expression, the Mann-Whitney test (nonparametric) was used. For the comparison of CT quantification of air- and smoke-exposed mice, one-way ANOVA test was used. Correlation between gene expression and FEV1%-based emphysema quantification was determined by linear regression. All statistical analyses were performed with the Prism software (GraphPad Software).
Supplementary Material
Acknowledgments
We thank X. Porter for the design of the smoke machine. Funding: Supported by VA Merit Fund HL072419 to F.K. and HL095382 to D.B.C.
Footnotes
Author contributions: F.K., D.B.C., and M.S. designed the experiments. M.S., X.Y., L.S., L.R., A.S., N.Z., and S.-H.C. performed the experiments. Y.Z. and S.H. analyzed microarray data. C.D. provided the transgenic mice. F.K., D.B.C., and M.S. wrote the paper.
Citation: M. Shan, X. Yuan, L. Song, L. Roberts, N. Zarinkamar, A. Seryshev, Y. Zhang, S. Hilsenbeck, S.-H. Chang, C. Dong, D. B. Corry, F. Kheradmand, Cigarette smoke induction of osteopontin (SPP1) mediates TH17 inflammation in human and experimental emphysema. Sci. Transl. Med. 4, 117ra9 (2012).
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Shafey O, Eriksen M, Ross H, Mackay J. The Tobacco Atlas. Vol. 3. American Cancer Society; Atlanta, GA: 2009. [Google Scholar]
- 2.Wilson DO, Weissfeld JL, Balkan A, Schragin JG, Fuhrman CR, Fisher SN, Wilson J, Leader JK, Siegfried JM, Shapiro SD, Sciurba FC. Association of radiographic emphysema and airflow obstruction with lung cancer. Am. J. Respir. Crit. Care Med. 2008;178:738–744. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J. Global Initiative for Chronic Obstructive Lung Disease, Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 4.Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am. J. Respir. Crit. Care Med. 2002;166:675–679. doi: 10.1164/rccm.2112096. [DOI] [PubMed] [Google Scholar]
- 5.Grumelli S, Corry DB, Song LZ, Song L, Green L, Huh J, Hacken J, Espada R, Bag R, Lewis DE, Kheradmand F. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med. 2004;1:e8. doi: 10.1371/journal.pmed.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Stefano A, Caramori G, Gnemmi I, Contoli M, Vicari C, Capelli A, Magno F, D'Anna SE, Zanini A, Brun P, Casolari P, Chung KF, Barnes PJ, Papi A, Adcock I, Balbi B. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin. Exp. Immunol. 2009;157:316–324. doi: 10.1111/j.1365-2249.2009.03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelsen SG, Aksoy MO, Georgy M, Hershman R, Ji R, Li X, Hurford M, Solomides C, Chatila W, Kim V. Lymphoid follicle cells in chronic obstructive pulmonary disease over-express the chemokine receptor CXCR3. Am. J. Respir. Crit. Care Med. 2009;179:799–805. doi: 10.1164/rccm.200807-1089OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, Green L, Hacken-Bitar J, Huh J, Bakaeen F, Coxson HO, Cogswell S, Storness-Bliss C, Corry DB, Kheradmand F. Antielastin autoimmunity in tobacco smoking–induced emphysema. Nat. Med. 2007;13:567–569. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 9.Shan M, Cheng HF, Song LZ, Roberts L, Green L, Hacken-Bitar J, Huh J, Bakaeen F, Coxson HO, Storness-Bliss C, Ramchandani M, Lee SH, Corry DB, Kheradmand F. Lung myeloid dendritic cells coordinately induce TH1 and TH17 responses in human emphysema. Sci. Transl. Med. 2009;1:4ra10. doi: 10.1126/scitranlsmed.3000154. [DOI] [PubMed] [Google Scholar]
- 10.Toh ML, Miossec P. The role of T cells in rheumatoid arthritis: New subsets and new targets. Curr. Opin. Rheumatol. 2007;19:284–288. doi: 10.1097/BOR.0b013e32805e87e0. [DOI] [PubMed] [Google Scholar]
- 11.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, Raskin L, Desai B, Faubion WA, de Waal Malefyt R, Pierce RH, McClanahan T, Kastelein RA. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J. Exp. Med. 2009;206:525–534. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atluri D, Iduru S, Veluru C, Mullen K. A levitating tattoo in a hepatitis C patient on treatment. Liver Int. 2010;30:583–584. doi: 10.1111/j.1478-3231.2009.02173.x. [DOI] [PubMed] [Google Scholar]
- 14.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 15.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat. Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 16.Maeno T, Houghton AM, Quintero PA, Grumelli S, Owen CA, Shapiro SD. CD8+ T cells are required for inflammation and destruction in cigarette smoke-induced emphysema in mice. J. Immunol. 2007;178:8090–8096. doi: 10.4049/jimmunol.178.12.8090. [DOI] [PubMed] [Google Scholar]
- 17.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, Zheng T, Zhu Z, Homer RJ, Riese RJ, Chapman HA, Jr, Shapiro SD, Elias JA. Interferon g induction of pulmonary emphysema in the adult murine lung. J. Exp. Med. 2000;192:1587–1600. doi: 10.1084/jem.192.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat. Rev. Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.GeurtsvanKessel CH, Lambrecht BN. Division of labor between dendritic cell subsets of the lung. Mucosal Immunol. 2008;1:442–450. doi: 10.1038/mi.2008.39. [DOI] [PubMed] [Google Scholar]
- 21.Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, Abello MV, Novitskaya I, Pierson KC, Gonzalez J, Krueger JG, Lowes MA. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J. Invest. Dermatol. 2009;129:79–88. doi: 10.1038/jid.2008.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ, Cantor H. Eta-1 (osteopontin): An early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 23.Murugaiyan G, Mittal A, Weiner HL. Increased osteopontin expression in dendritic cells amplifies IL-17 production by CD4+ T cells in experimental autoimmune encephalomyelitis and in multiple sclerosis. J. Immunol. 2008;181:7480–7488. doi: 10.4049/jimmunol.181.11.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinohara ML, Kim JH, Garcia VA, Cantor H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: Role of intracellular osteopontin. Immunity. 2008;29:68–78. doi: 10.1016/j.immuni.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bandi V, Jakubowycz M, Kinyon C, Mason EO, Atmar RL, Greenberg SB, Murphy TF. Infectious exacerbations of chronic obstructive pulmonary disease associated with respiratory viruses and non-typeable Haemophilus influenzae. FEMS Immunol. Med. Microbiol. 2003;37:69–75. doi: 10.1016/S0928-8244(03)00100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motz GT, Eppert BL, Wesselkamper SC, Flury JL, Borchers MT. Chronic cigarette smoke exposure generates pathogenic T cells capable of driving COPD-like disease in Rag2−/− mice. Am. J. Respir. Crit. Care Med. 2010;181:1223–1233. doi: 10.1164/rccm.200910-1485OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 28.Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu. Rev. Physiol. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- 29.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O'Shea JJ. Lymphoid tissue inducer–like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong C. Differentiation and function of pro-inflammatory Th17 cells. Microbes Infect. 2009;11:584–588. doi: 10.1016/j.micinf.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm. Bowel Dis. 2006;12:382–388. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 32.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, Quinn C, Blankenship D, Dhawan R, Cush JJ, Mejias A, Ramilo O, Kon OM, Pascual V, Banchereau J, Chaussabel D, O'Garra A. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saetta M, Baraldo S, Corbino L, Turato G, Braccioni F, Rea F, Cavallesco G, Tropeano G, Mapp CE, Maestrelli P, Ciaccia A, Fabbri LM. CD8+ve cells in the lungs of smokers with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1999;160:711–717. doi: 10.1164/ajrccm.160.2.9812020. [DOI] [PubMed] [Google Scholar]
- 34.Saetta M, Mariani M, Panina-Bordignon P, Turato G, Buonsanti C, Baraldo S, Bellettato CM, Papi A, Corbetta L, Zuin R, Sinigaglia F, Fabbri LM. Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2002;165:1404–1409. doi: 10.1164/rccm.2107139. [DOI] [PubMed] [Google Scholar]
- 35.Patarca R, Saavedra RA, Cantor H. Molecular and cellular basis of genetic resistance to bacterial infection: The role of the early T-lymphocyte activation-1/osteopontin gene. Crit. Rev. Immunol. 1993;13:225–246. [PubMed] [Google Scholar]
- 36.Iwata D, Kitamura M, Kitaichi N, Saito Y, Kon S, Namba K, Morimoto J, Ebihara A, Kitamei H, Yoshida K, Ishida S, Ohno S, Uede T, Onoé K, Iwabuchi K. Prevention of experimental autoimmune uveoretinitis by blockade of osteopontin with small interfering RNA. Exp. Eye Res. 2010;90:41–48. doi: 10.1016/j.exer.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Braitch M, Constantinescu CS. The role of osteopontin in experimental autoimmune encephalomyelitis (EAE) and multiple sclerosis (MS). Inflamm. Allergy Drug Targets. 2010;9:249–256. doi: 10.2174/187152810793358778. [DOI] [PubMed] [Google Scholar]
- 38.Denhardt DT, Noda M, O'Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: Regulation of inflammation, tissue remodeling, and cell survival. J. Clin. Invest. 2001;107:1055–1061. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rangaswami H, Bulbule A, Kundu GC. Osteopontin: Role in cell signaling and cancer progression. Trends Cell Biol. 2006;16:79–87. doi: 10.1016/j.tcb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Renkl AC, Wussler J, Ahrens T, Thoma K, Kon S, Uede T, Martin SF, Simon JC, Weiss JM. Osteopontin functionally activates dendritic cells and induces their differentiation toward a Th1-polarizing phenotype. Blood. 2005;106:946–955. doi: 10.1182/blood-2004-08-3228. [DOI] [PubMed] [Google Scholar]
- 41.Mamane Y, Heylbroeck C, Génin P, Algarté M, Servant MJ, LePage C, DeLuca C, Kwon H, Lin R, Hiscott J. Interferon regulatory factors: The next generation. Gene. 1999;237:1–14. doi: 10.1016/s0378-1119(99)00262-0. [DOI] [PubMed] [Google Scholar]
- 42.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N. Engl. J. Med. 2009;360:2445–2454. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- 43.Kuempel ED, Wheeler MW, Smith RJ, Vallyathan V, Green FH. Contributions of dust exposure and cigarette smoking to emphysema severity in coal miners in the United States. Am. J. Respir. Crit. Care Med. 2009;180:257–264. doi: 10.1164/rccm.200806-840OC. [DOI] [PubMed] [Google Scholar]
- 44. [21 May 2009];GOLD Executive Committee, Global strategy for the diagnosis and prevention of COPD. http://www.goldcopd.org/
- 45.Coxson HO, Rogers RM. Quantitative computed tomography of chronic obstructive pulmonary disease. Acad. Radiol. 2005;12:1457–1463. doi: 10.1016/j.acra.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 46.Froese AR, Ask K, Labiris R, Farncombe T, Warburton D, Inman MD, Gauldie J, Kolb M. Three-dimensional computed tomography imaging in an animal model of emphysema. Eur. Respir. J. 2007;30:1082–1089. doi: 10.1183/09031936.00000507. [DOI] [PubMed] [Google Scholar]
- 47.Goswami S, Angkasekwinai P, Shan M, Greenlee KJ, Barranco WT, Polikepahad S, Seryshev A, Song LZ, Redding D, Singh B, Sur S, Woodruff P, Dong C, Corry DB, Kheradmand F. Divergent functions for airway epithelial matrix metalloproteinase 7 and retinoic acid in experimental asthma. Nat. Immunol. 2009;10:496–503. doi: 10.1038/ni.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.