Summary
Although BRAF and MEK inhibitors have proven clinical benefits in melanoma, most patients develop resistance. We report a de novo MEK2-Q60P mutation and BRAF gain in a melanoma from a patient who progressed on the MEK inhibitor trametinib and did not respond to the BRAF inhibitor dabrafenib. We also identified the same MEK2-Q60P mutation along with BRAF amplification in a xenograft tumor derived from a second melanoma patient resistant to the combination of dabrafenib and trametinib. Melanoma cells chronically exposed to trametinib acquired concurrent MEK2-Q60P mutation and BRAF-V600E amplification, which conferred resistance to MEK and BRAF inhibitors. The resistant cells had sustained MAPK activation and persistent phosphorylation of S6K. A triple combination of dabrafenib, trametinib, and the PI3K/mTOR inhibitor GSK2126458 led to sustained tumor growth inhibition. Hence, concurrent genetic events that sustain MAPK signaling can underlie resistance to both BRAF and MEK inhibitors, requiring novel therapeutic strategies to overcome it.
Introduction
Melanoma is the most lethal skin cancer, and its incidence continues to increase worldwide. Deregulation of MAPK signaling is a hallmark of melanoma. In particular, mutant V600-BRAF melanoma cells are dependent on MEK/ERK signaling (Ribas and Flaherty, 2011; Solit et al., 2006). Based on improved overall survival, two BRAF inhibitors (BRAFi), vemurafenib and dabrafenib, and the allosteric MEK inhibitor (MEKi) trametinib, have received FDA approval for the treatment of metastatic BRAF-V600E (V600E) melanoma. Additionally, trametinib in combination with dabrafenib significantly improves progression free survival compared to monotherapy (Flaherty et al., 2012). Nevertheless, the long-term efficacy of these compounds is limited by the emergence of drug resistance (Sosman et al., 2012). Several mechanisms of resistance to BRAFi have been identified (Abel et al., 2013; Das Thakur et al., 2013; Johannessen et al., 2010; Nazarian et al., 2010; Poulikakos et al., 2011; Roesch et al., 2013; Shi et al., 2012b; Villanueva et al., 2010). Resistance to MEKi has been linked to mutations in MAP2K1 (MEK1) (Emery et al., 2009; Wagle et al., 2011; Trunzer et al., 2013) and a MAP2K (MEK2) E207K mutation was identified in a melanoma cell line with decreased sensitivity to selumetinib (Nikolaev et al., 2012). Given the heterogeneity of melanoma, additional resistance mechanisms are likely to arise. Moreover, it is not yet known if the same mechanisms underlie resistance to combined BRAF and MEK inhibition. As most patients with metastatic BRAF-V600E mutant melanoma will be treated with BRAF and MEK inhibitors, delineating the spectrum of resistance mechanisms is critical to devise optimal therapeutic regimens.
Results
A de novo MEK2 mutation and BRAF gain is associated with resistance to MEK and BRAF inhibitors
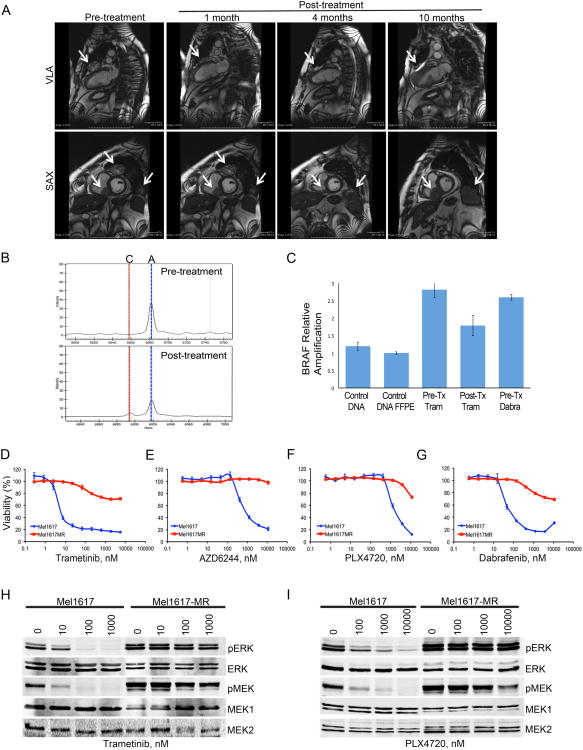
To identify genetic alterations associated with drug resistance in clinical specimens, serial biopsies were obtained from a BRAF-V600E metastatic melanoma patient enrolled on the trametinib first-in-human study MEK111054 (Infante et al., 2012; Falchook et al., 2012) prior to treatment with trametinib and at different times after treatment initiation. Paired biopsies showed a pharmacodynamic response with striking decreases in pERK and Ki67 after 2 weeks of treatment (Figure S1A). The patient achieved a confirmed partial response with ∼57% tumor reduction and remained on study for 36 weeks prior to discontinuation due to disease progression (Figure 1A). A post-progression biopsy was obtained from the same chest wall mass just prior to enrollment in the dabrafenib first-in-human study, BRF112680. Sequenom analysis of the tumor samples demonstrated a MAP2K2 c.179A>C p.Gln60Pro (MEK2-Q60P) mutation in the post-progression sample, which was not present in the trametinib pre-dose or day 15 samples (Figure 1B). The patient also had gain of the region on chromosome 7 containing BRAF, in pre-treatment, on-treatment, and progression samples (Figure 1C). The patient's best response while receiving dabrafenib was progressive disease at approximately week 8, suggesting that the MEK2-Q60P mutation, and potentially the gain of BRAF, conferred resistance to both MEK and BRAF inhibitors in this patient.
Figure 1. A de novo MEK2 mutation in trametinib-resistant melanoma.
(A) MRI images from a 62-year-old Caucasian male with metastatic melanoma, with involvement of the lungs, chest wall, heart, and liver prior to treatment with trametinib and at different times after treatment initiation. VLA, vertical long-axis; SAX, short-axis (B) Sequenom iPLEX assay depicting nucleotide determination on pre-treatment and at progression samples. Specific assay depicted is for MEK2-Q60P mutation. ‘A’=wild type nucleotide; ‘C’=mutant nucleotide. (C) Fold amplification of BRAF in human samples determined by qPCR. Normal DNA control; FFPE, formalin fixed paraffin embedded sample. Pre-tx-Tram, trametinib pre-treatment; Post-tx-Tram, progression on trametinib; Pre-tx-Dabra, dabrafenib pre-treatment. (D-G) Melanoma cells were treated with MEK (D-E) or BRAF (F-G) inhibitors for 72h. Cell viability was calculated relative to the untreated cells. Data are represented as mean ± SEM with n=3. (H-I) Mel1617 parental and trametinib resistant (MR) sublines were treated with trametinib (H) or PLX4720 (I) for 20h. Protein lysates were analyzed by immunoblotting. See also Fig. S1
Modeling resistance to MEK and BRAF inhibitors in vitro
We modeled the emergence of drug resistance in BRAF-V600E melanoma cells by chronically exposing them to trametinib. Cells chronically exposed to the MEK inhibitor (MR) were substantially less sensitive to trametinib than the isogenic parental cells and were cross-resistant to selumetinib (AZD6244), vemurafenib/PLX4720, and dabrafenib (Figures 1D-G, S1B-E, and data not shown). Viability in response to chemotherapy was similar in parental and resistant sublines (Figure S1F). MEK and BRAF inhibitors efficiently blocked ERK phosphorylation in the parental but not in the resistant cells (Figures 1H-I, S1G-K).
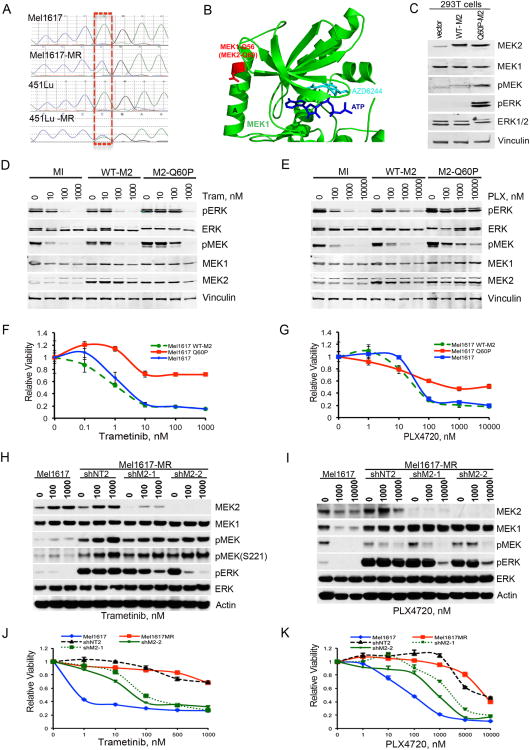
Sequencing of the MAPK2K1/2 genes identified the same MAP2K2 mutation c.179A>C (MEK2-Q60P) as that observed in the patient's melanoma in two of the five resistant sublines independently generated (Figure 2A and data not shown). The glutamine at position 60 is located within a negative regulatory region of MEK2, Helix A; substitutions of proline into the Helix A of MEK1 have been shown to cause kinase activation (Emery et al., 2009; Senawong et al., 2008; Wagle et al., 2011). A sequence alignment of MEK1 and MEK2 reveals that the MEK2-Q60P trametinib resistant mutant identified in this study is analogous to the MEK1-Q56P AZD6244-resistant mutant identified by random insertion mutagenesis (Emery et al., 2009). The structure of MEK1 bound in complex to ATP and the allosteric MEK inhibitor AZD6244 reveals that the MEK1-Q56 (MEK2-Q60) residue is in a regulatory A helix that sits against the N-terminal kinase lobe that binds both ATP and the allosteric inhibitor (Figure 2B). Residues within the A-helix are too far from ATP and inhibitor to interact directly with the ligands but are close enough to the N-terminal kinase lobe to alter the ATP binding site. We therefore propose that the MEK2-Q60P drug resistant mutation likely functions by allosterically altering the ATP binding site in a way that increases the intrinsic kinase activity of MEK2. Accordingly, pMEK and pERK levels were ∼3 and 20-fold higher in 293T cells ectopically expressing MEK2-Q60P compared to WT MEK2 (Figure 2C, Table S1).
Figure 2. A MEK2-Q60P mutation decreases sensitivity to BRAF and MEK inhibitors.
(A) Chromatogram of Sanger sequencing depicting the de novo Q60P mutation (c.179A>C, p.Q60P) identified in exon 2 in BRAF-V600E melanoma cells resistant to trametinib. (B) Structure of MEK1 (Protein Data Bank accession No. 3EQC) bound to ATP and the MEK inhibitor selumetinib (AZD6244) highlighting the position of the MEK1-Q56P (MEK2-Q60P; red stick) mutation. Only the N-terminal kinase lobe of MEK1 is shown as a cartoon with the ATP and AZD6244 molecules shown as stick figures. The figure was generated with PyMol. (C) 293T cells were mock-infected, or infected with WT-MEK2, or mutant MEK2-Q60P. Cells were serum starved for 48h. Cell lysates were analyzed by immunoblotting. (D-G) Mel1617 parental cells were mock-infected (MI), or infected with a lentivirus carrying wild type MEK2 (WT-M2), or mutant MEK2-Q60P (M2-Q60P). (D-E) Immunoblotting analysis of cells treated with trametinib (Tram) or PLX4720 (PLX) for 20h. (F-G) Cell viability was determined by MTT assays and calculated relative to the untreated cells. Data are represented as mean ± SEM, n=6. (H-K) Mel1617-MR cells were infected with lentiviral vectors expressing a non-targeting shRNA (shNT2) or MEK2 shRNA (shM2-1, shM2-2). Cell lysates were analyzed by immunoblotting (H-I). Relative cell viability was determined by MTT assays (J-K). Data are represented as mean ± SEM, n=5. See also Fig. S2 and Table S1.
Melanoma cells ectopically expressing MEK2-Q60P required higher concentrations of trametinib for MAPK inhibition; PLX4720 had virtually no effect on pERK inhibition (Figure 2D-E). Whereas overexpression of WT MEK2 did not alter the effect of BRAF or MEK inhibitors on cell viability, overexpression of MEK2-Q60P caused a >10-fold decrease in sensitivity to these compounds (Figures 2F-G, S2A). BRAFi had minimal effects on pMEK and pERK levels even in low serum conditions (data not shown). These data indicate that MEK2-Q60P is associated with an attenuated response to BRAF/MEK inhibitors and does not require substantial mitogenic stimulation.
To further examine the role of MEK2-Q60P in modulating sensitivity to MEK and BRAF inhibitors, we silenced MEK2 in Mel1617-MR cells. MEK2 depletion partially restored sensitivity to these drugs (Figures 2H-K, S2B-E). In contrast, silencing of MEK1 in Mel1617-MR had no significant effect on MAPK activity and drug sensitivity (Figures S2F-M). Furthermore, silencing of MEK1/2 in parental cells had minimal effects on drug sensitivity (Figure S2N-Q).
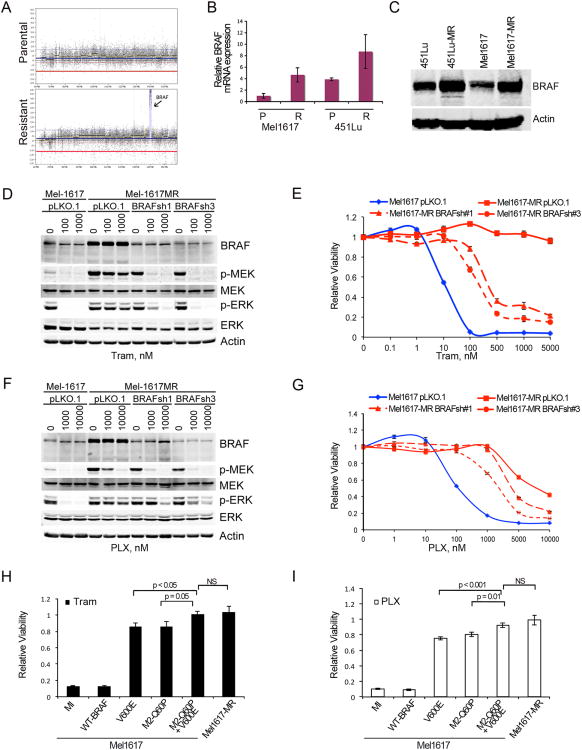
Considering that MEK2 depletion in resistant cells only partially restored drug sensitivity, we postulated that additional factors could be underlying resistance to BRAF/MEK inhibitors in our trametinib-resistant cells. To explore this possibility, we performed array based comparative genomic hybridization (aCGH). The resistant cells had a localized 20-fold amplification on chromosome 7, targeting the BRAF locus (Figure 3A). The mutant BRAF-V600E allele was amplified compared to the wild-type allele with a mutant: wild-type ratio of ∼10:1; BRAF mRNA and protein levels were also higher (Figure 3B-C). Depletion of BRAF to levels equivalent to those found in the parental cells did not fully restore sensitivity to BRAF or MEK inhibitors (Figure 3D-G). No other secondary mutations or known mechanisms of resistance to BRAF or MEK inhibitors were identified in these cells (Figure S3A-C and data not shown).
Figure 3. BRAF-V600E overexpression and mutant MEK2 promote drug resistance.
(A) Targeted amplification of the BRAF locus on chromosome 7 demonstrated using aCGH in trametinib-resistant cells. (B) BRAF mRNA levels in Mel1617 and 451Lu sublines assessed by quantitative real time PCR. Data are represented as mean ± SEM (n=3); p<0.05 (C) BRAF protein levels were analyzed by immunoblotting in Mel1617 parental and resistant sublines. (D-G) Mel1617-MR cells were infected with vector control (pLKO.1) or BRAF shRNA. MAPK signaling was assessed by immunoblotting (D, F) and cell viability by MTT assays (E, G). Data are represented as mean ± SEM (n=7). Data was analyzed using Wilcoxon rank sum test; p<0.001 when comparing Mel1617-MR-pLK0.1 with Mel1617-MR expressing BRAFshRNA at ≥100 nM of tram (E) or ≥5000 nM PLX (G). (H-I) Mel1617 ectopically expressing WT-BRAF, BRAF-V600E or MEK-Q60P, and trametinib resistant Mel1617 (MR) cells were treated with 0.1 μM of Trametinib (H) or 1 μM PLX4720 (PLX; I). Cell viability was calculated relative to the untreated control. Data are represented as mean ± SD (n=6); p<0.01 when comparing Q60P/V600E with Q60P or V600E and p>0.05 when comparing Q60P/V600E with MR cells. See also Fig S3.
To further explore the role of MEK2-Q60P and BRAF-V600E amplification, we overexpressed BRAF-V600E and/or MEK2-Q60P in parental cells (Figure 3H-I, S3D). Ectopic expression of BRAF-V600E or MEK2Q60P in Mel1617 cells decreased sensitivity to PLX4720 (75.4% and 85.9% surviving cells respectively). Concomitant expression of BRAF-V600E and MEK2-Q60P further increased the level of resistance to PLX4720 (104% surviving cells; p= 0.0006 for V600E vs. Q60P plus V600E; p=0.016 for Q60P vs. Q60P plus V600E; p=0.358 for Q60P plus V600E vs. MR cells). Similar results were obtained with trametinib. Altogether these data suggest that concurrent MEK2 mutations and BRAF-V600E amplification enhance the MAPK pathway and confer resistance to both BRAF and MEK inhibitors.
MEK2-Q60P and BRAF amplification confer resistance to the combination of BRAF and MEK inhibitors in vivo
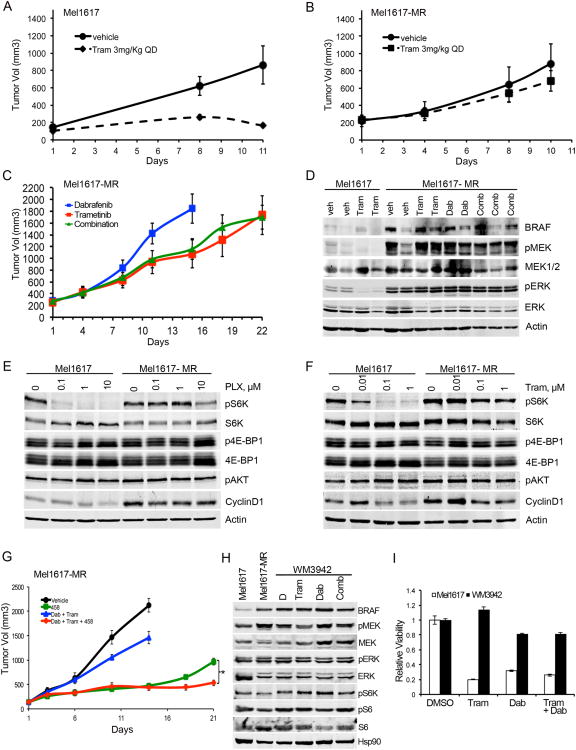
To evaluate the significance of drug resistance in vivo, we injected parental cells, resistant cells, and cells ectopically expressing MEK2-Q60P at low or high levels into NOD-SCID-IL2-γ-null mice (Figures 4A-B, S4A-F). Whereas trametinib inhibited MAPK signaling and growth of tumors derived from parental cells, it had virtually no effect on drug resistant tumors or tumors expressing high levels of MEK2-Q60P. Notably, the combination of trametinib and dabrafenib, although partially effective in vitro, did not decrease growth of trametinib-resistant tumors in vivo (Figures 4C, S4G-K and data not shown). Analysis of MAPK activity in the xenograft tumors showed that neither single agent nor the combination therapy affected MAPK signaling in the trametinib-resistant tumors (Figure 4D).
Figure 4. Targeting S6K can halt the growth of trametinib-resistant tumors in vivo.
Mice bearing tumors derived from Mel1617 (A) or Mel-1617-MR (B) sublines were treated with vehicle or trametinib (3 mg/kg po qd; n=5/group). Tumor volumes were plotted over time (mean ± SEM). Tumor growth trends were significantly different between treatment groups in Mel1617 parental (p<0.001) but no significant difference was observed in Mel1617-MR (p=0.11) (C) Mice bearing trametinib-resistant tumors were treated with dabrafenib (Dabr; 30 mg/kg), trametinib (Tram; 3 mg/kg) or the combination of both drugs at the same doses. Dabrafenib-treated tumors grew more rapidly and animals were sacrificed at day 15. Mice were treated with trametinib or the combination for 22 days. There was no significant difference between treatment groups (p=0.727). (D) Immunoblotting analysis of tumors isolated 4h after the last treatment. (E-F) Parental and MR sublines were treated with PLX4032 or trametinib for 22 h. Cell lysates were analyzed by immunoblotting. (G) Mice bearing trametinib-resistant tumors were treated with vehicle, 458 (3 mg/kg), dabrafenib (Dab; 30 mg/kg) plus trametinib (Tram; 0.3 mg/kg) or a combination of three drugs at the same doses. Tumors in mice treated with vehicle or dabrafenib plus trametinib grew more rapidly and animals were sacrificed at day 15. Mice were treated with 458 or the triple combination for 21 days. Tumor growth was significantly faster in the Dab + Tram group (p<0.0001) and the 458 single agent group (*p=0.005) compared with the triple combination (Dab+Tram+458). Tumor growth rate of the Dab + Tram group was significantly faster than the group treated with 458 (p<0.001). (H-I) A short-term culture derived from a CRPDX, WM3942, was treated with the indicated inhibitors for 24h and analyzed by immunoblotting (H) or 72h for MTT assays (I). See also Fig. S4.
Interestingly, MEK or BRAF inhibition led to decreased pS6K levels in the parental cells but not in the resistant cells (Figures 4E-F, S4L-M). Persistent MAPK signaling was coupled to phosphorylation of S6K, while inhibition of MAPK blocked S6K phosphorylation. These data suggest that persistent MAPK signaling contributes to sustained S6K phosphorylation in the resistant cells. To determine the therapeutic value of targeting PI3K/mTOR/S6K in overcoming resistance to BRAF and MEK inhibitors we used a dual PI3K/mTOR inhibitor GSK2126458 (458; Figure S4N). Resistant xenograft tumors were treated with 458 as a single agent or in combination with dabrafenib and trametinib (Figure 4G). The PI3K/mTOR inhibitor halted the growth of trametinib-resistant tumors. However, the effect of 458 was only transient and the tumors resumed growth after two weeks of treatment. In contrast, treatment with a triple combination of dabrafenib, trametinib, and 458 led to sustained tumor growth inhibition with no apparent toxicity. Distinguishing between sustained and transient tumor growth inhibition is important, as we aim at identifying therapies associated with long-term responses. Although a double combination with PI3K inhibitors plus MEK or BRAF inhibitors may work to some extent, it could be associated with higher toxicity than the triple combination, as it has been reported that simultaneous treatment with BRAF and MEK inhibitors is much better tolerated than treatment with either inhibitor as single agent (Flaherty et al., 2012). These studies provide proof-of-principle that effective triple combinatorial strategies targeting two or more pathways can have a favorable risk benefit profile and should be further explored as a valuable strategy to treat melanoma and overcome drug resistance.
Further supporting the clinical relevance of our findings, we identified the same MEK2-Q60P mutation along with BRAF-V600E amplification (BRAF-V600E: wild-type ratio of ∼18:1) in a patient-derived xenograft tumor generated from a biopsy of a second melanoma patient who progressed on the combination of dabrafenib and trametinib (CRPDX; Figure 4H and S4O). The tumor sample was isolated from a chest wall subcutaneous metastasis from a BRAF-V600E-melanoma patient enrolled in the phase I/II of dabrafenib in combination with trametinib and injected subcutaneously into NSG mice. The patient had achieved a confirmed partial response and progression free survival of ∼6 months prior to discontinuation due to disease progression. Treatment of a short-term culture derived from the CRPDX with trametinib, dabrafenib or their combination, did not inhibit MAPK signaling, phosphorylation of S6K, or viability of these cells (Figure 4H-I). Altogether our data suggest that concurrent MEK2-Q60P mutation and BRAF overexpression can confer resistance to combined BRAF and MEK inhibition.
Discussion
We identified a de novo MEK2-Q60P mutation and BRAF gain in a progression sample from a patient resistant to trametinib, a xenograft tumor-derived from a second patient resistant to the combination of BRAF and MEK inhibitors, and two melanoma sublines chronically treated with trametinib. We posit that both genetic events confer resistance to trametinib and dabrafenib by increasing MAPK signaling to levels that cannot be inhibited by BRAF and MEK inhibitors.
Although various mechanisms of resistance to MEK or BRAF inhibitors have been identified, the contribution of concomitant mechanisms that sustain addiction to the MAPK pathway and confer resistance to the combination of BRAF and MEK inhibitors has not been previously reported. Although MEK1 mutations have been previously identified (Emery et al., 2009; Nikolaev et al., 2012; Wagle et al., 2011), not all MEK1 mutations confer drug resistance (Shi et al., 2012a; Trunzer et al., 2013) and MEK2 mutations have not been previously reported in patients resistant to MEK and/or BRAF inhibitors. Our findings suggest that prospective analysis of patient samples will need to include both genetic and genomic characterization of tumors, so that all potential types of aberrations associated with resistance, such as concurrent MEK mutations and BRAF amplifications, can be identified. Functional characterization of MEK1/2 mutations and other genetic events that can alter MAPK signaling output will provide useful information to guide selection of therapy for patients with metastatic melanoma.
Combination therapy with BRAF and MEK inhibitors appears to be more effective than single-agent approaches (Emery et al., 2009; Greger et al., 2012; Hegedus et al., 2012; Su et al., 2012; Flaherty et al., 2012); however, this combination could have limited activity in resistant tumors, particularly in the context of concurrent resistance mechanisms that hyperactivate the MAPK pathway. Our studies suggest that this combination is likely to be more effective if used as first-line therapy before resistance emerges. Moreover, effective therapies are sorely needed for patients who progress on BRAF/MEK inhibitors. Targeting the MAPK pathway downstream of MEK at the level of ERK, S6K, or RSK is a potential approach to overcome resistance (Hatzivassiliou et al., 2012; Morris et al., 2013). We have demonstrated that a triple combination strategy using BRAF, MEK and PI3K/mTOR inhibitors led to sustained tumor growth control, with no overt signs of toxicity. This type of strategy will need to be further refined and evaluated. Various issues that could be explored include alternative dose scheduling (e.g. intermittent dosing; Das Thakur et al., 2013), drug sequencing, drug combinations comprising specific inhibitors of downstream targets, and efficacy in tumors bearing other mechanisms of BRAF- and/or MEK-inhibitor resistance or other tumor types. Alternative combination strategies, such as the one we tested, warrant preclinical and clinical investigation as potential new approaches to treat patients refractory to BRAF and MEK inhibitors.
Experimental Procedures
Detailed experimental procedures are described in the supplemental information section.
Patient samples
Tumor specimens and clinical information were obtained under institutional review board-approved studies at the Sarah Cannon Research Institute (Nashville, TN) and the Hospital of the University of Pennsylvania. Patients provided informed written consent.
Cell lines and viability assays
Human melanoma sublines Mel1617 and 451Lu were derived from the same cell line and are BRAF-V600E mutant. WM3942 was derived from a CRPDX tumor. Cell viability was measured using MTT or Alamar Blue as previously described (Villanueva et al., 2010)
Western blotting
Protein lysates were prepared and analyzed as previously described (Villanueva et al., 2010). All antibodies used were from Cell Signaling Technology (Beverly, MA, USA), except β-Actin, which was purchased from Sigma (St. Louis, MO), COT and Cyclin D1 from Santa Cruz Biotechnology (Dallas, TX), and BRAF from Millipore.
MEK2 constructs, shRNA, and lentivirus infection
The MEK2 cDNA clone was obtained from OpenBiosystems (Lafayette, CO). The MEK2-Q60P point mutation was generated using Stratagene's QuickChangeII XL site-directed mutagenesis kit (Invitrogen) according to the manufacturer's instructions.
Tumor Xenografts
All animal studies were performed in accordance with institutional guidelines in NOD/LtSscidIL2Rγnull mice (NSG).
Supplementary Material
Highlights.
MEK2Q60P mutation and BRAF amplification confer BRAF & MEK inhibitor resistance
ERK and S6K are persistently phosphorylated in BRAF & MEKi-resistant tumor cells
A combination of BRAF, MEK and PI3K/mTOR inhibitors overcomes drug resistance in vivo
Acknowledgments
We would like to thank Dario Altieri, Maureen Murphy, Gideon Bollag (Plexxikon), J. Kong, A. Cipolla, M. Halloran, R. Letrero, K. D'Andrea, M. Xiao, T. Brafford, G. Zhang, B. Shannan, P. Hembach, Xiangfan Yin, S. Hodawadekar, and A. Vanvakidou. We thank the flow cytometry, molecular screening, imaging, and animal core facilities at the Wistar Institute. These studies were partially funded by grants from the National Cancer Institute (PO1 CA114046, CA093372, P30 CA010815), Pennsylvania Department of Health, The V Foundation, Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, NCI T32 CA009615 (MW), and research funds from GlaxoSmithKline.
DJD, TMG, and AMM are employees and shareholders of GSK.
JRI serves as uncompensated advisor for GSK.
Footnotes
Accession Numbers: The Gene Expression Omnibus accession number for the CGH data reported in this article is GSE49430
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel EV, Basile KJ, Kugel CH, 3rd, Witkiewicz AK, Le K, Amaravadi RK, Karakousis GC, Xu X, Xu W, Schuchter LM, et al. Melanoma adapts to RAF/MEK inhibitors through FOXD3-mediated upregulation of ERBB3. The Journal of clinical investigation. 2013;123:2155–2168. doi: 10.1172/JCI65780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Thakur M, Salangsang F, Landman AS, Sellers WR, Pryer NK, Levesque MP, Dummer R, McMahon M, Stuart DD. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature. 2013;494:251–255. doi: 10.1038/nature11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery CM, Vijayendran KG, Zipser MC, Sawyer AM, Niu L, Kim JJ, Hatton C, Chopra R, Oberholzer PA, Karpova MB, et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20411–20416. doi: 10.1073/pnas.0905833106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchook GS, Lewis KD, Infante JR, Gordon MS, Vogelzang NJ, Demarini DJ, Sun P, Moy C, Szabo SA, Roadcap LT, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:782–789. doi: 10.1016/S1470-2045(12)70269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, Hamid O, Schuchter L, Cebon J, Ibrahim N, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger JG, Eastman SD, Zhang V, Bleam MR, Hughes AM, Smitheman KN, Dickerson SH, Laquerre SG, Liu L, Gilmer TM. Combinations of BRAF, MEK, and PI3K/mTOR inhibitors overcome acquired resistance to the BRAF inhibitor GSK2118436 dabrafenib, mediated by NRAS or MEK mutations. Molecular cancer therapeutics. 2012;11:909–920. doi: 10.1158/1535-7163.MCT-11-0989. [DOI] [PubMed] [Google Scholar]
- Hatzivassiliou G, Liu B, O'Brien C, Spoerke JM, Hoeflich KP, Haverty PM, Soriano R, Forrest WF, Heldens S, Chen H, et al. ERK inhibition overcomes acquired resistance to MEK inhibitors. Mol Cancer Ther. 2012;11:1143–1154. doi: 10.1158/1535-7163.MCT-11-1010. [DOI] [PubMed] [Google Scholar]
- Hegedus C, Truta-Feles K, Antalffy G, Varady G, Nemet K, Ozvegy-Laczka C, Keri G, Orfi L, Szakacs G, Settleman J, et al. Interaction of the EGFR inhibitors gefitinib, vandetanib, pelitinib and neratinib with the ABCG2 multidrug transporter: Implications for the emergence and reversal of cancer drug resistance. Biochemical pharmacology. 2012;84:260–267. doi: 10.1016/j.bcp.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Infante JR, Fecher LA, Falchook GS, Nallapareddy S, Gordon MS, Becerra C, DeMarini DJ, Cox DS, Xu Y, Morris SR, et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:773–781. doi: 10.1016/S1470-2045(12)70270-X. [DOI] [PubMed] [Google Scholar]
- Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, Emery CM, Stransky N, Cogdill AP, Barretina J, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris EJ, Jha S, Restaino CR, Dayananth P, Zhu H, Cooper A, Carr D, Deng Y, Jin W, Black S, et al. Discovery of a Novel ERK Inhibitor with Activity in Models of Acquired Resistance to BRAF and MEK Inhibitors. Cancer discovery. 2013 doi: 10.1158/2159-8290.CD-13-0070. [DOI] [PubMed] [Google Scholar]
- Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, Chen Z, Lee MK, Attar N, Sazegar H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev SI, Rimoldi D, Iseli C, Valsesia A, Robyr D, Gehrig C, Harshman K, Guipponi M, Bukach O, Zoete V, et al. Exome sequencing identifies recurrent somatic MAP2K1 and MAP2K2 mutations in melanoma. Nature genetics. 2012;44:133–139. doi: 10.1038/ng.1026. [DOI] [PubMed] [Google Scholar]
- Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, Moriceau G, Shi H, Atefi M, Titz B, Gabay MT, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480:387–390. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A, Flaherty KT. BRAF targeted therapy changes the treatment paradigm in melanoma. Nat Rev Clin Oncol. 2011;8:426–433. doi: 10.1038/nrclinonc.2011.69. [DOI] [PubMed] [Google Scholar]
- Roesch A, Vultur A, Bogeski I, Wang H, Zimmermann KM, Speicher D, Korbel C, Laschke MW, Gimotty PA, Philipp SE, et al. Overcoming Intrinsic Multidrug Resistance in Melanoma by Blocking the Mitochondrial Respiratory Chain of Slow-Cycling JARID1B(high) Cells. Cancer Cell. 2013;23:811–825. doi: 10.1016/j.ccr.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senawong T, Phuchareon J, Ohara O, McCormick F, Rauen KA, Tetsu O. Germline mutations of MEK in cardio-facio-cutaneous syndrome are sensitive to MEK and RAF inhibition: implications for therapeutic options. Human molecular genetics. 2008;17:419–430. doi: 10.1093/hmg/ddm319. [DOI] [PubMed] [Google Scholar]
- Shi H, Moriceau G, Kong X, Koya RC, Nazarian R, Pupo GM, Bacchiocchi A, Dahlman KB, Chmielowski B, Sosman JA, et al. Preexisting MEK1 exon 3 mutations in V600E/KBRAF melanomas do not confer resistance to BRAF inhibitors. Cancer Discov. 2012a;2:414–424. doi: 10.1158/2159-8290.CD-12-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Moriceau G, Kong X, Lee MK, Lee H, Koya RC, Ng C, Chodon T, Scolyer RA, Dahlman KB, et al. Melanoma whole-exome sequencing identifies (V600E)B-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nature communications. 2012b;3:724. doi: 10.1038/ncomms1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, Ye Q, Lobo JM, She Y, Osman I, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, McArthur GA, Hutson TE, Moschos SJ, Flaherty KT, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. The New England journal of medicine. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su F, Bradley WD, Wang Q, Yang H, Xu L, Higgins B, Kolinsky K, Packman K, Kim MJ, Trunzer K, et al. Resistance to selective BRAF inhibition can be mediated by modest upstream pathway activation. Cancer Res. 2012;72:969–978. doi: 10.1158/0008-5472.CAN-11-1875. [DOI] [PubMed] [Google Scholar]
- Trunzer K, Pavlick AC, Schuchter L, Gonzalez R, McArthur GA, Hutson TE, Moschos SJ, Flaherty KT, Kim KB, Weber JS, et al. Pharmacodynamic Effects and Mechanisms of Resistance to Vemurafenib in Patients With Metastatic Melanoma. J Clin Oncol. 2013 doi: 10.1200/JCO.2012.44.7888. [DOI] [PubMed] [Google Scholar]
- Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, Wubbenhorst B, Xu X, Gimotty PA, Kee D, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–695. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, Kehoe SM, Johannessen CM, Macconaill LE, Hahn WC, et al. Dissecting Therapeutic Resistance to RAF Inhibition in Melanoma by Tumor Genomic Profiling. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 doi: 10.1200/JCO.2010.33.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.