Abstract
Colorectal cancer (CRC) is considered to develop through the conventional adenoma-carcinoma sequence. However, the existence of de novo carcinogenesis, without any intervening precursor lesions, has been suggested for certain morphologically different tumors lacking polypoid characteristics. The presence of such tumors, along with their correlation with cardinal clinicopathological parameters, such as stage, grade and site, was retrospectively investigated in a series of 119 surgically treated CRC cases. The absence of particular polypoid characteristics (adenomatous remnants or coexisting polyps in the tumor vicinity) in combination with an infiltrative (or ulceroinfiltrative) growth pattern, were the criteria defining the nonpolypoid origin. The recorded frequencies of remnants, coexisting polyps and infiltrative tumors were 7, 5, 9 and 32%, respectively. The incidence of cases meeting the above-mentioned criteria was 28.5%. These nonpolypoid lesions exhibited a predilection for proximal anatomical site (P=0.04), probably associated with their infiltrative pattern. Most importantly, de novo lesions (unlike polypoid) were rarely found among cases with indolent tumor characteristics (stage I or grade I, P=0.008), showing a considerably different overall pattern of distribution by stage and grade as compared to that of polypoid tumors (P=0.03). The fact that nonpolypoid CRCs appeared to be clinicopathologically different from their polypoid counterparts is supportive of possible de novo origin and suggestive of a likely worse clinical behavior. The impact of these findings should be investigated to determine potential applications in the diagnosis, treatment and surveillance of these lesions.
Keywords: polypoid, nonpolypoid, colorectal cancer, de novo carcinogenesis
Introduction
Colorectal cancer (CRC), one of the most common malignancies in the Western world (1), is considered to develop from benign precursor lesions (i.e., adenomatous polyps) through the ademona-carcinoma sequence (2). This term refers to a progressive malignant transformation, the phenotypic expression of underlying stepwise genetic alterations, according to the model developed by Fearon and Vogelstein (3). Notwithstanding the existence of certain genetically different tumors following alternative tumorigenic pathways (with various precursor-serrated adenomas) (4), this model remains the dominant carcinogenic mechanism of CRC.
However, the hypothesis of another tumorigenic pathway, that of de novo carcinogenesis, suggesting the development of a tumor from normal (or intact) colonic mucosa, without the intervening step of an adenoma, has been a matter of discussion for decades (5–11). By definition, de novo lesions are characterized by the lack of any adenomatous remnant (i.e., the only indisputable evidence of the origin of adenoma) (7,9). However, the application of this criterion is hampered by the obliteration of adenomatous elements during tumor growth. Therefore, their detection is confined to a minority of CRC (∼15–20%) (6,7), mostly those diagnosed in early stages and thought to retain their initial structural characteristics (7,9,10). This objective difficulty may explain the wide variation in the reported frequencies of de novo tumors in the literature (range, 1–80%) (6–13). It also necessitates the implementation of additional criteria, potentially associated with these lesions, including small size (<1 or 2 cm), the limited invasion of the bowel wall (T1 lesions) and, mostly, the nonpolypoid growth pattern (presenting in various forms: flat, depressed and infiltrative) (6–11). Despite the lack of absolute specificity for any of these criteria (9), their application may be helpful in the identification of de novo tumors (6,7,10,13).
At the genetic level, de novo carcinogenesis has been associated with specific molecular characteristics, such as a reduced proportion of Ki-Ras mutation (9,13–15) [a key genetic event of the adenoma-carcinoma sequence (3)]. Moreover, de novo lesions exhibited a predilection for proximal tumor location (7,16), an association strongly suggesting genetic disparity, given the considerable predominance of the microsatellite instability (MSI) and CpG island methylator phenotype (CIMP) tumorigenic pathways among proximal tumors (4). Other genetic and epigenetic alterations correlated with de novo tumors have also been detected (15,17–19), although inconsistently (9,11).
Clinically, de novo tumors may represent a more aggressive (fast-growing at an early stage) subtype of CRC (14,15,20). Consequently, their identification may be important in the planning of treatment and follow-up (10,12,14).
In this study, we investigated the presence of de novo tumors, particularly among cases referred for surgery [being the large majority of CRC (1)], using a combination of histomorphological criteria for their identification (see Materials and methods). We also examined their association with particular clinicopathological parameters affecting CRC prognosis (i.e., stage, grade and site).
Materials and methods
Study population
The sample initially examined included 147 CRC cases, surgically treated between 2000 and 2003 in the Second Surgical Department of Tzaneio Hospital in Piraeus (Piraeus, Greece). Subsequent to excluding cases with recurrences, hereditary cancer, synchronous tumors of double location and unclear pathology reports, 119 patients were finally deemed eligible for the conducted retrospective investigation. None of the 119 patients had undergone neoadjuvant therapy, not performed during the study period.
De novo determination
Tumor specimens were examined for de novo origin based on the following criteria: i) lack of adenomatous remnants; ii) absence of coexisting polyps in the surgical specimen over a 10 cm distance from the tumor (potentially implying ‘field cancerization’, i.e., carcinogenic molecular alterations of the intestinal epithelium close to the tumor site). Similar genetic alterations in cancers of other anatomic areas (head and neck) have been detected within this distance (15). The existence of polyps beyond this limit was considered coincidental (i.e., not correlated with the primary lesion) and therefore was not recorded; and iii) apparently nonpolypoid growth pattern, based on morphological and histological appearance, in particular infiltrative lesions (without overhanging edge and showing massive infiltration of tumor cells) (6), including even those with a relatively limited ulceration (7,20) but always excluding protruding exophytic tumors (with overhanging edge) (6). The latter type was also identified on the basis of tumor thickness (at least 2-fold greater than that of the adjacent normal mucosa) (20).
Tumor size and extent of invasion were not included in the examining criteria since only three cases of lesions were <2 cm or presented with T1 invasion, characteristics also thought to facilitate de novo investigation. Moreover, flat and depressed lesions were not identified in our sample. These tumors are usually found in early CRC (not invading beyond the submucosa), since in an advanced disease their characteristics are frequently altered, being virtually indistinguishable from those with polypoid origin (21).
Tumors fulfilling all the aforementioned criteria were considered nonpolypoid (possibly de novo). By contrast, cases exhibiting either remnants or an apparently exophytic growth pattern were designated as ‘polypoid’. Lesions with coexisting polyps in their vicinity were also classified as ‘polypoid’, provided they were not infiltrative. Otherwise, they were classified as cases of ‘unclear origin’, a category comprising tumors without remnants but with a mixed or unclear gross configuration (mostly due to extended ulceration).
Therefore, our stratification is based on the tumor growth pattern [according to the Japanese classification for CRC (22), slightly modified by integrating infiltrative with ulceroinfiltrative and ulcerated with unclassified lesions] and in combination with the presence/absence of remnants and coexisting polyps.
Clinicopathological classification
Tumors were classified as stage I, II, III, IV and grade G1, G2, G3 (well, moderate and poor), following the TNM and World Health Organization (WHO) classifications, respectively. We also divided cases into proximal (right-sided) and distal (left-sided), with regard to the splenic flexure (7,16). Moreover, in order to examine the combined effect of stage and grade on de novo distribution, we stratified cases into three additional subsets: indolent (stage I or G1), unfavorable (stage IV or G3) and intermediate (stages II–III, G2). The absence of tumors with entirely conflicting characteristics (stage I/G3 or stage IV/G1) rendered any exclusion unnecessary.
Statistical analysis
The distribution of the particular criteria suggesting de novo origin among the various clinicopathological categories (stage, grade and site) was analyzed using the χ2 test (with Yates’s correction when necessary) and Fisher’s exact test, depending on the dataset. These tests were also used for the subsequent analysis of the distribution of tumors considered de novo (according to the aforementioned criteria) among the same clinicopathological categories. The tests were two-sided and P≤0.05 was considered to indicate a statistically significant difference.
Results
Prevalence of the examining variables and classification according to tumor origin
Table I shows the clinicopathological characteristics of the sample as well as the detection rates of the parameters investigated in this study. Adenomatous remnants were detected in 9 (7.5%), while coexisting polyps were observed in 11 cases (9.2%). The recorded frequencies of the exophytic and infiltrative (or ulceroinfiltrative) growth patterns were 20 and 32%, respectively. The remaining 48% of tumors exhibited a mixed or unclear growth pattern. Representative histologic images of lesions with remnants and of the various patterns are shown in Fig. 1.
Table I.
Clinicopathological characteristics.
| Characteristics | No. of cases (n=119) | % |
|---|---|---|
| Age (years) | ||
| <70 | 56 | 47 |
| >70 | 63 | 53 |
| Gender | ||
| Male | 69 | 38 |
| Female | 50 | 42 |
| Site | ||
| Proximal | 36 | 30 |
| Distal | 83 | 70 |
| TNM stage | ||
| I | 12 | 10 |
| II | 50 | 42 |
| III | 44 | 37 |
| IV | 13 | 11 |
| Grade | ||
| Well | 7 | 6 |
| Moderate | 103 | 86.5 |
| Poor | 9 | 7.5 |
| Combined stage-grade | ||
| Indolent (stage I, G1) | 16 | 13.5 |
| Intermediate (stage II–III, G2) | 82 | 69 |
| Unfavorable (stage IV, G3) | 21 | 17.5 |
| Growth pattern | ||
| Exophytic | 23 | 20 |
| Infiltrative | 38 | 32 |
| Ulcerated or mixed | 58 | 48 |
| Remnants | 9 | 7.5 |
| Coexisting polyps | 11 | 9.0 |
| Combined polypoid characteristicsa | 8 | 7.0 |
Exophytic lesions with remnants or polyps.
Figure 1.
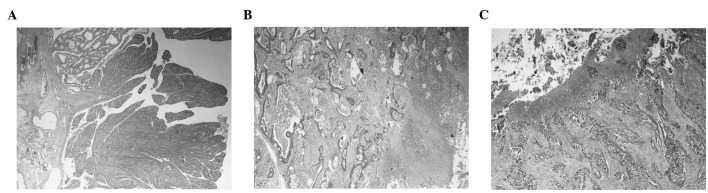
Histologic images (low power view) of (A) exophytic, (B) ulceroinfiltrative and (C) ulcerated adenocarcinomas. (A) Exophytic lesion accompanied by adenomatous remnant (magnification, ×20) is shown. (B) Ulceroinfiltrative tumor (magnification, ×40) is shown. (C) Ulcerated lesion (magnification, ×40) is shown.
Based on these results and according to the aforementioned criteria we classified 27% of the cases as ‘apparently polypoid’ (those with remnants, coexisting polyps and/or exophytic pattern), 28.5% as potentially de novo (infiltrative, always without remnants or coexisting polyps) and 44.5% as ‘of unclear origin’ (Fig. 2).
Figure 2.
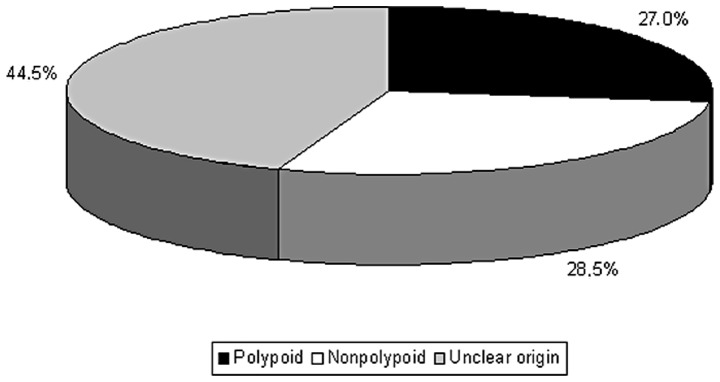
Distribution of tumors according to their origin in our sample (119 cases). Polypoid (lesions with exophytic pattern and/or remnants or polyps), 27%. Nonpolypoid (infiltrative lesions, without remnants or polyps), 28.5%. Unclear origin (cases with extensive ulceration, mixed configuration or infiltrative lesions with coexisting polyps), 44.5%.
Associations of the examining variables with clinicopathological characteristics
As shown in Table II, remnants were more frequently detected among stage I tumors compared to the other stages in combination (II–IV, 33 vs. 5.5%, P=0.003) or individually (II, III and IV, P=0.01, 0.049 and 0.04, respectively). The remnants also exhibited a trend towards well-differentiation (22 vs. 6%, P=0.09). These were observed in a markedly higher proportion in exophytic lesions compared to other growth patterns (22 vs. 4%, P=0.015). Notably, this predilection remained significant in the particular comparison of exophytic tumors with infiltrative lesions (P=0.048), however, not with those exhibiting an unclear pattern (P=0.07). The presence of coexisting polyps was also more commonly recorded in stage I tumors. However, this trend was not statistically significant. In addition, no statistically significant difference was observed in the incidence of polyps in the other clinicopathological categories.
Table II.
Distribution of remnants and co-existing polyps in various clinicopathological categories.
| Remnants
|
Polyps
|
|||||
|---|---|---|---|---|---|---|
| Category (n)a | No. | Percentage | P-value | No. | Percentage | P-value |
| Growth pattern | ||||||
| Exophytic (23) | 5 | 22 | 0.02b | 3 | 13 | NSb |
| Infiltrative (38) | 1 | 2.6 | 3 | 8 | ||
| Ulcerated (58) | 3 | 5 | 5 | 8 | ||
| Stage | ||||||
| I (12) | 4 | 33 | 0.003b | 3 | 25 | NS (0.14b) |
| II (50) | 2 | 4 | 3 | 6 | ||
| III (44) | 3 | 7 | 2 | 4.5 | ||
| IV (13) | - | 0 | 3 | 23 | ||
| Grade | ||||||
| Well (G1) (7) | 2 | 29 | NS (0.09)b | - | 0 | NSb |
| Moderate (G2) (103) | 6 | 6 | 11 | 11 | ||
| Poor (G3) (9) | 1 | 11 | - | 0 | ||
| Site | ||||||
| Proximal (36) | 3 | 8.5 | NSb | 2 | 5.5 | NSb |
| Distal (83) | 6 | 7.2 | 9 | 11 | ||
| Total (119) | 9 | 7.5 | 11 | 9.2 | ||
Total cases of each subset. Percentages in the next columns were calculated upon this number.
First subset of each particular category (i.e. exophytic, stage I, G1, proximal) vs. other subsets, for instance, stage I vs. stages II–IV. NS, not significant.
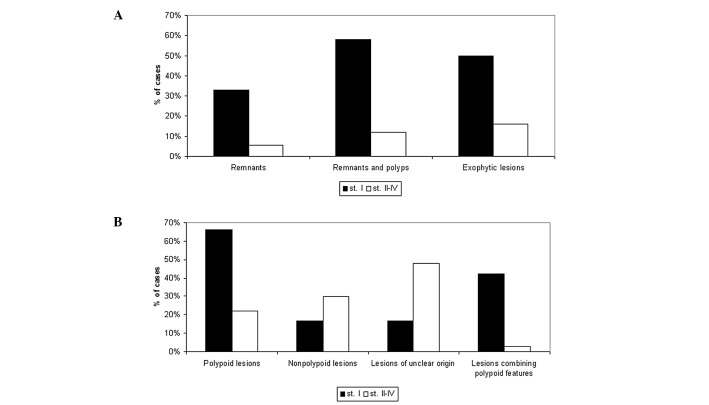
Moreover, remnants and polyps (considered together) displayed clear predilection, mostly for stage I disease (58 vs. 12%, P<0.001) and, to a lesser extent, for exophytic pattern (35 vs. 12,5%, P=0.025). Also, while half of stage I tumors were exophytic, the incidence of this growth pattern among stages II–IV was only 16% (P=0.014). Overall, polypoid characteristics (remnants, polyps and exophytic lesions) were detected in a markedly higher proportion in stage I compared to stages II–IV (66 vs. 22%, P=0.003). In addition, tumors combining these characteristics (exophytic lesions with remnants or polyps) were predominantly found in stage I (42 vs. 2.8%, P<0.0001) (Fig. 3).
Figure 3.
Comparison of various tumor characteristics (A) and categories of origin (B) between stage I and stages II–IV. (A) As indicated, the proportion of remnants, remnants and coexisting polyps or exophytic lesions was markedly higher in stage I compared to stages II–IV (P=0.003, <0.001 and 0.014, respectively). (B) The prevalence of polypoid lesions was markedly higher in stage I compared to stages II–IV (P=0.003). Conversely, lesions of an unclear origin were recorded in a markedly lower proportion in stage I compared to other stages (P=0.04). No statistically significant difference was observed in the incidence of de novo lesions between stage I and stages II–IV. Moreover, a considerably higher incidence of cases combining polypoid characteristics (exophytic pattern with remnants or polyps) was recorded in stage I (42 vs. 2.8%, P<0,0001).
The distribution of growth pattern by stage, grade and site is demonstrated in detail in Table III. Apart from the abovementioned findings regarding stage, exophytic lesions exhibited a trend for well-differentiation and a total lack of poor grade. However, the two findings were not statistically significant. Infiltrative lesions exhibited a tendency for proximal location (P=0.052) becoming significant for tumors finally classified as de novo (P=0.04). As shown in Fig. 4, the pattern of segmental distribution in nonpolypoid cases was prominently different compared to polypoid and cases of unclear origin.
Table III.
Distribution of growth pattern by stage, grade and site.
| Exophytic
|
Growth pattern Infiltrative/ulceroinfiltrative
|
Ulcerated and/or mixed
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Category (n)a | No. | Percentage | P-value | No. | Percentage | P-value | No. | Percentage | P-value |
| Stage | |||||||||
| I (12) | 6 | 50.0 | 0.014b | 3 | 25.0 | NSb | 3 | 25.0 | NSb |
| II (50) | 9 | 18.0 | 14 | 28.0 | 27 | 54.0 | |||
| III (44) | 6 | 13.5 | 16 | 36.5 | 22 | 50.0 | |||
| IV (13) | 2 | 15.5 | 5 | 38.5 | 6 | 46.0 | |||
| Grade | |||||||||
| Well (7) | 3 | 43.0 | 0.1b | 2 | 28.5 | 2 | 28.5 | NSb | |
| Moderate (103) | 20 | 19.5 | 32 | 31.5 | 51 | 50.0 | |||
| Poor (9) | - | 0.0 | 0.13c | 4 | 44.5 | NSc | 5 | 55.5 | NSc |
| Site | |||||||||
| Proximal (36) | 5 | 14.0 | NSb | 16 | 44.5 | 0.052b | 15 | 41.5 | NSb |
| Distal (83) | 18 | 21.5 | 22 | 26.5 | 43 | 52.0 | |||
| Total (119) | 23 | 20.0 | 38 | 32.0 | 58 | 48.0 | |||
Total cases of each subset. Percentages in the next columns were calculated upon this number.
First subset (stage I, G1, proximal) vs. others.
Poor grade vs. others. NS, not significant. P<0.15, although not significant it was included in the table to indicate corresponding trends.
Figure 4.
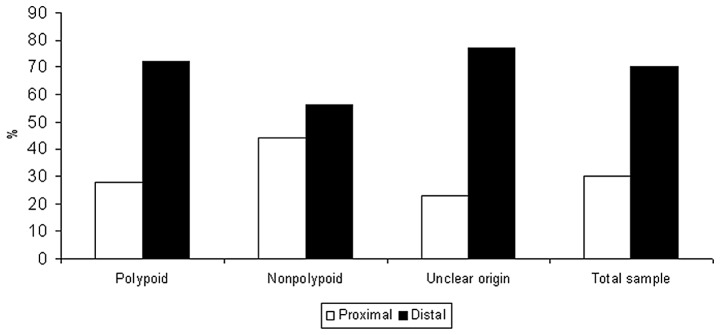
Segmental distribution according to tumor origin is shown. The proportion of proximal tumors was markedly higher among nonpolypoid tumors compared to that observed among polypoid and unclarified lesions (P= 0.04), showing a relatively similar pattern of segmental distribution. Notably, while polypoid tumors were the subset better approaching the anatomical distribution of the total sample, nonpolypoid lesions were widely diverging from that distribution.
Clinicopathological distribution of de novo tumors
Table IV shows the distribution of lesions classified as de novo by stage, grade and site, exhibiting the aforementioned predilection for proximal location as well as a higher (albeit not significantly) detection rate in cases with poor grade or unfavorable characteristics (stage IV or G3). However, the observed total pattern of distribution of these lesions varying between 12.5% (indolent cases) and 33% (unfavorable cases) clearly differed from that observed for polypoid tumors, ranging from 62.5 to 19% in the same categories (P=0.03, Fig. 5). This disparity was more evident in the indolent subset (P=0.008).
Table IV.
Distribution of de novo tumors in various clinicopathological categories.
|
De novo lesions
|
|||
|---|---|---|---|
| Category (n)a | No. | Percentage | P-valueb |
| Stage | |||
| I (12) | 2 | 17 | NSc |
| II (50) | 14 | 28 | |
| III (44) | 15 | 34 | |
| IV (13) | 3 | 23 | |
| Grade | |||
| Well (G1) (7) | 2 | 29 | NSd |
| Moderate (G2) (103) | 28 | 27 | |
| Poor (G3) (9) | 4 | 44 | |
| Site | |||
| Proximal (36) | 15 | 42 | 0.04e |
| Distal (83) | 19 | 23 | |
| Combined stage-grade | |||
| Indolent (16) | 2 | 12.5 | NS (0.12)f |
| Intermediate (82) | 25 | 30 | |
| Unfavorable (21) | 7 | 33 | |
| Total (119) | 34 | 28.5 | |
Total cases of each subset. Percentages in the next columns were calculated upon this number.
All comparisons were performed between the observed frequencies of non-polypoid lesions in the various categories. The distribution of polypoid and tumors of unclear origins is not shown in this table.
Stages I–II vs. III–IV.
G3 vs. G1–G2.
Proximal vs. distal.
Indolent vs. intermediate-unfavorable. NS, not significant.
Figure 5.
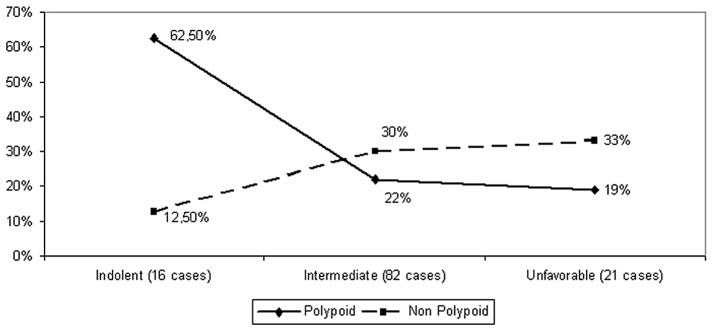
Disparate distribution of polypoid and non-polypoid lesions among combined stage-grade categories is shown. The frequency of polypoid tumors was reduced from 62.5% in the indolent subset to a mere 19% in the unfavorable category. Conversely, the proportion of non-polypoid lesions increased from 12.5 to 33% in the same categories (P=0.03 for the comparison of overall distributions and P=0.008 for the particular comparison of the observed proportions in the indolent subset).
Discussion
The aim of this study was to identify de novo tumors on the basis of certain histomorphological criteria (lack of remnants or coexisting polyps, nonpolypoid configuration). We also examined their correlation with particular clinicopathological characteristics (stage, grade and site). The study was conducted in a sample mainly comprising ‘advanced’ CRC (i.e., tumors invading beyond the submucosa), consistent with certain previous studies (7,12,13,20,23), although in contrast to most Japanese (5,6,10,18,19) and certain Western studies (8,11) focusing on early (T1) lesions.
Consistent with the findings reported by Chen et al, the ascertained incidence of potentially de novo tumors in our series was 28.5% (12). However, the incidence of such lesions is widely varying (even within a given study) according to the criteria occasionally used for their detection. In the large-scale study by Bedenne et al(7)de novo tumors (defined as those lacking remnants) were found in 40% of the cases, ranging from 17% in small T1 lesions to almost 100% in tumors with an endophytic (nonpolypoid) growth pattern. Largely diverging frequencies were also reported by Shimoda et al(6) (25% in early and 80% in advanced lesions). Moreover, in the study by Goto et al(10), the proportion of de novo lesions was 23% in early (T1) CRC, increasing to 32% when small advanced lesions were additionally included in the analyzed sample. These discrepancies might be attributed in part to the difficulty in the colonoscopic detection of early de novo lesions and their potentially rapid growth, leading to their underestimation in early CRC and, concurrently to a diagnostic delay (14,21) resulting in their overrepresentation in advanced CRC.
The presence of infiltrative growth pattern seems to be suggestive of de novo origin, as indicated by the rarity in the appearance of remnants among infiltrative lesions (2.5%), consistent with previous results (6,7). However, a number of those may actually arise from adenomas, with their gross configuration being altered into nonpolypoid during their evolution (10,23), although this has been disputed (6,13). Nevertheless, the effect of this bias (if any) may be counteracted by the potentially simultaneous presence of certain tumors with nonpolypoid route among cases classified as of unclear origin (due to their extended ulceration).
The low proportion (27%) of lesions with apparently polypoid characteristics in our sample is probably not representative of the actual incidence of CRC of adenoma origin, although similar results have been occasionally reported (6,24). Tumors of ‘unclear origin’, accounting for 44.5% of our cohort, likely arise from polyps (for the most part) (23), as possibly suggested by their predilection for distal tumor site (similar to polypoid and unlike de novo lesions). Supportive of this assumption was also the recorded high incidence of polypoid lesions in stage I (the earliest disease cases in our sample), 67% consistent with findings of a previous study in a similar subset (T2 lesions) (25). Moreover, this tendency towards earlier stage (considerably stronger for cases combining polypoid characteristics) is possibly indicative of more favorable behavior and/or easier colonoscopic detection of polypoid lesions and should be investigated in larger samples.
Consistent with results of previous studies (7,16), we found lesions possibly developing de novo, in a significantly higher proportion among proximal tumors. This trend was particularly associated with the infiltrative growth pattern, more frequently observed proximally. Given the known clinicopathological and molecular differences of proximal CRC (4,16,26,27), the recorded link between nonpolypoid lesions and proximal location supports the assumption of a different tumorigenic mechanism for de novo CRC. Moreover, this association suggests a higher malignant potential of de novo tumors, anticipated to the corresponding clinicopathological pattern reported for proximal tumors [higher stage and grade, worse outcome (16,27,28)]. The recorded rarity of de novo lesions in the indolent subset is also supportive of this state and consistent with previous findings (20,29). It is also potentially correlated with the proximal predilection of these tumors, considering the milder clinical manifestations and the lower efficacy of colonoscopy observed in proximal CRC, resulting in later stage presentation (16,28).
From the clinical aspect, these results require more awareness and persistence in the colonoscopic detection of nonpolypoid lesions (particularly in the proximal segment), more intensive surveillance of the colonoscopically treated cases and surgical treatment for selected patients. These suggestions are also applicable for cancers alternatively evolving through a corresponding nonpolypoid adenoma, instead of direct de novo development (14). The clinical significance of nonpolypoid lesions, emphasized in recent studies (21,30), is expected to be further elucidated by the ongoing prospective Japan Polyp Study (31), evaluating CRC surveillance strategies after initial colonoscopic removal of the detected neoplasia.
To the best of our knowledge, the current study is the first using the presence of coexisting polyps as a discriminative criterion between polypoid and nonpolypoid CRC. This application was based on the ‘field cancerization theory’, involving the dissemination of genetic changes characteristic of the primary tumor over a wide distance from the edges of the lesion (15,32,33). Therefore, coexisting polyps, likely originating from the same changes, are indicative of a polypoid origin of the primary tumor. Additional investigation of the molecular alterations potentially occurring in the seemingly healthy colonic mucosa near the tumor is necessary to determine the exact distance required for the definite characterization of a given polyp as actually correlated with the primary lesion.
A limitation to our study was the relatively small sample size [although comparable to other relevant studies (10,11,13,20,25)], potentially affecting results, particularly in subset analysis. Another limitation could be the almost complete lack of small and/or T1 lesions in our samples, potentially hampering de novo identification. However, such investigation in tumors invading beyond the submucosa (as we did), was also conducted by other scientists (7,12,13,20,25), reporting frequencies of de novo lesions ranging from 11 to 40%. Our findings (28.5%) were within this range. Additionally, consistent with our findings, the incidence of nonpolypoid small T1 tumors has been found to be markedly low (<5%) in previous studies (9,11,16,34), suggesting a rather limited utility of the particular criteria for de novo identification (particularly among CRC referred for surgery).
In conclusion, our study was suggestive of a potential de novo origin for a considerable proportion of CRC, concurrently indicating the predilection of these lesions for proximal tumor location and introducing an additional approach (that of coexisting polyps) potentially facilitating de novo identification. The clinical impact of our findings should be examined, especially with regard to possible associations between de novo lesions and important prognostic variables. The tendency for a worse behavior of nonpolypoid lesions, as suggested by their increasing incidence with disease progression and aggressiveness may necessitate adequate readjustments in the diagnosis, treatment and surveillance of these cases.
Acknowledgments
The authors would like to thank Dr F. Georgiadis for his help, Mrs. N. Vathi and Mr. V. Anthoulis for their valuable assistance in the preparation of the manuscript.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Morson B. President’s address. The polyp-cancer sequence in the large bowel. Proc R Soc Med. 1974;67:451–457. [PMC free article] [PubMed] [Google Scholar]
- 3.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;6:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 4.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–130. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 5.Kuramoto S, Oohara T. Minute cancers arising de novo in the human large intestine. Cancer. 1988;61:829–834. doi: 10.1002/1097-0142(19880215)61:4<829::aid-cncr2820610431>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 6.Shimoda T, Ikegami M, Fujisaki J, Matsui T, Aizawa S, Ishikawa E. Early colorectal carcinoma with special reference to its development de novo. Cancer. 1989;64:1138–1146. doi: 10.1002/1097-0142(19890901)64:5<1138::aid-cncr2820640529>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 7.Bedenne L, Faivre J, Boutron MC, Piard F, Cauvin MJ, Hillon P. Adenoma-carcinoma sequence or de novo carcinogenesis? A study of adenomatous remnants in population-based series of large bowel cancers. Cancer. 1992;69:883–888. doi: 10.1002/1097-0142(19920215)69:4<883::aid-cncr2820690408>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 8.Stolte M, Bethke B. Colorectal mini-de novo carcinoma: a reality in Germany too. Endoscopy. 1995;27:286–290. doi: 10.1055/s-2007-1005694. [DOI] [PubMed] [Google Scholar]
- 9.Mueller JD, Bethke B, Stolte M. Colorectal de novo carcinoma: a review of its diagnosis, histopathology, molecular biology, and clinical relevance. Virchows Arch. 2002;440:453–460. doi: 10.1007/s00428-002-0623-z. [DOI] [PubMed] [Google Scholar]
- 10.Goto H, Oda Y, Murakami Y, et al. Proportion of de novo cancers among colorectal cancers in Japan. Gastroenterology. 2006;131:40–46. doi: 10.1053/j.gastro.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Hornic J, Farraye F, Odze R. Clinicopathologic and immunohistochemical study of small apparently ‘de novo’ colorectal adenocarcinomas. Am J Surgh Pathol. 2007;31:207–215. doi: 10.1097/01.pas.0000213383.17418.a9. [DOI] [PubMed] [Google Scholar]
- 12.Chen CD, Yen MF, Wang WM, Wong JM, Chen TH. A case-cohort study for the disease natural history of adenoma-carcinoma and de novo carcinoma and surveillance of colon and rectum after polypectomy: implication for efficacy of colonoscopy. Br J Cancer. 2003;88:1866–1873. doi: 10.1038/sj.bjc.6601007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaneko K, Kurahashi T, Makino R, et al. Pathological features and genetic alterations in colorectal carcinomas with characteristics of nonpolypoid growth. Br J Cancer. 2004;91:312–318. doi: 10.1038/sj.bjc.6601965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kashida H, Kudo S. Early colorectal cancer: concept, diagnosis, and management. Int J Clin Oncol. 2006;11:1–8. doi: 10.1007/s10147-005-0550-5. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka T. Colorectal carcinogenesis: review of human and experimental animal studies. J Carcinog. 2009;8:5. doi: 10.4103/1477-3163.49014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nawa T, Kato J, Kawamoto H, et al. Differences between right-and left-sided colon cancer in patient characteristics, cancer morphology and histology. J Gastroenderol Hepatol. 2008;23:418–423. doi: 10.1111/j.1440-1746.2007.04923.x. [DOI] [PubMed] [Google Scholar]
- 17.Ogawa T, Yoshida T, Tsuruta T, Saigenji K, Okayasu I. Genetic instability on chromosome 17 in the epithelium of non-polypoid colorectal carcinomas compared to polypoid lesions. Cancer Sci. 2006;97:1335–1342. doi: 10.1111/j.1349-7006.2006.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nosho K, Yamamoto H, Takahashi T, et al. Genetic and epigenetic profiling in early colorectal tumors and prediction of invasive potential in pT1 (early invasive) colorectal cancers. Carcinogenesis. 2007;28:1364–1370. doi: 10.1093/carcin/bgl246. [DOI] [PubMed] [Google Scholar]
- 19.Yasugi A, Yashima K, Hara A, et al. Fhit, Mlh1, p53 and phenotypic expression in the early stage of colorectal neoplasms. Oncol Rep. 2008;19:41–47. [PubMed] [Google Scholar]
- 20.Nasir A, Boulware D, Kaiser H, et al. Flat and polypoid adenocarcinomas of the colorectum: a comparative histomorphologic analysis of 47 cases. Hum Pathol. 2004;35:604–611. doi: 10.1016/j.humpath.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Soetikno R, Kaltenbach T, Rouse R, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299:1027–1035. doi: 10.1001/jama.299.9.1027. [DOI] [PubMed] [Google Scholar]
- 22.Yasutomi M, Baba S, Hojo K, editors. Japanese classification of colorectal cancinoma. Kanehara & Co, Ltd; Tokyo: 1997. [Google Scholar]
- 23.Matsui T, Yao T, Iwashita A. Natural history of early colorectal cancer. World J Surg. 2000;24:1022–1028. doi: 10.1007/s002680010153. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura K. De novo cancer and adenoma-carcinoma sequence of the colorectum-clinicopathological differences between de novo carcinoma and carcinoma with the sequence. Nihon Geka Gakkai Zaasshi. 1999;100:766–775. (In Japanese). [PubMed] [Google Scholar]
- 25.Goi T, Kawasaki M, Hirono Y, Katayama K, Yamaguchi A. Clinicopathological analysis of invading muscularis propria (T2) cancers ≤20 mm in diameter. Int Surg. 2008;93:1–5. [PubMed] [Google Scholar]
- 26.Sugai T, Habano W, Jiao YF, Tsukahara M, Takeda Y, Otsuka K, Nakamura S. Analysis of molecular alterations in left- and right-sided colorectal carcinomas reveals distinct pathways of carcinogenesis. J Mol Diagn. 2006;8:193–201. doi: 10.2353/jmoldx.2006.050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papagiorgis PC, Zizi AE, Tseleni S, et al. Site impact on colorectal cancer biological behavior in terms of clinicopathological and molecular features. J BUON. 2011;16:84–92. [PubMed] [Google Scholar]
- 28.Wong R. Proximal tumors are associated with greater mortality in colon cancer. J Gen Intern Med. 2010;25:1157–1163. doi: 10.1007/s11606-010-1460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurisu Y, Shimoda T, Ochiai A, Nakanishi Y, Hirata I, Katsu KI. Histologic and immunohistochemical analysis of early submucosal invasive carcinoma of the colon and rectum. Pathol Int. 1999;49:608–616. doi: 10.1046/j.1440-1827.1999.00928.x. [DOI] [PubMed] [Google Scholar]
- 30.Matsuda T, Saito Y, Hotta K, Sano Y, Fujii T. Prevalence and clinicopathological features of nonpolypoid colorectal neoplasms: should we pay more attention to indentifying flat and depressed lesions? Dig Endoscopy. 2010;22:S57–S62. doi: 10.1111/j.1443-1661.2010.00967.x. [DOI] [PubMed] [Google Scholar]
- 31.Sano Y, Fujii T, Oda Y, et al. A multicenter randomized controlled trial designed to evaluate follow-up surveillance strategies for colorectal cancer: the Japan Polyp Study. Dig Endoscop. 2004;16:376–378. [Google Scholar]
- 32.Dakubo GD, Jakupciak JP, Birch-Machin MA, Parr RL. Clinical implications and utility of field cancerization. Cancer Cell Int. 2007;7:2. doi: 10.1186/1475-2867-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aivado M, Gynes M, Golerov V, Schmidt WU, Röher HD, Goretzki PE. ‘Field cancerization’ - an additional phenomenon in development of colon tumors? K-ras codon mutations in normal colonic mucosa of patients with colorectal neoplasms. Chirurg. 2000;71:1230–1234. doi: 10.1007/s001040051207. (In German). [DOI] [PubMed] [Google Scholar]
- 34.Weil R, Ohana G, Haplern M, Estlein D, Anvi A, Wolloch Y. Small nonpolypoid colorectal carcinoma. World J Surg. 2002;26:503–508. doi: 10.1007/s00268-001-0257-3. [DOI] [PubMed] [Google Scholar]