Abstract
Unhealthy dietary patterns are associated with poorer lung function. It is not known whether this is due to low consumption of antioxidant-rich fruit and vegetables, or is a consequence of higher intakes of harmful dietary constituents such as processed meat.
We examined the individual and combined associations of processed meat, fruit and vegetable consumption and dietary total antioxidant capacity (TAC) with lung function among 1551 men and 1391 women in the Hertfordshire Cohort Study, UK. Diet was assessed by food frequency questionnaire.
After controlling for confounders, processed meat consumption was negatively associated with forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and FEV1/FVC in men and women, while fruit and vegetable consumption and dietary TAC were positively associated with FEV1 and FVC, but not FEV1/FVC. In men the negative association between processed meat consumption and FEV1 was more marked in those who had low fruit and vegetable consumption (Pinteraction=0.035), and low dietary TAC (Pinteraction=0.025). The deficit in FEV1/FVC associated with processed meat consumption was larger in men who smoked (Pinteraction=0.022).
Higher processed meat consumption is associated with poorer lung function, especially in men who have lower fruit and vegetable consumption or dietary TAC, and among current smokers.
Keywords: Dietary balance, total antioxidant capacity, fruit and vegetables, processed meat, lung function
INTRODUCTION
Healthy dietary patterns appear to have protective effects on lung function in older age [1-3]. For example, we have previously described a healthy “prudent” dietary pattern, characterized by high consumption of fruit, vegetables, oily fish and wholemeal cereals, but low consumption of white bread, added sugar, full-fat dairy products, chips and processed meat, that is associated with better lung function and reduced prevalence of chronic obstructive pulmonary disease (COPD) among older people in the UK [1]. The apparently beneficial effects of healthy dietary patterns on lung function have been largely attributed to the effects of antioxidants in fruit and vegetables. Epidemiological studies have reported positive associations of antioxidant-rich foods, and nutrients including vitamin C, vitamin E, β-carotene and flavonoids, with lung function [4-6]. Antioxidants are thought to play a protective role in the pathogenesis of lung impairment by scavenging free radicals and other oxygen species that cause cellular damage and inflammation [7-9].
However, one aspect of dietary patterns that may be overlooked is that they describe a balance of foods - and are characterised both by relatively high and by relatively low consumption of individual food items. Potentially one of the most important types of food that is less common in healthy diets is processed meat (such as bacon, ham, sausage and other cured meats). There is growing evidence that a high consumption of processed meat is associated with poorer lung function and an increased risk of COPD, including exacerbations [10-13]. This could be due to its high nitrite content. Nitrites are added as a preservative, an antimicrobial agent and a colour fixative, and generate reactive nitrogen species that can cause oxidative and nitrative damage to the lung [14]. Processed meat is also rich in advanced glycation end products (AGEs) [15] which can increase oxidative stress and inflammation [16]. Thus, the size of the effect of processed meat consumption on lung function may also depend on other factors which influence pulmonary oxidant/antioxidant balance such as dietary antioxidant intake and smoking. The protective effect of a healthy “prudent” dietary pattern on lung function could therefore reflect a favourable balance of protective antioxidants and harmful pro-oxidant foods in the diet. To our knowledge the role of the balance of such foods in the diet has not been considered before.
In a large cohort of older men and women, we investigated associations between lung function and key foods (processed meat and fruit and vegetables) that may contribute to pulmonary oxidant/antioxidant balance. Our particular aim was to consider the nature of their individual and combined associations with lung function. In addition, since foods other than fruit and vegetables contribute to overall antioxidant status, we also considered the role of the total antioxidant capacity (TAC) of the diet [17].
METHODS
The Hertfordshire Cohort Study (HCS)
Details of the HCS have been published elsewhere [18]. From 1911–1948, midwives recorded information on all infants born in the county of Hertfordshire, UK. In 1998, 3822 men and 3284 women (born 1931–1939) were traced. Permission to contact 3126 men (82%) and 2973 women (91%) was obtained from their general practitioner; 1684 men (54%) and 1541 women (52%) agreed to a home interview; 1579 men (94%) and 1418 women (92%) attended a clinic for further investigations. In total, 1551 men and 1391 women completed spirometry tests. The study had ethical approval from the Bedfordshire and Hertfordshire local research ethics committee and the West Hertfordshire local research ethics committee. Written informed consent was obtained from all participants.
Dietary assessment
Diet over a 3-month period before the home interview was assessed using a food frequency questionnaire (FFQ) that was administered by a trained research nurse [19,20]. The FFQ included 129 foods and food groups. Ten predefined frequency responses were listed, ranging from “never” to “6+ per day”. Information on frequency of consumption and quantities consumed of different types of alcoholic beverages was obtained separately. Energy intake from foods and alcoholic beverages was calculated by multiplying the frequency of consumption of a portion of each food item by its energy content, according to the UK national food composition database or manufactures’ composition data [21,22].
For the analyses we grouped foods listed on the FFQ as follows. Processed meat: bacon and gammon, ham, corned beef, spam and luncheon meat, sausage, meat pies; fruit: fresh fruit (including citrus, apples, bananas, grapes), fruit juices, dried fruit, tinned and cooked fruit; vegetables: fresh & frozen vegetables (including cabbage, cauliflower, peas and root vegetables), salad vegetables, pulses, vegetarian foods and tinned vegetables. Weekly consumption (servings/week) of processed meat and fruit and vegetables were calculated as the sum of the individual frequencies of the foods within these groups.
Dietary TAC was estimated using published composition data where TAC was assessed using an oxygen radical absorbance capacity (ORAC) assay, that measured the degree of inhibition of peroxy-radical-induced oxidation in vitro [23,24]. ORAC was selected as one of the most widely used methods because of its biological relevance to the in vivo antioxidant efficacy [23,24]. A total dietary TAC score was calculated for each participant by multiplying the TAC values of each food/beverage by their reported frequency of consumption and then summing these values. A full description of the assignment of TAC values to each food item and calculation method is given in the online appendix.
Lung function
Lung function was measured using a Micro Spirometer (CareFusion UK, Gillingham, UK) in the seated position without noseclips. After at least one practice blow, three forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) readings were recorded. The highest FEV1 and FVC values from satisfactory manoeuvres were used in the analyses; these did not necessarily come from the same blow. A bronchodilator was not given before spirometry was performed. For FEV1, 85.8% of the men and 92.2% of the women had a difference of ≤0.15 L between their two highest readings; for FVC, the corresponding figures were 80.4% and 88.6%. However, we did not exclude those with a difference of >0.15 L [25]. We calculated standardized residuals of lung function by using Global Lung function Initiative 2012 regression equations (which is based on lung function data from lifelong non-smokers) [26]. COPD was defined as FEV1/FVC less than the lower limit of normal (i.e. the Z-score was <−1.645) [26].
Statistical analysis
All statistical analyses were performed using Stata version 12 (Statacorp LP, College Station, TX, USA). Univariate and multiple linear regression analysis were used to examine the relationships between consumption of processed meat, fruit and vegetables and dietary TAC with lung function outcomes; logistic regression was used to analyse COPD. For the regression analysis, we controlled for the effects of age and height, and the following potential confounders: smoking status (never, ex, or current), pack-years smoked, exposure to tobacco smoke in the home, age left education, social class, body fat mass, physical activity score, dietary supplement use, use of inhaled or oral steroids, use of paracetamol, alcohol consumption and energy intake. A detailed full description of the confounders is given in the online appendix. Tests for trend associations were based on continuously distributed variables and after adjustment for potential confounders.
We evaluated the combined associations of processed meat consumption and dietary antioxidants (i.e., fruit and vegetables and dietary TAC) on lung function, examining both their independent and interactive associations. We also stratified dietary associations by smoking status and tested for interactions. We analyzed men and women separately. Where dietary variables were categorized into fifths (for tables) or thirds (for figures), we used cut-offs that were defined using the distributions for the whole population.
RESULTS
The men and women studied were of similar social class, but the men were more likely to be current or ex-smokers, to smoke more heavily, and to drink alcohol (Table 1). The men were less likely than the women to be taking oral or inhaled steroids, paracetamol or dietary supplements. They had higher processed meat consumption and total energy intakes than women, but lower consumption of fruit and vegetables (all p<0.05). Dietary TAC was mainly derived from fruit (35.8%), tea (15.4%), vegetables (14.9%), potatoes (13.4%) and cereal products (7.1%); dietary TAC did not differ between men and women. Men had a lower FEV1/FVC ratio and FEV1/FVC z-score and a higher FVC z-score and prevalence of COPD (both p<0.001).
TABLE 1. Subject characteristics of 1551 men and 1391 women who participated in the Hertfordshire Cohort Study, UK.
| Men (n=1551) |
Women (n=1391) |
|
|---|---|---|
| Age (years) | 65.7 (2.9) | 66.6 (2.7) |
| Height (cm) | 174.2 (6.4) | 160.9 (5.9) |
| Fat mass (kg) | 23.3 (19.0–28.4) | 28.2 (23.2–33.8) |
| Habitual activity score | 61.0 (15.3) | 59.1 (15.7) |
| Smoking status (n, %) | ||
| Never smoker | 507 (32.7) | 854 (61.4) |
| Ex-smoker | 806 (52.0) | 402 (28.9) |
| Current smoker | 238 (15.3) | 134 (9.6) |
| Pack-years among ever smokers | 23 (11–40) | 15 (5–29) |
| Exposed to tobacco smoke in the home (n, %) | 203 (13.5) | 163 (11.9) |
| Age left full-time education (n, %) | ||
| ≤ 14 years | 302 (19.5) | 241 (17.3) |
| ≥ 15 years | 1249 (80.5) | 1150 (82.7) |
| Social class (n, %) | ||
| I-IIINM | 611 (40.6) | 583 (41.9) |
| IIIM-V | 893 (59.4) | 807 (58.1) |
| Taking oral or inhaled steroid (n, %) | 107 (6.9) | 127 (9.1) |
| Taking paracetamol (n, %) | 105 (6.8) | 178 (12.8) |
| Taking dietary supplements (n, %) | 712 (45.9) | 824 (59.2) |
| Alcohol consumption (units/week) | 8.9 (2.3–19.5) | 1.5 (0.0–5.5) |
| Dietary intake | ||
| Processed meat (servings/week) | 4.0 (2.5–6.2) | 3.0 (1.9–4.5) |
| Fruit and vegetables (servings/week) | 42.6 (31.1–56.3) | 47.1 (35.5–60.4) |
| TAC (μmol/day) | 15915 (12425–19640) | 15915 (12641–19387) |
| Total energy intake (kcal/day) | 2433 (2093–2796) | 1973 (1705–2272) |
| Lung function | ||
| Maximum FEV1 (L) | 2.84 (0.60) | 1.98 (0.41) |
| Maximum FVC (L) | 4.04 (0.74) | 2.71 (0.50) |
| FEV1/FVC | 0.702 (0.089) | 0.732 (0.079) |
| GLI 2012 FEV1 (z-score)* | −0.74 (0.03) | −0.77 (0.03) |
| GLI 2012 FVC (z-score)* | −0.28 (0.03) | −0.42 (0.03) |
| GLI 2012 FEV1/FVC (z-score)* | −0.81 (0.03) | −0.68 (0.03) |
| Prevalence of COPD (n, %) | 274 (17.7) | 185 (13.3) |
Data are shown as n (%), mean (standard deviation) or median (interquartile range).
TAC, total antioxidant capacity; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; COPD, chronic obstructive pulmonary disease.
Calculated by the Global Lung Function Initiative 2012 regression equations (which is based on lung function data from lifelong non-smokers) [26].
Individual associations of processed meat, fruit and vegetable consumption and dietary TAC with lung function
After controlling for potential confounders, processed meat consumption was negatively associated with FEV1 in both sexes; this was especially marked in men [difference in FEV1 comparing top versus bottom fifth of consumption -170 mL (95% CI −250, −80)] (Tables 2 and 3). In contrast, fruit and vegetable consumption and dietary TAC were positively associated with FEV1 in both men and women. The patterns of association with FVC were similar to those for FEV1. Processed meat consumption was negatively associated with FEV1/FVC, but there were no associations with fruit and vegetable consumption or dietary TAC in either men or women (Tables 2 and 3). In men, processed meat consumption was positively associated with COPD (p for trend=0.013, online supplementary Table E1). Fruit and vegetable consumption and dietary TAC were not associated with COPD risk in either sex. The effect sizes for the associations between dietary exposures and lung function were compared for men and women, but they did not differ substantially (p for interaction all >0.05).
TABLE 2. Associations of processed meat, fruit and vegetable consumption and dietary total antioxidant capacity (TAC) with forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and FEV1/FVC among men in the Hertfordshire Cohort Study, UK.
| FEV1 (L) |
FVC (L) |
FEV1/FVC |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Regression coefficient | Mean (SD) | Regression coefficient | Mean (SD) | Regression coefficient | |||||
| Variable* | n | (95%CI)† | (95%CI)† | (95%CI)† | ||||||
| Processed meat (servings/week) | ||||||||||
| Q1 (< 2.0) | 264 | 2.95 (0.56) | Reference | 4.18 (0.73) | Reference | 0.709 (0.084) | Reference | |||
| Q2 (2.1-) | 268 | 2.90 (0.60) | −0.06 | (−0.15, 0.03) | 4.11 (0.70) | −0.07 | (−0.18, 0.03) | 0.706 (0.088) | −0.006 | (−0.020, 0.008) |
| Q3 (3.2-) | 271 | 2.81 (0.62) | −0.09 | (−0.18, 0.00) | 3.99 (0.77) | −0.13 | (−0.24, −0.03) | 0.704 (0.087) | −0.001 | (−0.015, 0.012) |
| Q4 (4.4-) | 350 | 2.85 (0.59) | −0.11 | (−0.19, −0.02) | 4.02 (0.71) | −0.13 | (−0.23, −0.03) | 0.707 (0.085) | −0.008 | (−0.022, 0.005) |
| Q5 (≥ 6.2) | 398 | 2.74 (0.62) | −0.17 | (−0.25, −0.08) | 3.97 (0.75) | −0.16 | (−0.26, −0.06) | 0.691 (0.096) | −0.016 | (−0.029, −0.003) |
| Effect per fifth increase | −0.04 | (−0.06, −0.02) | −0.04 | (−0.06, −0.01) | −0.004 | (−0.007, −0.001) | ||||
| P for trend‡ | <0.001 | 0.001 | 0.006 | |||||||
| Fruit and vegetables (servings/week) | ||||||||||
| Q1 (< 30.7) | 368 | 2.70 (0.65) | Reference | 3.93 (0.75) | Reference | 0.686 (0.103) | Reference | |||
| Q2 (30.7-) | 333 | 2.81 (0.64) | 0.00 | (−0.07, 0.08) | 4.00 (0.79) | −0.01 | (−0.11, 0.08) | 0.702 (0.087) | 0.003 | (−0.009, 0.015) |
| Q3 (40.5-) | 286 | 2.92 (0.56) | 0.07 | (−0.01, 0.15) | 4.13 (0.69) | 0.07 | (−0.03, 0.16) | 0.710 (0.086) | 0.007 | (−0.006, 0.020) |
| Q4 (49.4-) | 293 | 2.88 (0.57) | 0.04 | (−0.04, 0.12) | 4.05 (0.71) | 0.02 | (−0.08, 0.12) | 0.709 (0.081) | 0.008 | (−0.005, 0.020) |
| Q5 (≥ 62.3) | 271 | 2.95 (0.53) | 0.08 | (0.00, 0.17) | 4.16 (0.70) | 0.10 | (0.00, 0.20) | 0.709 (0.080) | 0.005 | (−0.008, 0.018) |
| Effect per fifth increase | 0.02 | (0.00, 0.04) | 0.02 | (−0.00, 0.05) | 0.002 | (−0.001, 0.004) | ||||
| P for trend‡ | 0.041 | 0.058 | 0.356 | |||||||
| Dietary TAC (μmol/day) | ||||||||||
| Q1 (< 11795) | 318 | 2.70 (0.65) | Reference | 3.93 (0.77) | Reference | 0.687 (0.103) | Reference | |||
| Q2 (11795-) | 313 | 2.81 (0.60) | 0.05 | (−0.03, 0.13) | 4.00 (0.72) | 0.02 | (−0.08, 0.12) | 0.701 (0.087) | 0.007 | (−0.006, 0.020) |
| Q3 (14547-) | 298 | 2.84 (0.61) | 0.07 | (−0.01, 0.16) | 4.01 (0.73) | 0.02 | (−0.08, 0.12) | 0.708 (0.089) | 0.014 | (0.001, 0.027) |
| Q4 (17210-) | 299 | 2.95 (0.54) | 0.11 | (0.02, 0.19) | 4.15 (0.67) | 0.07 | (−0.03, 0.18) | 0.710 (0.079) | 0.013 | (−0.001, 0.026) |
| Q5 (≥ 20551) | 323 | 2.92 (0.58) | 0.11 | (0.02, 0.20) | 4.13 (0.76) | 0.10 | (0.00, 0.21) | 0.707 (0.082) | 0.009 | (−0.004, 0.023) |
| Effect per fifth increase | 0.01 | (0.01, 0.05) | 0.03 | (0.00, 0.05) | 0.002 | (−0.001, 0.005) | ||||
| P for trend‡ | 0.031 | 0.045 | 0.311 | |||||||
Cut-off values for fifths of dietary consumption were derived from distributions among the whole population.
Adjusted for age, height, smoking status, pack-years, exposure to tobacco smoke in home, age left education, social class, body fat mass, physical activity score, dietary supplement use, inhaled or oral steroid use, paracetamol use, alcohol consumption and energy intake.
P values to test for linear trends were calculated by using dietary consumption as a continuous variable after controlling for potential confounders.
TABLE 3. Associations of processed meat, fruit and vegetable consumption and dietary total antioxidant capacity (TAC) with forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and FEV1/FVC among women in the Hertfordshire Cohort Study, UK.
| FEV1 (L) |
FVC (L) |
FEV1/FVC |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Regression coefficient | Mean (SD) | Regression coefficient | Mean (SD) | Regression coefficient | |||||
| Variable* | n | (95%CI)† | (95%CI)† | (95%CI)† | ||||||
| Processed meat (servings/week) | ||||||||||
| Q1 (< 2.0) | 438 | 2.06 (0.41) | Reference | 2.79 (0.51) | Reference | 0.737 (0.081) | Reference | |||
| Q2 (2.1-) | 313 | 1.99 (0.40) | −0.04 | (−0.09, 0.02) | 2.72 (0.51) | −0.02 | (−0.09, 0.04) | 0.733 (0.075) | −0.007 | (−0.018, 0.004) |
| Q3 (3.2-) | 239 | 1.93 (0.43) | −0.07 | (−0.13,−0.01) | 2.64 (0.51) | −0.07 | (−0.14, −0.01) | 0.730 (0.073) | −0.010 | (−0.022, 0.002) |
| Q4 (4.4-) | 242 | 1.91 (0.39) | −0.10 | (−0.15, −0.04) | 2.64 (0.47) | −0.10 | (−0.17, −0.04) | 0.725 (0.078) | −0.009 | (−0.021, 0.002) |
| Q5 (≥ 6.2) | 159 | 1.92 (0.42) | −0.05 | (−0.12, 0.01) | 2.64 (0.47) | −0.04 | (−0.12, 0.04) | 0.724 (0.087) | −0.012 | (−0.026, 0.002) |
| Effect per fifth increase | −0.02 | (−0.04, −0.01) | −0.02 | (−0.04, −0.00) | −0.003 | (−0.006, 0.000) | ||||
| P for trend‡ | 0.005 | 0.031 | 0.041 | |||||||
| Fruit and vegetables (servings/week) | ||||||||||
| Q1 (< 30.7) | 223 | 1.89 (0.44) | Reference | 2.61 (0.52) | Reference | 0.724 (0.094) | Reference | |||
| Q2 (30.7-) | 256 | 1.93 (0.42) | 0.03 | (−0.04, 0.09) | 2.65 (0.50) | 0.01 | (−0.06, 0.09) | 0.726 (0.078) | 0.007 | (−0.006, 0.021) |
| Q3 (40.5-) | 300 | 1.97 (0.39) | 0.04 | (−0.03, 0.10) | 2.67 (0.47) | 0.00 | (−0.07, 0.07) | 0.736 (0.073) | 0.013 | (0.000, 0.026) |
| Q4 (49.4-) | 296 | 2.01 (0.38) | 0.07 | (0.00, 0.13) | 2.73 (0.48) | 0.05 | (−0.02, 0.13) | 0.735 (0.073) | 0.011 | (−0.002, 0.025) |
| Q5 (≥ 62.3) | 316 | 2.07 (0.41) | 0.10 | (0.03, 0.16) | 2.83 (0.52) | 0.10 | (0.03, 0.17) | 0.733 (0.077) | 0.010 | (−0.003, 0.024) |
| Effect per fifth increase | 0.02 | (0.01, 0.04) | 0.03 | (0.01, 0.04) | 0.002 | (−0.001, 0.005) | ||||
| P for trend‡ | 0.001 | <0.001 | 0.637 | |||||||
| Dietary TAC (μmol/day ) | ||||||||||
| Q1 (< 11795) | 318 | 1.92 (0.42) | Reference | 2.61 (0.49) | Reference | 0.732 (0.088) | Reference | |||
| Q2 (11795-) | 313 | 1.94 (0.42) | 0.02 | (−0.04, 0.08) | 2.66 (0.50) | 0.02 | (−0.05, 0.09) | 0.725 (0.079) | 0.001 | (−0.012, 0.014) |
| Q3 (14547-) | 298 | 2.00 (0.39) | 0.05 | (−0.02, 0.11) | 2.70 (0.45) | 0.03 | (−0.04, 0.10) | 0.738 (0.073) | 0.009 | (−0.003, 0.022) |
| Q4 (17210-) | 299 | 2.02 (0.40) | 0.06 | (−0.01, 0.12) | 2.74 (0.51) | 0.05 | (−0.03, 0.12) | 0.737 (0.070) | 0.010 | (−0.003, 0.023) |
| Q5 (≥ 20551) | 323 | 2.03 (0.42) | 0.07 | (0.00, 0.14) | 2.81 (0.52) | 0.10 | (0.02, 0.18) | 0.724 (0.083) | 0.002 | (−0.012, 0.016) |
| Effect per fifth increase | 0.02 | (0.00, 0.03) | 0.02 | (0.00, 0.04) | 0.001 | (−0.002, 0.005) | ||||
| P for trend‡ | 0.003 | 0.001 | 0.646 | |||||||
Cut-off values for fifths of dietary consumption were derived from distributions among the whole population.
Adjusted for age, height, smoking status, pack-years, exposure to tobacco smoke in home, age left education, social class, body fat mass, physical activity score, dietary supplement use, inhaled or oral steroid use, paracetamol use, alcohol consumption and energy intake.
P values to test for linear trends were calculated by using dietary consumption as a continuous variable after controlling for potential confounders.
Combined associations of processed meat consumption and dietary antioxidants with lung function
Processed meat consumption was weakly correlated with fruit and vegetable consumption (Spearman correlation coefficients r=−0.06, p=0.01 for men and r=−0.07, p=0.01 for women), but not dietary TAC (r=−0.05, p=0.05 for men and r=−0.03, p=0.21 for women). We therefore investigated the independent associations between lung function and processed meat, fruit and vegetable consumption and dietary TAC by mutual adjustment in multivariate models. The negative association between processed meat consumption and FEV1 was independent of fruit and vegetable consumption and dietary TAC in men (both p for trend<0.001) and women (p for trend=0.013 and p for trend=0.014, respectively). Similarly, associations between processed meat consumption and FVC and FEV1/FVC in men and women, and COPD in men, were not confounded by fruit and vegetable consumption or dietary TAC (data not shown). In contrast, the positive associations of fruit and vegetable consumption and dietary TAC with FEV1 and FVC remained after adjustment for processed meat consumption in women, but these associations disappeared in men.
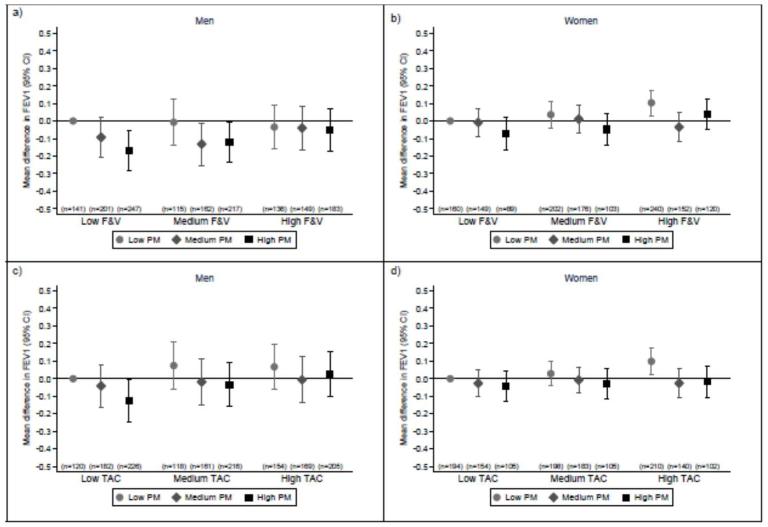
We then examined whether the association between processed meat consumption and FEV1 was modified by fruit and vegetable consumption and dietary TAC (Figure 1). In men, the association between processed meat consumption and FEV1 was more marked in those who had lower fruit and vegetable consumption (p for interaction=0.035, Figure 1a) and lower dietary TAC (p for interaction=0.025, Figure 1c). In women, the association between processed meat consumption and FEV1 did not differ according to fruit and vegetable consumption (p for interaction=0.633) or dietary TAC (p for interaction=0.412). There was no evidence of effect modification in relation to FVC (data not shown), FEV1/FVC (online supplementary Figure E1) or COPD (data not shown) in either men or women.
FIGURE 1. Interactions between processed meat consumption and fruit and vegetable consumption and dietary TAC on forced expiratory volume in 1 s (FEV1).
Interactions between processed meat (PM) consumption and fruit and vegetable (F&V) consumption (a for men and b for women) or dietary total antioxidant capacity (TAC) (c for men and d for women) on FEV1. Dietary variables were stratified by tertiles (low: <2.5 servings/week; medium 2.6–4.7 servings/week; and 4.9+ servings/week for processed meat, low: <37.5 servings/week; medium: 37.5–53.2 servings/week; and high: 53.2+ servings/week for fruit and vegetables and low: <13700 μmol/d; medium: 13700–18245 μmol/d; and high: 18245+ μmol/d for dietary TAC). Values are multivariate-adjusted regression coefficients for the difference in mean FEV1 compared to subjects with the lowest consumption of both processed meat and dietary antioxidants.
Interactions between dietary consumption and smoking status on lung function
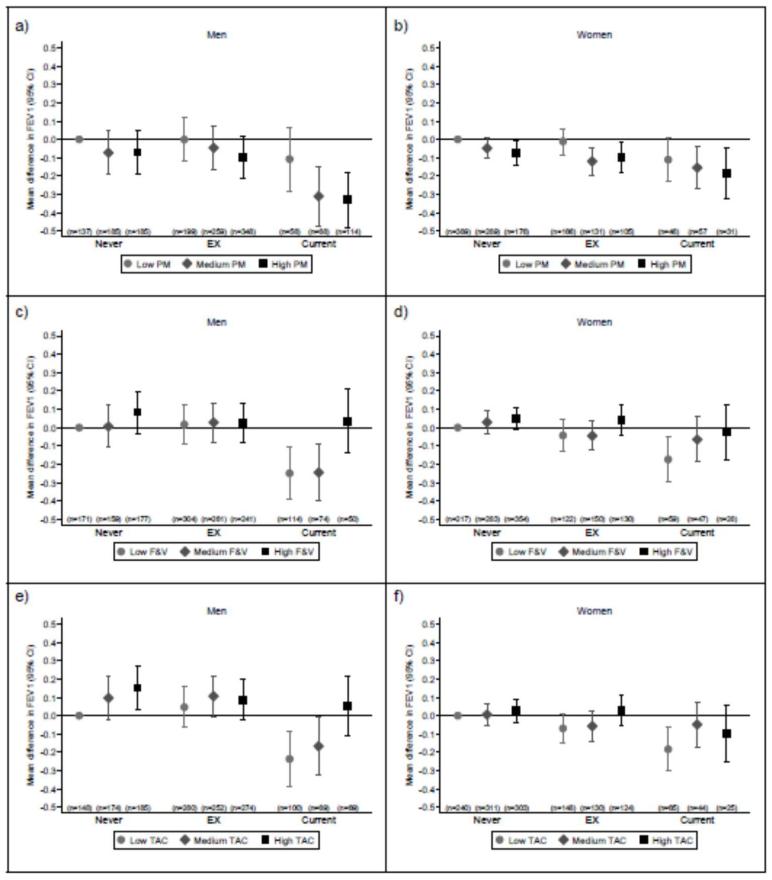
In both men and women there was some evidence that negative associations of processed meat consumption, and positive associations of fruit and vegetable consumption and dietary TAC with FEV1 were more marked in smokers, although the tests for interaction did not achieve conventional statistical significance (i.e., p<0.05, Figure 2). The patterns of association with FVC and COPD were similar to those for FEV1 (data not shown). However, for FEV1/FVC we found that the negative association with processed meat consumption among men was stronger in current smokers (p for interaction=0.022, online supplementary Figure E2), but no clear differences in the associations with fruit and vegetable consumption or dietary TAC according to smoking status were observed (p for interaction=0.108 and p=0.595, respectively). In women, there were no clear differences in the associations between dietary exposures and FEV1/FVC according to smoking status.
FIGURE 2. Interactions between dietary consumption and smoking status on forced expiratory volume in 1 s (FEV1).
Interactions between processed meat (PM) consumption (a for men and b for women), fruit and vegetable (F&V) consumption (c for men and d for women), dietary total antioxidant capacity (TAC) (e for men and f for women) and smoking status on FEV1. Dietary variables were stratified by tertiles (low: <2.5 servings/week; medium 2.6–4.7 servings/week; and 4.9+ servings/week for processed meat, low: <37.5 servings/week; medium: 37.5–53.2 servings/week; and high: 53.2+ servings/week for fruit and vegetables and low: <13700 μmol/d; medium: 13700–18245 μmol/d; and high: 18245+ μmol/d for dietary TAC). Values are multivariate-adjusted regression coefficients for the difference in mean FEV1 compared to never smokers with the lowest consumption of each dietary component.
DISCUSSION
Main findings
The main findings of this study were that higher processed meat consumption was associated with poorer lung function in older men and women, and that in men the negative association with FEV1 was greatest in those who also had lower fruit and vegetable consumption and low dietary TAC. These interactions suggest that the association between a healthier dietary pattern and lung function, as observed previously in this cohort [1] and in other populations [2,3], might not simply reflect the intake of antioxidants, but rather the relative intakes of protective and harmful constituents in the diet that influence pulmonary oxidant/antioxidant balance. To our knowledge, effects of the balance of foods in the diet in relation to lung function have not been described before.
Association of processed meat consumption with lung function
Our findings of poorer lung function among men and women who have a high consumption of processed meat are consistent with a growing number of epidemiological studies of lung function and COPD from the USA [10-12] and Europe [13]. In keeping with findings from the cross-sectional study of NHANES III [10], we confirmed a negative association of processed meat consumption with FEV1 and FEV1/FVC and a positive association with COPD, defined spirometrically. In contrast, we also found a negative association with FVC. A further difference between studies is our observation of an interaction between smoking and processed meat consumption in relation to FEV1/FVC in men, which was not seen in NHANES III. However, our study suggests that there may be gender-specific effects of diet on lung function that were not examined previously [10].
A number of potential mechanisms have been suggested to link processed meat consumption to poorer lung function. A key component of processed meat is its high nitrite content [10-13]. Nitrites are pro-oxidants and can generate strong oxidising reactive nitrogen species such as peroxynitrite [14], which can produce lung damage and contribute to airway inflammation. Tobacco smoke is another source of nitrites as well as oxidants, hence the interaction between processed meat consumption and smoking on lung function in men is biologically plausible. In addition, cured/processed meats are a rich source of AGEs [15]. AGEs contribute to increased oxidative stress and inflammation through binding with their cell surface receptor (RAGE) which activates NF-κB (nuclear factor-KappaB) [16,27]. Thus high consumption of foods rich in AGEs could plausibly increase lung inflammation and hence reduce lung function. Given the likely pro-oxidant effects of nitrites and AGEs in processed meat, it may be unsurprising that the association between processed meat consumption and FEV1 was modified by fruit and vegetable consumption and dietary TAC, at least in men. The significant trends in the associations of processed meat consumption with lung function, suggestive of a “dose-response”, the magnitude of the association with FEV1, and the plausible interactions with smoking and dietary antioxidants in men would support a causal interpretation.
Association of dietary antioxidants with lung function
Consistent with the growing recognition of potential beneficial effects of foods rich in antioxidants on lung function [6], we found that fruit and vegetable consumption and dietary TAC were positively associated with FEV1 and FVC. Comparable associations with dietary TAC have also been described in an Italian study where dietary TAC was positively associated with FEV1 and FVC, although these effects were seen only in women [28]. Dietary TAC reflects all antioxidants in the diet and even takes into account their synergistic effects [17,23,24], hence dietary TAC is expected to be more useful than a single food or single nutrient approach to examine relationships between antioxidants and health outcomes.
However, in our study the effects of dietary TAC on lung function were not markedly different from those of fruit and vegetables (Tables 2 and 3). One possible explanation is that our estimation of dietary TAC was inaccurate. This may partly be because of a lack of TAC values in the database we used to assign to the foods on the FFQ (coverage rate=44.8%), but also because the database, developed in the USA, may not be appropriate to estimate TAC in foods eaten in the UK, where growing conditions and cooking methods differ. To our knowledge dietary TAC using the ORAC assay has not been described in other UK cohort studies, and further data, using other estimates of dietary TAC, are needed to understand its importance for health.
Gender-specific effects of diet on lung function
Although higher processed meat consumption was associated with reduced lung function in both men and women, the effect modification by dietary antioxidants and smoking was observed only in men. It is not clear why the effect modification of diet differs between men and women, although comparable gender differences in oxidative stress have been described in another study [29].
Study limitations
Our study has a number of limitations. Firstly, the study is cross-sectional which limits causal inference. However, “reverse causation” does not seem a likely explanation for the main findings as we cannot see why individuals developing worse lung function would choose to eat more processed meat or fewer fruit and vegetables. Secondly, while we defined COPD spirometrically, which is the gold standard approach [30] and avoids potential problems of bias which might arise with self-reported COPD, we did not measure post-bronchodilator lung function. This raises the possibility that a small minority of individuals classified as having COPD by our spirometric definition may have had asthma. However, such misclassification of phenotype would seem less likely in males, in whom the association between processed meat consumption and FEV1/FVC was modified by smoking, the main risk factor for COPD. Thirdly, dietary information was collected using an administered FFQ. There are concerns that participants can over-report intake in response to FFQs, although their ability to describe types of diets and patterns of food consumption is well-established [20,22]. However, as measurement error and misclassification of exposure is likely to be random with respect to the study outcomes, this would be expected to attenuate associations; we therefore do not think that misreporting on the FFQ could explain the associations we describe. Finally, subjects with high fruit and vegetable consumption and dietary TAC or with low processed meat consumption may be more health conscious and have other healthy behaviours, which could potentially confound associations with lung function. Whilst we controlled for a large number of potential confounders, including detailed measures of smoking status, we cannot rule out unmeasured or residual confounding (such as the other environmental sources of oxidants/antioxidants, air pollution and occupational exposures) in an observational study of this kind.
Conclusion
Processed meat consumption was negatively associated with lung function in both men and women. This association was stronger among men with low fruit and vegetable consumption, low dietary TAC and among current smokers. The most important public health message to prevent reduced lung function remains smoking cessation. However the present findings suggest that the relative consumption of foods which influence pulmonary oxidant/antioxidant balance may also be important for optimising lung function, particularly in smokers. Whilst longitudinal data are needed, these findings provide further evidence to suggest that current dietary guidelines to promote “healthier” patterns of eating could play a protective role in slowing lung function decline and preventing COPD in older age.
Supplementary Material
Acknowledgment
We thank all the men and women who took part in the Hertfordshire Cohort Study (HCS), and the HCS research staff.
Support statement: This study was supported by the Medical Research Council, UK. Hitomi Okubo, Ph.D, was supported in part by the fellowship of Astellas Foundation for Research on Metabolic Disorders, Japan and the Naito Memorial Grant for Research Abroad from the Naito Foundation, Japan.
Footnotes
Statement of interest: None declared.
REFERENCES
- 1.Shaheen SO, Jameson KA, Syddall HE, et al. The relationship of dietary patterns with adult lung function and COPD. Eur Respir J. 2010;36:277–284. doi: 10.1183/09031936.00114709. [DOI] [PubMed] [Google Scholar]
- 2.Varraso R, Fung TT, Hu FB, et al. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US men. Thorax. 2007;62:786–791. doi: 10.1136/thx.2006.074534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varraso R, Fung TT, Barr RG, et al. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US women. Am J Clin Nutr. 2007;86:488–495. doi: 10.1093/ajcn/86.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romieu I, Trenga C. Diet and obstructive lung diseases. Epidemiol Rev. 2001;23:268–287. doi: 10.1093/oxfordjournals.epirev.a000806. [DOI] [PubMed] [Google Scholar]
- 5.Carey IM, Strachan DP, Cook DG. Effects of changes in fresh fruit consumption on ventilatory function in healthy British adults. Am J Respir Crit Care Med. 1998;158:728–733. doi: 10.1164/ajrccm.158.3.9712065. [DOI] [PubMed] [Google Scholar]
- 6.Schünemann HJ, Freudenheim JL, Grant BJ. Epidemiologic evidence linking antioxidant vitamins to pulmonary function and airway obstruction. Epidemiol Rev. 2001;23:248–267. doi: 10.1093/oxfordjournals.epirev.a000805. [DOI] [PubMed] [Google Scholar]
- 7.Kelly FJ. Vitamins and respiratory disease: antioxidant micronutrients in pulmonary health and disease. Proc Nutr Soc. 2005;64:510–526. doi: 10.1079/pns2005457. [DOI] [PubMed] [Google Scholar]
- 8.Mak JC. Pathogenesis of COPD. Part II. Oxidative-antioxidative imbalance. Int J Tuberc Lung Dis. 2008;12:368–374. [PubMed] [Google Scholar]
- 9.Rahman I, MacNee W. Oxidant/antioxidant imbalance in smokers and chronic obstructive pulmonary disease. Thorax. 1996;51:348–350. doi: 10.1136/thx.51.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang R, Paik DC, Hankinson JL, et al. Cured meat consumption, lung function, and chronic obstructive pulmonary disease among United States adults. Am J Respir Crit Care Med. 2007;175:798–804. doi: 10.1164/rccm.200607-969OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang R, Camargo CA, Jr, Varraso R, et al. Consumption of cured meats and prospective risk of chronic obstructive pulmonary disease in women. Am J Clin Nutr. 2008;87:1002–1008. doi: 10.1093/ajcn/87.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varraso R, Jiang R, Barr RG, et al. Prospective study of cured meats consumption and risk of chronic obstructive pulmonary disease in men. Am J Epidemiol. 2007;166:1438–1445. doi: 10.1093/aje/kwm235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Batlle J, Mendez M, Romieu I, et al. Cured meat consumption increases risk of readmission in COPD patients. Eur Respir J. 2012;40:555–560. doi: 10.1183/09031936.00116911. [DOI] [PubMed] [Google Scholar]
- 14.Folkerts G, Kloek J, Muijsers RB, et al. Reactive nitrogen and oxygen species in airway inflammation. Eur J Pharmacol. 2001;429:251–262. doi: 10.1016/s0014-2999(01)01324-3. [DOI] [PubMed] [Google Scholar]
- 15.Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, Yong A, Striker GE, Vlassara H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110:911–916. doi: 10.1016/j.jada.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uribarri J, Cai W, Peppa M, Goodman S, Ferrucci L, Striker G, Vlassara H. Circulating glycotoxins and dietary advanced glycation endproducts: two links to inflammatory response, oxidative stress, and aging. J Gerontol A Biol Sci Med Sci. 2007;62:427–433. doi: 10.1093/gerona/62.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serafini M, Del Rio D. Understanding the association between dietary antioxidants, redox status and disease: is the Total Antioxidant Capacity the right tool? Redox Rep. 2004;9:145–152. doi: 10.1179/135100004225004814. [DOI] [PubMed] [Google Scholar]
- 18.Syddall HE, Aihie Sayer A, Dennison EM, et al. Cohort profile: the Hertfordshire cohort study. Int J Epidemiol. 2005;34:1234–1242. doi: 10.1093/ije/dyi127. [DOI] [PubMed] [Google Scholar]
- 19.Bingham SA, Gill C, Welch A, et al. Comparison of dietary assessment methods in nutritional epidemiology: weighed records v. 24 h recalls, food-frequency questionnaires and estimated-diet records. Br J Nutr. 1994;72:619–643. doi: 10.1079/bjn19940064. [DOI] [PubMed] [Google Scholar]
- 20.Robinson S, Syddall H, Jameson K, et al. Current patterns of diet in community-dwelling older men and women: results from the Hertfordshire Cohort Study. Age Ageing. 2009;38:594–599. doi: 10.1093/ageing/afp121. [DOI] [PubMed] [Google Scholar]
- 21.Holland B, Welch AA, Unwin ID, et al. McCance and Widdowson’s The Composition of Foods. 5th edition, and supplements to this edition. Royal Society of Chemistry; Cambridge: 1991. [Google Scholar]
- 22.Robinson SM, Jameson KA, Batelaan SF, et al. Diet and its relationship with grip strength in community-dwelling older men and women: the Hertfordshire cohort study. J Am Geriatr Soc. 2008;56:84–90. doi: 10.1111/j.1532-5415.2007.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu X, Beecher GR, Holden JM, et al. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J Agric Food Chem. 2004;52:4026–4037. doi: 10.1021/jf049696w. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Department of Agriculture, Agricultural Research Service. USDA Database for the Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods, Release 2. Nutrient Data Laboratory; Maryland: 2010. [Google Scholar]
- 25.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 26.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gerontol A Biol Sci Med Sci. 2010;65:963–975. doi: 10.1093/gerona/glq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.di Giuseppe R, Arcari A, Serafini M, et al. Total dietary antioxidant capacity and lung function in an Italian population: a favorable role in premenopausal/never smoker women. Eur J Clin Nutr. 2012;66:61–68. doi: 10.1038/ejcn.2011.148. [DOI] [PubMed] [Google Scholar]
- 29.Ochs-Balcom HM, Grant BJ, Muti P, et al. Oxidative stress and pulmonary function in the general population. Am J Epidemiol. 2005;162:1137–1145. doi: 10.1093/aje/kwi339. [DOI] [PubMed] [Google Scholar]
- 30.Celli BR, MacNee W, ATS/ERS Task Force Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.