Abstract
Luteinizing hormone (LH) and human chorionic gonadotropin (hCG) are integral components of the hypothalamic–pituitary–gonadal axis, which controls sexual maturation and functionality. In the absence of signaling through their shared receptor, fetal sexual differentiation and post-natal development cannot proceed normally. Although they share a high degree of homology, the physiologic roles of these hormones are unique, governed by differences in expression pattern, biopotency and regulation. Whereas LH is a key regulator of gonadal steroidogenesis and ovulation, hCG is predominantly active in pregnancy and fetal development. Emerging evidence has revealed endogenous functions not previously ascribed to hCG, including participation in ovulation and fertilization, implantation, placentation and other activities in support of successful pregnancy. Spontaneous and induced mutations in LH, hCG and their mutual receptor have contributed substantially to our understanding of reproductive development and function. The lack of naturally occurring, functionally significant mutations in the β-subunit of hCG reinforce its putative role in establishment of pregnancy. Rescue of reproductive abnormalities resulting from aberrant gonadotropin signaling is possible in certain clinical contexts, depending on the nature of the underlying defect. By understanding the physiologic roles of LH and hCG in normal and pathologic states, we may better harness their diagnostic, prognostic and therapeutic potential.
Keywords: Function, hCG, infertility, LH, LHCGR, mutation, ovulation, polymorphism
Introduction
Luteinizing hormone (LH) and human chorionic gonadotropin (hCG) serve different roles in reproductive physiology: LH contributes to the driving force behind gonadal steroidogenesis and regulation of ovulation, whereas hCG (in the absence of pathologic abnormalities) is secreted in large amounts only during pregnancy [1]. The expression profile differences manifest early in fetal development and are maintained throughout the life span [2–4]. Although LH and hCG share the same receptor, many of their actions remain distinct [1,5,6]. A wealth of information regarding the endogenous functions of LH and hCG has been ascertained through naturally occurring and induced mutations, yet data are still emerging that shed new light on the involvement of LH and hCG in various physiologic processes.
By understanding how LH and hCG affect normal and abnormal human development and reproduction, we may be able to improve on current treatments for reproductive disorders while uncovering other areas where assessment of these gonadotropins may be of use, such as the management of certain cancers. This review describes the current state of knowledge regarding the physiology of LH and hCG throughout the stages of life and the impact of disrupting their normal expression profiles and functionality.
Physiology of LH and hCG through the life stages
Fetal period
Fluctuations in circulating LH and hCG concentrations are observed throughout fetal development (Figure 1) [1,3,6,7]. In the male fetus, initially high levels of plasma hCG decrease sharply between weeks 10 and 20 and then gradually decline for the remainder of gestation [6]. Expression of LH, in contrast, increases from week 10, reaching a peak before week 20 and decreasing slowly thereafter. The amount of hCG present at its nexus far exceeds that of LH [1,6]. Although fetal cells that are destined to become Leydig cells begin to produce testosterone as early as 6–7 weeks of gestation, it is not until after this period that hCG and LH are believed to be important to the process [8]. Indeed, the LH/choriogonadotropin receptor (LHCGR) is not detected in Leydig cells until 10–12 weeks after conception [6]. As the more abundant and biologically active species, hCG likely plays a larger role in testosterone production by Leydig cells than does LH during early fetal development [6,9]. Testosterone is responsible for virilization of the reproductive tract, promoting the formation of the ductus deferens, epididymides, seminal vesicles and ejaculatory ducts [1,6]. Dihydrotestosterone, a derivative of testosterone, is active in the differentiation of the prostate and external genitalia. At ∼15–20 weeks of gestation, testosterone regulation shifts to the fetal pituitary gland, as LH secretion takes over from placental hCG in driving testosterone production [9]. This transition is evident in LH-deficient anencephalic male fetuses, in whom normal masculinization of the reproductive tract occurs while high levels of hCG are present, but further development of the external genitalia is hindered by the lack of LH when hCG levels decrease [9]. The pattern of pituitary LH expression during fetal development mirrors that of serum LH levels [3].
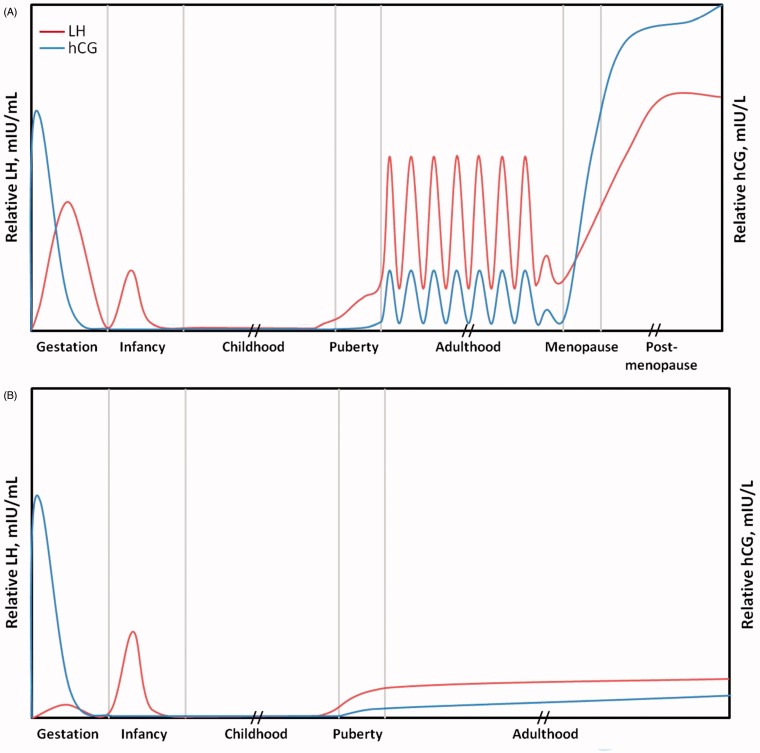
Figure 1.
Relative expression of luteinizing hormone (LH) and human chorionic gonadotropin (hCG) during the stages of life in females (A) and males (B). Note that patterns of LH and hCG expression will vary substantially from person to person. This figure is a representation of which molecule is predominant at the different stages of life. It is not an exact measurement.
Peak concentrations of pituitary and circulating LH are higher in female fetuses relative to that of males [3,7]. It has been postulated that negative feedback from testosterone and inhibin in males lead to their lower LH levels, which is borne out by the higher levels of testosterone and inhibin B detected during gestation in males compared with females [7]. Development of the female fetal reproductive tract does not depend on the presence of LH or hCG. In fact, LHCGR is not expressed by the developing ovary until after the 16th week of gestation [6]. In general, minimal steroidogenesis occurs in the ovary during fetal development.
Detection of LHCGR in fetal organs, including the kidney, liver, lung, pancreas and gastrointestinal tract, has led to speculation that hCG may have a further role in fetal organ growth and differentiation [5,10]. No specific function in fetal organ development has yet been ascribed to hCG [5].
Infancy and childhood
In term infants, circulating LH and hCG concentrations are below the limits of detection [7]. After delivery, both sexes experience a surge in gonadotropin levels due to the withdrawal of maternal-derived estrogen. The magnitude of LH elevation is greater in male than in female infants [2,11,12]. In male infants, LH peaks at 1–3 months of age, declining rapidly thereafter and reaching a nadir at ∼4–9 months of age. Testosterone levels, which are also elevated at 3 months of age in boys, decrease more precipitously, falling below the limit of detection by 6 months of age [12]. The testosterone peak may be driven by a coinciding increase in Leydig cell number and is believed to trigger germ cell proliferation and sexual differentiation of the central nervous system.
Infant girls demonstrate greater variability in LH expression patterns [12]. Generally, a peak in LH concentration is reached at 1–3 months of age, followed by a decrease to childhood levels and a possible small resurgence at 1 year [2,12]. The significance of transient hypothalamic–pituitary–gonadal axis activation is as yet unknown. After the temporary surge in boys and girls, gonadotropin levels remain low until prepuberty. This period is sometimes referred to as the juvenile pause [6,13].
Prepuberty and puberty
In the year or two preceding puberty, the gonadotropin-releasing hormone pulse generator begins to mature and escape its central inhibition. In response, there is a gradual increase in the frequency and amplitude of nocturnal LH pulsatile secretion [14,15]. With pubertal progression, this diurnal pattern of LH secretion abates, and LH is released in a more regular pulsatile manner throughout the day [13,16]. Concurrently, the proportion of bioactive to immunoreactive LH increases [17,18]. Phillips et al. [19] studied the shift in LH and follicle-stimulating hormone (FSH) isohormone profile in puberty. In boys, there was an increased prevalence of acidic FSH and LH isoforms observed at pubertal stage II. In contrast, the median charge of LH was largely unchanged in girls during pubertal stages I through IV.
The increase in gonadotropin activity during puberty drives gonadal steroidogenesis – primarily the production of testosterone by Leydig cells in males and testosterone and progesterone by thecal cells in females [1]. Most of the testosterones generated by thecal cells are converted to estradiol by granulosa cells. Testosterone, estradiol and adrenal androgens produce the physical changes associated with puberty, including the development of secondary sex characteristics. As estradiol production increases in girls, stimulation of the endometrium occurs, eventually leading to menarche (∼2–3 years after the onset of puberty). The hypothalamic–pituitary–ovarian axis matures in middle-to-late puberty, with the development of estradiol’s positive feedback effect on stimulating an LH surge [13]. More regular, ovulatory cycles do not become established until several years after menarche.
Adulthood
In women, LH and hCG play crucial roles in ovulation and pregnancy (discussed in the next section). After puberty, the pattern of LH secretion during the menstrual cycle becomes more regular in normo-ovulatory women. In women with polycystic ovarian syndrome (PCOS), however, higher basal levels of LH are observed with increased pulse frequency and amplitude throughout the cycle [20–22]. The lack of variation in LH secretion pattern contributes to the anovulatory cycles frequently found in women with PCOS.
In men, LH stimulates testosterone production by Leydig cells. Secretion of LH is pulsatile, but it does not demonstrate the cyclic variations observed in women. Low levels of hCG (presumably of pituitary origin) are also present in men [4], the function of which is unknown. Although not the key impetus for endogenous steroidogenesis, in conditions such as male hypogonadism and infertility, exogenous hCG may be administered to drive testosterone production [1].
Menopause and post-menopause
A decline in ovarian function during the perimenopausal period, which can last from 2--8 years [13], is marked by a gradual increase in FSH, a pronounced decrease in the follicular pool, and a reduction in early follicular phase inhibin secretion [1]. As menopause approaches, estradiol and estrone levels decline [1]. The subsequent lack of ovarian steroid negative feedback results in a sharp increase in LH and FSH secretion, with a gradual decline during the post-menopausal period [1,6].
During the perimenopausal period, serum hCG levels increase substantially in women [23]. The finding that hCG measurements in women 55 years of age and older are significantly greater than those in non-pregnant women aged 18–40 years or 41–55 years (p < 0.0001) could have diagnostic implications when interpreting hCG tests. After the onset of menopause, hCG levels seem to stabilize. The elevated levels of hCG in perimenopausal and menopausal women are believed to be pituitary in origin [23]. It should be noted that LH and hCG levels during and after the menopausal transition demonstrate a high degree of interindividual variability.
Although loss of reproductive capability akin to female menopause does not occur in men, they do experience a progressive decline in spermatogenesis and fertility with age. Leydig cells decrease in number and are less capable of producing testosterone in response to LH or hCG stimulation [6,24]. Aging is generally associated with a decrease in circulating total and free serum testosterone levels, but this phenomenon is not observed in all men [6,24,25]. On an average, testosterone levels decrease by 1–2% per year [25]. Levels of LH and hCG, in contrast, gradually increase with age [25,26]. The increases in LH and hCG levels in men are much less pronounced than those observed during the perimenopausal and post-menopausal intervals in women. Nonetheless, reference ranges for normal LH and hCG values in men are typically stratified by age group.
Roles of LH and hCG during ovulation and pregnancy
Ovulation
Luteinizing hormone regulates ovulation through changes in expression levels and the proportions of glycosylated isoforms. Both LH and hCG exist as compilations of isohormones that differ with respect to post-translational modification (predominantly glycosylation) and state of degradation, which influences their biological activity and half-life in circulation [27,28]. Early in the follicular phase, estradiol exerts a negative feedback effect on LH; however, some LH activity occurs to stimulate thecal cell production of androgens that serve as substrates for estradiol production by granulosa cells. As estradiol levels rise in the late follicular phase, the relationship with LH becomes one with a positive feedback effect, leading to the LH surge and subsequent ovulation [6]. This transition corresponds with a shift toward less glycosylated and more biologically active LH isoforms [29,30]. With ovulation, the ovulatory oocyte progresses through to meiosis II while the corpus luteum forms in the ovarian stroma. LH drives progesterone production and secretion from the corpus luteum and, if pregnancy occurs, hCG takes over progesterone regulation. The hyperglycosylated isoform of hCG, which is abundant in early pregnancy, does not seem to play a role in the promotion of progesterone secretion [31].
Low levels of pituitary hCG parallel the dynamics of LH secretion during the menstrual cycle [4]. Pituitary hCG secretion is responsive to stimulation by GnRH analogs and is suppressed by oral contraceptives [32–34]. The role of this pituitary hCG is unclear, but the pattern of expression is consistent with overlapping function to that of LH [5]. It has been suggested that hCG expression may elevate the peak range of LH, thereby aiding in the promotion of ovulation [35]. In addition, lingering hCG could enhance progesterone production at luteal phase onset.
Fertilization and implantation
The presence of LHCGR in human fallopian tubes and in sperm suggests a role for LH and hCG in fertilization [36,37]. Consistent with this putative function, expression of LHCGR is greater in fallopian tubes during the luteal phase compared with the proliferative phase of the menstrual cycle or fallopian tubes from postpartum or post-menopausal women [36]. Studies using a porcine model demonstrated that LH was capable of inducing relaxation of the oviduct, most notably during the periovulatory interval [38]. It is not known what the function of gonadotropin signaling is in sperm; however, a recent study reported correlations between polymorphisms in LHCGR and sperm quality (e.g. concentration and motility) in cattle [39]. Investigators have speculated that hCG present in the female reproductive tract could activate the LHCGR on sperm, thus promoting motility and capacitation [37].
Blastocyst secretion of hCG may contribute to endometrial cross-talk to enable implantation [40,41]. Endometrial expression of LHCGR increases mid-luteal phase, during a short period in which the endometrium is receptive to implantation (i.e. the implantation window) [41]. Investigators hypothesize that a blastocyst capable of producing locally high levels of hCG could extend the implantation window, thereby increasing the chances for successful pregnancy [40]. A similar phenomenon of increased endometrial LHCGR expression during the implantation window has been observed in mice [42]. Given that the mouse genome does not include a chorionic gonadotropin gene, LH would need to functionally substitute for hCG in this system. Preliminary evidence suggests that this may indeed be the case – a bioactive LH signal has been reported at the time of murine blastocyst implantation.
During early pregnancy, hCG is also believed to support implantation and placentation by modulating endometrial tissue remodeling, fostering maternal immunotolerance of fetal tissue, promoting neoangiogenesis and increasing the uterine natural killer cell population [5,40,41]. These functions all contribute to endometrial receptivity – a key component to successful pregnancy. Emerging evidence suggests that hCG expressed by the blastocyst stimulates receptors on the endometrium, eliciting a variety of effects on downstream molecules, including growth factors (vascular endothelial growth factor [VEGF], transforming growth factor beta), cytokines (leukemia inhibitory factor) and proteinases (matrix metalloproteinase 9 [MMP-9]), which contribute to different aspects of endometrial receptivity [40,41,43]. For example, hCG-mediated upregulation of VEGF is believed to promote angiogenesis, whereas stimulation of MMP-9 expression by hCG is involved in regulation of tissue remodeling. The role of hCG in fostering endometrial receptivity appears to begin even before embryonic hCG expression. It has been shown that epithelial hCG is expressed and produced in human endometrium biopsy specimens during the early to mid-secretory phase of the menstrual cycle [44]. Elucidating the precise role of hCG in endometrial functions related to implantation remains an active subject of investigation.
Maintenance of pregnancy
Expression of hCG continues throughout pregnancy, with peak levels occurring at ∼10 weeks’ gestation [5]. As many as 20–30 isoforms of hCG can be detected during pregnancy [45]. Hyperglycosylated hCG (hCG-H) secreted by root or extravillous cytotrophoblasts is the predominant species early in gestation, but it is gradually surpassed in proportion by regular hCG, which is produced by fused and differentiated syncytiotrophoblast cells [5]. It has been well established that in early pregnancy, hCG sustains progesterone production by the corpus luteum. Placental production of progesterone takes over in the later part of the first trimester, at a time when hCG levels are in decline [6]. Beyond its role in luteal support, hCG is believed to promote angiogenesis, thereby supporting blood flow to the placenta and nutrition for the fetus [46,47]. Emerging evidence also suggests that hCG is involved in umbilical cord growth and development, synchronizing uterine growth with that of the developing fetus and relaxation of myometrial contractions [5]. In addition, the presence of LHCGR in the brain has led to speculation that gonadotropin signaling is involved in the nausea and vomiting experienced during pregnancy [48].
Quantification of hCG species is a valuable diagnostic and prognostic tool during pregnancy. For example, measuring hCG-H levels, which increase rapidly during the initial stages of pregnancy, allows for earlier detection than standard pregnancy tests. A recent study showed that an ultrasensitive hCG-H assay was capable of detecting pregnancy 6 days after embryo transfer in women undergoing in vitro fertilization [49]. At 12 days, lower levels of hCG-H distinguished biochemical from clinical pregnancies. Insufficient hCG-H levels (i.e. urinary hCG-H <50% of total hCG) have also been detected in a large proportion of early pregnancy losses in spontaneously conceived cycles [50]. Low circulating concentrations of another form of hCG, the disassociated β-subunit, have been linked to an increased probability of ectopic pregnancy in symptomatic patients [51]. During the later stages of pregnancy, inadequate amounts hCG-H are associated with the development of preeclampsia [52]. Preeclampsia itself is attributed to abnormal placentation and trophoblast invasion [53]. On the other end of the spectrum, elevated levels of hCG-H or hCG free β-subunit in maternal serum during the first trimester are markers of Down syndrome pregnancy [54]. Triploid abnormalities may be discernible as early as the pronuclear stage by the presence of hCG-H [55]. Notably, most methods currently used for hCG quantification in clinical laboratories underestimate hCG-H levels.
Pathology of LH, hCG and LHCGR
Loss- or gain-of-function mutations have helped to establish the extent to which gonadotropin signaling is necessary for sexual development and reproductive success, while also providing insights into pathologic processes. These gene alterations may be naturally occurring, as is the case for human mutations/polymorphisms, or induced in model systems (most commonly in mice). The effects of targeted mutations in LH or overexpression of hCG on reproductive structure and function in mice are presented in Table 1. A description of human phenotypes associated with changes in LH, hCG and LHCGR functionality or expression are provided below and summarized in Table 2.
Table 1.
Phenotypes in knockout and transgenic mice.
| Model | Gender | Reproductive phenotype |
|---|---|---|
| Disrupted pituitary glycoprotein hormone α-subunit [56] | Female | Prepubertal external genitalia, underdeveloped ovaries and uterus, limited follicle development, absence of ovulation, failure of vaginal orifice opening, infertility |
| Male | Prepubertal external genitalia, reduced testes size, impaired spermatogenesis, testosterone deficiency, infertility | |
| LH receptor null [57,58] | Female | Underdeveloped genitalia, ambiguous vaginal opening, arrested follicular growth, decreased estradiol and progesterone levels, infertility |
| Male | Underdeveloped genitalia, abdominal testes, micropenis, Leydig cell hypotrophy, disarray of seminiferous tubules, spermatogenic arrest, modestly elevated estradiol levels, infertility | |
| LH β-subunit null [59] | Female | Hypogonadism, decreased serum estradiol and progesterone levels, defects in folliculogenesis, gene expression abnormalities, uterine hypoplasia, infertility |
| Male | Decreased testicular size, Leydig cell hypoplasia, gene expression abnormalities, reduced testosterone levels, impaired spermatogenesis, infertility | |
| hCG α-subunit overexpression [60] | Female and male | Normal and fertile* |
| hCG β-subunit overexpression [60–62] | Female | Precocious puberty, enhanced ovarian steroidogenesis, abnormal uterine structure, hyperprolactinemia, infertility† |
| Male | Mild hypogonadism, fertile† Infertility* | |
| hCG α- and β-subunit overexpression [60,61] | Female | Elevated levels of serum estradiol, hemorrhagic and cystic ovaries, thecal layer enlargement, stromal cell proliferation, infertility*, tumorigenesis* |
| Male | Increased testicular androgen production, focal Leydig cell hypertrophy, progressive seminiferous tubule degeneration, urethral obstruction, infertility† Leydig cell hyperplasia, very high serum testosterone levels, reduced testis size, enlarged seminal vesicles, infertility* |
LH, luteinizing hormone; hCG, human chorionic gonadotropin.
Transgene expression was driven by the ubiquitin promoter.
Mice expressing high levels of hCG, with transgene expression driven by the metallothionein 1 promoter.
Table 2.
Phenotypes associated with mutations in human LHB, CGB and LHCGR genes*.
| Gene and type of mutation | Sex | Phenotype | Effect on fertility |
|---|---|---|---|
| LHB | |||
| Inactivating | Women | Oligomenorrhea, secondary amenorrhea | Infertile |
| Men | Hypogonadism, Leydig cell hypoplasia, testosterone deficiency, azoospermia | Infertile | |
| Polymorphisms | Women | Endometriosis, hyperprolactinemia, luteal insufficiency, menstrual disorders, PCOS, premature ovarian failure | Reduced fertility |
| Men | Increased prostate cancer risk | Unknown | |
| CGB | |||
| Polymorphism | Women | Recurrent miscarriage | Reduced fertility |
| LHCGR | |||
| Activating | Men | Familial male-limited gonadotropin-independent precocious puberty Leydig cell adenoma | Not affected Reduced fertility |
| Inactivating | Women | Oligomenorrhea/amenorrhea, empty follicle syndrome | Infertile |
| Men | Leydig cell hypoplasia | Infertile | |
| Polymorphism | Women and men | Risk factor for certain cancers | Unknown |
LHB, luteinizing hormone β-polypeptide; CGB, chorionic gonadotropin β-polypeptide; LHCGR, luteinizing hormone/choriogondotropin receptor; PCOS, polycystic ovary syndrome.
Sources: references [63–81].
Human mutations/variants
LHB
Naturally occurring mutations that result in inactive LH are rare in humans, particularly in women. Only two cases in female patients with inactivating LHB mutations have been described to date [63,64]. Characterization of one of these individuals revealed normal development and appropriate pubertal milestones followed by secondary amenorrhea and infertility [63]. Uterine volume was normal, although the endometrium was atrophic. The absence of detectable LH did not seem to adversely affect FSH, hCG, testosterone, or prolactin levels; estradiol and progesterone levels were in the low-to-normal range for the follicular phase of the menstrual cycle. Supplementation with estrogen and progesterone was capable of inducing endometrial thickening and follicle enlargement; however, normal ovulation was not restored and no corpus luteum was observed. The second patient was reported to have experienced menarche at age 14 years, with subsequent oligomenorrhea and secondary amenorrhea [64]. Residual LH activity described in an affected male sibling suggests that this mutation did not result in the complete absence of LH, although serum levels were below the limit of detection. The reproductive findings in these women confirm that adequate LH is not required for normal sexual differentiation and pubertal development, but it is necessary for ovulation and development of a corpus luteum.
Men with homozygous inactivating LHB mutations have a more severe phenotype characterized by hypogonadism, severe testosterone deficiency and azoospermia [63,65,66]. Leydig cells are absent or hypoplastic. Sexual differentiation and development proceed normally through gestation and childhood, but pubertal maturation does not occur. Male heterozygotes display a milder phenotype, with normal pubertal development but altered steroidogenesis and increased risk of infertility [65]. Long-term treatment with hCG is capable of rescuing the hypogonadal phenotype in men with inactivating LHB mutations, resulting in virilization, increased testicular volume, elevated testosterone levels and improved spermatogenesis [65–67]. Restoration of spermatogenesis allowed one of these men to father a child through intracytoplasmic sperm injection [67]. There has been one reported case of an LHB mutation that does not completely abrogate LH activity and is compatible with complete spermatogenesis even in the absence of virilization and in the presence of low serum testosterone levels [64]. Despite a reduced number of Leydig cells compared with an age-matched control, sperm production in this patient was quantitatively normal. These observations suggest that the LH threshold for sperm production is relatively low.
One common variant of LHB (often referred to as “variant LH”) has been detected in 7.1–41.9% of the world’s population [82,83]. This polymorphic isoform contains two point mutations, resulting in a functional LH with a shorter half-life and possibly decreased bioactivity relative to normal LH. Amino acid sequencing of variant LH suggests a closer homology to hCG than to normal LH. Current LH antibody assays are less able to detect variant LH, which may pose difficulty in ovulation testing. In certain patient cohorts, variant LH has been linked to ovulatory disorders, including hyperprolactinemia, premature ovarian failure, menstrual disorders, luteal insufficiency and PCOS [68–70]. Variant LH allele carriers may be at increased or reduced risk for developing ovulatory dysfunction, depending on the specific disorder and population evaluated. Separate (non-variant LH) polymorphisms have also been associated with PCOS susceptibility and phenotype [71,72], as well as with endometriosis, premature ovarian failure and luteal insufficiency [71].
hCG
Sequence changes that influence the function of hCG are rare. It has been suggested that mutations with a significant effect on hCG would not be compatible with successful pregnancy and are, therefore, not observed in the population at large [73]. This hypothesis is consistent with the postulated importance of hCG signaling from the blastocyst for implantation and the additional functions of hCG during gestation. It seems intuitive then that polymorphisms in the hCG β-subunit (i.e. the CGB gene) are associated with increased risk of recurrent miscarriage [73].
LHCGR
A variety of naturally occurring activating and inactivating mutations have been identified in the LHCGR gene. In men, activating mutations of LHCGR result in autosomal dominant familial male-limited gonadotropin-independent precocious puberty (i.e. testotoxicosis) [74]. Affected individuals develop Leydig cell hyperplasia and demonstrate evidence of pubertal development in early childhood. Of the variety of gain-of-function LHCGR mutations reported, only D578H is known to cause Leydig cell adenomas in addition to precocious puberty [84]. Whereas the other LHCGR activating mutations have a familial or germline origin, D578H is somatic [74]. Surprisingly, women with activating LHCGR mutations display no reproductive abnormalities in structure or function. Women with inactivating mutations of LHCGR, on the other hand, have a similar phenotype to that of inactivating LHB mutations, including oligomenorrhea/amenorrhea and infertility [75]. In addition, an LHCGR mutation that is believed to reduce receptor expression and binding capacity has been implicated in empty follicle syndrome [76]. Bentov et al. [85] recently reported a reproductive phenotype in a woman who was heterozygous for an inactivating LHCGR mutation. Previously identified loss-of-function LHCGR mutations had only generated overt reproductive pathology in the homozygous state (i.e. autosomal recessive inheritance). This patient presented with amenorrhea and had a history of irregular periods, endometriosis and recurrent ovarian cysts. Attempts at in vitro fertilization resulted in poor egg yield and multiple biochemical pregnancies.
Inactivating mutations of LHCGR cause Leydig cell hypoplasia in men [74]. There are degrees of retained LHCGR activity among the inactivating mutations, which influences the patient’s phenotype. Genetically, males with complete loss of LHCGR-mediated signaling develop Type I Leydig cell hypoplasia, wherein male sexual differentiation does not progress normally during fetal development, resulting in psuedohermaphroditism. Partial loss of LHCGR function yields a milder and more variable form of Leydig cell hypoplasia (Type II) that may include micropenis and hypospadias. In one unusual case of Leydig cell hypoplasia Type II, a patient presented with hypogonadism, azoospermia and pubertal delay, despite otherwise normal prepubertal male development [86]. Functional analysis revealed that the LHCGR mutation in this patient rendered the receptor insensitive to LH but not to hCG [87]. Consequently, unlike typical inactivating LHCGR mutations, treatment with hCG was effective for treating hypogonadism and restoring spermatogenesis in this patient [86]. This case underscores the inherit divergence in the physiologic roles of endogenous hCG and LH: continued hCG sensitivity allowed for normal fetal sexual differentiation, but lack of LH response prevented pubertal development and sperm production.
Sequence alterations in components of the gonadotropin signaling pathway have also been linked to cancer risk and prognosis. Polymorphisms in LHCGR, for example, are associated with an increased risk of testicular germ cell cancer and endometriod adenocarcinoma [77,88], whereas variant LH is considered a weak risk factor for prostate cancer [78]. Moreover, increased LHCGR activity resulting from a common polymorphism has been correlated with shortened disease-free survival in patients with breast cancer [79,80]. Investigators postulate that the negative influence on cancer outcome of this LHCGR allele results from an increase in estrogen exposure. Further investigation is needed to better understand the role of aberrant gonadotropin signaling in cancer etiology.
Conclusions
Despite a high level of homology and action at a common receptor, LH and hCG play unique roles in development and reproduction. LH and hCG act during specific windows of opportunity during fetal and post-natal development, at times coordinately, to promote proper sexual differentiation, maturation and functionality. Although long known to support pregnancy through its effect on progesterone production, hCG is increasingly recognized for its multitude of activities that foster implantation and subsequent maintenance of pregnancy. Loss-of-function mutations prove that the absence of one gonadotropin is not fully compensated by endogenous production of the other, yet rescue through exogenous administration is possible. Success of supplementation with hCG or downstream hormones in the gonadotropin cascade for spurring pubertal development, treating hypogonadism or managing infertility depends on the nature of the underlying molecular abnormality. The ongoing study of LH and hCG physiology will aid in the development of diagnostic tests, prognostic assessments and therapeutic approaches relevant to human disease and reproduction.
Declaration of interest
Financial support for manuscript development was provided by Ferring Pharmaceuticals, Inc., Parsippany, NJ. Crystal Murcia, PhD, and Jill McCollam, PharmD, of The JB Ashtin Group, Inc., prepared this manuscript for publication based on the authors’ initial draft and direction. The authors alone are responsible for the content of this article. The authors report no conflicts of interest.
References
- 1.Strauss JE, III, Barbieri RL. Yen & Jaffe’s reproductive endocrinology: physiology, pathophysiology, and clinical management. 6th. Philadelphia, PA: Saunders Elsevier; 2009. [Google Scholar]
- 2.Forest MG, Sizonenko PC, Cathiard AM, Bertrand J. Hypophyso-gonadal function in humans during the first year of life. 1. Evidence for testicular activity in early infancy. J Clin Invest. 1974;53:819–28. doi: 10.1172/JCI107621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan SL, Grumbach MM. Pituitary and placental gonadotrophins and sex steroids in the human and sub-human primate fetus. Clin Endocrinol Metab. 1978;7:487–511. doi: 10.1016/s0300-595x(78)80006-1. [DOI] [PubMed] [Google Scholar]
- 4.Odell WD, Griffin J. Pulsatile secretion of human chorionic gonadotropin in normal adults. N Engl J Med. 1987;317:1688–91. doi: 10.1056/NEJM198712313172702. [DOI] [PubMed] [Google Scholar]
- 5.Cole LA. Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol. 2010;8:102–15. doi: 10.1186/1477-7827-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, eds. Williams textbook of endocrinology. 12th ed. Philadelphia, PA: Elsevier Saunders; 2011.
- 7.Debieve F, Beerlandt S, Hubinont C, Thomas K. Gonadotropins, prolactin, inhibin A, inhibin B, and activin A in human fetal serum from midpregnancy and term pregnancy. J Clin Endocrinol Metab. 2000;85:270–4. doi: 10.1210/jcem.85.1.6249. [DOI] [PubMed] [Google Scholar]
- 8.Svechnikov K, Landreh L, Weisser J, et al. Origin, development and regulation of human Leydig cells. Horm Res Paediatr. 2010;73:93–101. doi: 10.1159/000277141. [DOI] [PubMed] [Google Scholar]
- 9.Scott HM, Mason JI, Sharpe RM. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev. 2009;30:883–925. doi: 10.1210/er.2009-0016. [DOI] [PubMed] [Google Scholar]
- 10.Abdallah MA, Lei ZM, Li X, et al. Human fetal nongonadal tissues contain human chorionic gonadotropin/luteinizing hormone receptors. J Clin Endocrinol Metab. 2004;89:952–6. doi: 10.1210/jc.2003-030917. [DOI] [PubMed] [Google Scholar]
- 11.Winter JS, Faiman C, Hobson WC, et al. Pituitary-gonadal relations in infancy. I. Patterns of serum gonadotropin concentrations from birth to four years of age in man and chimpanzee. J Clin Endocrinol Metab. 1975;40:545–51. doi: 10.1210/jcem-40-4-545. [DOI] [PubMed] [Google Scholar]
- 12.Andersson AM, Toppari J, Haavisto AM, et al. Longitudinal reproductive hormone profiles in infants: peak of inhibin B levels in infant boys exceeds levels in adult men. J Clin Endocrinol Metab. 1998;83:675–81. doi: 10.1210/jcem.83.2.4603. [DOI] [PubMed] [Google Scholar]
- 13.Park SJ, Goldsmith LT, Weiss G. Age-related changes in the regulation of luteinizing hormone secretion by estrogen in women. Exp Biol Med (Maywood) 2002;227:455–64. doi: 10.1177/153537020222700709. [DOI] [PubMed] [Google Scholar]
- 14.Penny R, Olambiwonnu NO, Frasier SD. Episodic fluctuations of serum gonadotropins in pre- and post-pubertal girls and boys. J Clin Endocrinol Metab. 1977;45:307–11. doi: 10.1210/jcem-45-2-307. [DOI] [PubMed] [Google Scholar]
- 15.Dunkel L, Alfthan H, Stenman UH, et al. Developmental changes in 24-hour profiles of luteinizing hormone and follicle-stimulating hormone from prepuberty to midstages of puberty in boys. J Clin Endocrinol Metab. 1992;74:890–7. doi: 10.1210/jcem.74.4.1548356. [DOI] [PubMed] [Google Scholar]
- 16.Veldhuis JD, Pincus SM, Mitamura R, et al. Developmentally delimited emergence of more orderly luteinizing hormone and testosterone secretion during late prepuberty in boys. J Clin Endocrinol Metab. 2001;86:80–9. doi: 10.1210/jcem.86.1.7127. [DOI] [PubMed] [Google Scholar]
- 17.Lucky AW, Rich BH, Rosenfield RL, et al. LH bioactivity increases more than immunoreactivity during puberty. J Pediatr. 1980;97:205–13. doi: 10.1016/s0022-3476(80)80475-6. [DOI] [PubMed] [Google Scholar]
- 18.Reiter EO, Biggs DE, Veldhuis JD, Beitins IZ. Pulsatile release of bioactive luteinizing hormone in prepubertal girls: discordance with immunoreactive luteinizing hormone pulses. Pediatr Res. 1987;21:409–13. doi: 10.1203/00006450-198704000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Phillips DJ, Albertsson-Wikland K, Eriksson K, Wide L. Changes in the isoforms of luteinizing hormone and follicle-stimulating hormone during puberty in normal children. J Clin Endocrinol Metab. 1997;82:3103–6. doi: 10.1210/jcem.82.9.4254. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Rudaz MC, Ropelato MG, Escobar ME, et al. Augmented frequency and mass of LH discharged per burst are accompanied by marked disorderliness of LH secretion in adolescents with polycystic ovary syndrome. Eur J Endocrinol. 1998;139:621–30. doi: 10.1530/eje.0.1390621. [DOI] [PubMed] [Google Scholar]
- 21.Silfen ME, Denburg MR, Manibo AM, et al. Early endocrine, metabolic, and sonographic characteristics of polycystic ovary syndrome (PCOS): comparison between nonobese and obese adolescents. J Clin Endocrinol Metab. 2003;88:4682–8. doi: 10.1210/jc.2003-030617. [DOI] [PubMed] [Google Scholar]
- 22.Carmina E, Campagna AM, Lobo RA. A 20-year follow-up of young women with polycystic ovary syndrome. Obstet Gynecol. 2012;119:263–9. doi: 10.1097/AOG.0b013e31823f7135. [DOI] [PubMed] [Google Scholar]
- 23.Snyder JA, Haymond S, Parvin CA, et al. Diagnostic considerations in the measurement of human chorionic gonadotropin in aging women. Clin Chem. 2005;51:1830–5. doi: 10.1373/clinchem.2005.053595. [DOI] [PubMed] [Google Scholar]
- 24.Vermeulen A, Kaufman JM. Ageing of the hypothalamo-pituitary-testicular axis in men. Horm Res. 1995;43:25–8. doi: 10.1159/000184233. [DOI] [PubMed] [Google Scholar]
- 25.Araujo AB, Wittert GA. Endocrinology of the aging male. Best Pract Res Clin Endocrinol Metab. 2011;25:303–19. doi: 10.1016/j.beem.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alfthan H, Haglund C, Dabek J, Stenman UH. Concentrations of human choriogonadotropin, its beta-subunit, and the core fragment of the beta-subunit in serum and urine of men and nonpregnant women. Clin Chem. 1992;38:1981–7. [PubMed] [Google Scholar]
- 27.Bergendah M, Veldhuis JD. Is there a physiological role for gonadotrophin oligosaccharide heterogeneity in humans? III. Luteinizing hormone heterogeneity: a medical physiologist’s perspective. Hum Reprod. 2001;16:1058–64. doi: 10.1093/humrep/16.6.1058. [DOI] [PubMed] [Google Scholar]
- 28.Arey BJ, Lopez FJ. Are circulating gonadotropin isoforms naturally occurring biased agonists? Basic and therapeutic implications. Rev Endocr Metab Disord. 2011;12:275–88. doi: 10.1007/s11154-011-9188-y. [DOI] [PubMed] [Google Scholar]
- 29.Anobile CJ, Talbot JA, McCann SJ, et al. Glycoform composition of serum gonadotrophins through the normal menstrual cycle and in the post-menopausal state. Mol Hum Reprod. 1998;4:631–9. doi: 10.1093/molehr/4.7.631. [DOI] [PubMed] [Google Scholar]
- 30.Wide L, Bakos O. More basic forms of both human follicle-stimulating hormone and luteinizing hormone in serum at midcycle compared with the follicular or luteal phase. J Clin Endocrinol Metab. 1993;76:885–9. doi: 10.1210/jcem.76.4.8473400. [DOI] [PubMed] [Google Scholar]
- 31.Crochet JR, Shah AA, Schomberg DW, Price TM. Hyperglycosylated human chorionic gonadotropin does not increase progesterone production by luteinized granulosa cells. J Clin Endocrinol Metab. 2012;97:E1741–4. doi: 10.1210/jc.2012-2027. [DOI] [PubMed] [Google Scholar]
- 32.Seppala M, Rutanen EM, Jalanko H, et al. Pregnancy-specific beta 1-glycoprotein and chorionic gonadotropin-like immunoreactivity during the latter half of the cycle in women using intrauterine contraception. J Clin Endocrinol Metab. 1978;47:1216–19. doi: 10.1210/jcem-47-6-1216. [DOI] [PubMed] [Google Scholar]
- 33.Stenman UH, Alfthan H, Ranta T, et al. Serum levels of human chorionic gonadotropin in nonpregnant women and men are modulated by gonadotropin-releasing hormone and sex steroids. J Clin Endocrinol Metab. 1987;64:730–6. doi: 10.1210/jcem-64-4-730. [DOI] [PubMed] [Google Scholar]
- 34.Odell WD, Griffin J. Pulsatile secretion of chorionic gonadotropin during the normal menstrual cycle. J Clin Endocrinol Metab. 1989;69:528–32. doi: 10.1210/jcem-69-3-528. [DOI] [PubMed] [Google Scholar]
- 35.Cole LA. New discoveries on the biology and detection of human chorionic gonadotropin. Reprod Biol Endocrinol. 2009;7:8–44. doi: 10.1186/1477-7827-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lei ZM, Toth P, Rao CV, Pridham D. Novel coexpression of human chorionic gonadotropin (hCG)/human luteinizing hormone receptors and their ligand hCG in human fallopian tubes. J Clin Endocrinol Metab. 1993;77:863–72. doi: 10.1210/jcem.77.3.7690366. [DOI] [PubMed] [Google Scholar]
- 37.Eblen A, Bao S, Lei ZM, et al. The presence of functional luteinizing hormone/chorionic gonadotropin receptors in human sperm. J Clin Endocrinol Metab. 2001;86:2643–8. doi: 10.1210/jcem.86.6.7533. [DOI] [PubMed] [Google Scholar]
- 38.Gawronska B, Paukku T, Huhtaniemi I, et al. Oestrogen-dependent expression of LH/hCG receptors in pig Fallopian tube and their role in relaxation of the oviduct. J Reprod Fertil. 1999;115:293–301. doi: 10.1530/jrf.0.1150293. [DOI] [PubMed] [Google Scholar]
- 39.Sun LP, Du QZ, Song YP, et al. Polymorphisms in luteinizing hormone receptor and hypothalamic gonadotropin-releasing hormone genes and their effects on sperm quality traits in Chinese Holstein bulls. Mol Biol Rep. 2012;39:7117–23. doi: 10.1007/s11033-012-1543-x. [DOI] [PubMed] [Google Scholar]
- 40.Licht P, Fluhr H, Neuwinger J, et al. Is human chorionic gonadotropin directly involved in the regulation of human implantation? Mol Cell Endocrinol. 2007;269:85–92. doi: 10.1016/j.mce.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 41.Perrier d’Hauterive S, Berndt S, Tsampalas M, et al. Dialogue between blastocyst hCG and endometrial LH/hCG receptor: which role in implantation? Gynecol Obstet Invest. 2007;64:156–60. doi: 10.1159/000101740. [DOI] [PubMed] [Google Scholar]
- 42.Gridelet V, Tsampalas M, Berndt S, et al. Evidence for cross-talk between the LH receptor and LH during implantation in mice. Reprod Fertil Dev. 2013;25:511–22. doi: 10.1071/RD11241. [DOI] [PubMed] [Google Scholar]
- 43.Berndt S, Blacher S, Munaut C, et al. Hyperglycosylated human chorionic gonadotropin stimulates angiogenesis through TGF-beta receptor activation. FASEB J. 2013;27:1309–21. doi: 10.1096/fj.12-213686. [DOI] [PubMed] [Google Scholar]
- 44.Zimmermann G, Ackermann W, Alexander H. Expression and production of human chorionic gonadotropin (hCG) in the normal secretory endometrium: evidence of CGB7 and/or CGB6 beta hCG subunit gene expression. Biol Reprod. 2012;86:87–100. doi: 10.1095/biolreprod.111.092429. [DOI] [PubMed] [Google Scholar]
- 45.Wide L, Lee JY, Rasmussen C. A change in the isoforms of human chorionic gonadotropin occurs around the 13th week of gestation. J Clin Endocrinol Metab. 1994;78:1419–23. doi: 10.1210/jcem.78.6.7515388. [DOI] [PubMed] [Google Scholar]
- 46.Zygmunt M, Herr F, Keller-Schoenwetter S, et al. Characterization of human chorionic gonadotropin as a novel angiogenic factor. J Clin Endocrinol Metab. 2002;87:5290–6. doi: 10.1210/jc.2002-020642. [DOI] [PubMed] [Google Scholar]
- 47.Berndt S, Blacher S, Perrier d’Hauterive S, et al. Chorionic gonadotropin stimulation of angiogenesis and pericyte recruitment. J Clin Endocrinol Metab. 2009;94:4567–74. doi: 10.1210/jc.2009-0443. [DOI] [PubMed] [Google Scholar]
- 48.Lei ZM, Rao CV, Kornyei JL, et al. Novel expression of human chorionic gonadotropin/luteinizing hormone receptor gene in brain. Endocrinology. 1993;132:2262–70. doi: 10.1210/endo.132.5.8477671. [DOI] [PubMed] [Google Scholar]
- 49.Strom CM, Bonilla-Guererro R, Zhang K, et al. The sensitivity and specificity of hyperglycosylated hCG (hhCG) levels to reliably diagnose clinical IVF pregnancies at 6 days following embryo transfer. J Assist Reprod Genet. 2012;29:609–14. doi: 10.1007/s10815-012-9774-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sasaki Y, Ladner DG, Cole LA. Hyperglycosylated human chorionic gonadotropin and the source of pregnancy failures. Fertil Steril. 2008;89:1781–6. doi: 10.1016/j.fertnstert.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 51.Borrelli PT, Butler SA, Docherty SM, et al. Human chorionic gonadotropin isoforms in the diagnosis of ectopic pregnancy. Clin Chem. 2003;49:2045–9. doi: 10.1373/clinchem.2003.022095. [DOI] [PubMed] [Google Scholar]
- 52.Bahado-Singh RO, Oz AU, Kingston JM, et al. The role of hyperglycosylated hCG in trophoblast invasion and the prediction of subsequent pre-eclampsia. Prenat Diagn. 2002;22:478–81. doi: 10.1002/pd.329. [DOI] [PubMed] [Google Scholar]
- 53.Uzan J, Carbonnel M, Piconne O, et al. Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag. 2011;7:467–74. doi: 10.2147/VHRM.S20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palomaki GE, Neveux LM, Haddow JE, Wyatt P. Hyperglycosylated-hCG (h-hCG) and Down syndrome screening in the first and second trimesters of pregnancy. Prenat Diagn. 2007;27:808–13. doi: 10.1002/pd.1778. [DOI] [PubMed] [Google Scholar]
- 55. Butler SA, Luttoo J, Freire MO, et al. Human chorionic gonadotropin (hCG) in the secretome of cultured embryos: hyperglycosylated hCG and hCG-free beta subunit are potential markers for infertility management and treatment. Reprod Sci 2013;20:1038--45. [DOI] [PubMed]
- 56.Kendall SK, Samuelson LC, Saunders TL, et al. Targeted disruption of the pituitary glycoprotein hormone alpha-subunit produces hypogonadal and hypothyroid mice. Genes Dev. 1995;9:2007–19. doi: 10.1101/gad.9.16.2007. [DOI] [PubMed] [Google Scholar]
- 57.Lei ZM, Mishra S, Zou W, et al. Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Mol Endocrinol. 2001;15:184–200. doi: 10.1210/mend.15.1.0586. [DOI] [PubMed] [Google Scholar]
- 58.Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol. 2001;15:172–83. doi: 10.1210/mend.15.1.0582. [DOI] [PubMed] [Google Scholar]
- 59.Ma X, Dong Y, Matzuk MM, Kumar TR. Targeted disruption of luteinizing hormone beta-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci USA. 2004;101:17294–9. doi: 10.1073/pnas.0404743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matzuk MM, DeMayo FJ, Hadsell LA, Kumar TR. Overexpression of human chorionic gonadotropin causes multiple reproductive defects in transgenic mice. Biol Reprod. 2003;69:338–46. doi: 10.1095/biolreprod.102.013953. [DOI] [PubMed] [Google Scholar]
- 61.Rulli SB, Ahtiainen P, Makela S, et al. Elevated steroidogenesis, defective reproductive organs, and infertility in transgenic male mice overexpressing human chorionic gonadotropin. Endocrinology. 2003;144:4980–90. doi: 10.1210/en.2003-0403. [DOI] [PubMed] [Google Scholar]
- 62.Rulli SB, Kuorelahti A, Karaer O, et al. Reproductive disturbances, pituitary lactotrope adenomas, and mammary gland tumors in transgenic female mice producing high levels of human chorionic gonadotropin. Endocrinology. 2002;143:4084–95. doi: 10.1210/en.2002-220490. [DOI] [PubMed] [Google Scholar]
- 63.Lofrano-Porto A, Barra GB, Giacomini LA, et al. Luteinizing hormone beta mutation and hypogonadism in men and women. N Engl J Med. 2007;357:897–904. doi: 10.1056/NEJMoa071999. [DOI] [PubMed] [Google Scholar]
- 64.Achard C, Courtillot C, Lahuna O, et al. Normal spermatogenesis in a man with mutant luteinizing hormone. N Engl J Med. 2009;361:1856–63. doi: 10.1056/NEJMoa0805792. [DOI] [PubMed] [Google Scholar]
- 65.Weiss J, Axelrod L, Whitcomb RW, et al. Hypogonadism caused by a single amino acid substitution in the beta subunit of luteinizing hormone. N Engl J Med. 1992;326:179–83. doi: 10.1056/NEJM199201163260306. [DOI] [PubMed] [Google Scholar]
- 66.Valdes-Socin H, Salvi R, Daly AF, et al. Hypogonadism in a patient with a mutation in the luteinizing hormone beta-subunit gene. N Engl J Med. 2004;351:2619–25. doi: 10.1056/NEJMoa040326. [DOI] [PubMed] [Google Scholar]
- 67.Valdes-Socin H, Salvi R, Thiry A, et al. Testicular effects of isolated luteinizing hormone deficiency and reversal by long-term human chorionic gonadotropin treatment. J Clin Endocrinol Metab. 2009;94:3–4. doi: 10.1210/jc.2008-1584. [DOI] [PubMed] [Google Scholar]
- 68.Suganuma N, Furui K, Furuhashi M, et al. Screening of the mutations in luteinizing hormone beta-subunit in patients with menstrual disorders. Fertil Steril. 1995;63:989–95. doi: 10.1016/s0015-0282(16)57535-9. [DOI] [PubMed] [Google Scholar]
- 69.Tapanainen JS, Koivunen R, Fauser BC, et al. A new contributing factor to polycystic ovary syndrome: the genetic variant of luteinizing hormone. J Clin Endocrinol Metab. 1999;84:1711–15. doi: 10.1210/jcem.84.5.5702. [DOI] [PubMed] [Google Scholar]
- 70.Takahashi K, Ozaki T, Okada M, et al. Increased prevalence of luteinizing hormone beta-subunit variant in patients with premature ovarian failure. Fertil Steril. 1999;71:96–101. doi: 10.1016/s0015-0282(98)00409-9. [DOI] [PubMed] [Google Scholar]
- 71.Takahashi K, Karino K, Kanasaki H, et al. Influence of missense mutation and silent mutation of LHbeta-subunit gene in Japanese patients with ovulatory disorders. Eur J Hum Genet. 2003;11:402–8. doi: 10.1038/sj.ejhg.5200968. [DOI] [PubMed] [Google Scholar]
- 72.Liu N, Ma Y, Wang S, et al. Association of the genetic variants of luteinizing hormone, luteinizing hormone receptor and polycystic ovary syndrome. Reprod Biol Endocrinol. 2012;10:36–42. doi: 10.1186/1477-7827-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nagirnaja L, Venclovas C, Rull K, et al. Structural and functional analysis of rare missense mutations in human chorionic gonadotrophin beta-subunit. Mol Hum Reprod. 2012;18:379–90. doi: 10.1093/molehr/gas018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Segaloff DL. Diseases associated with mutations of the human lutropin receptor. Prog Mol Biol Transl Sci. 2009;89:97–114. doi: 10.1016/S1877-1173(09)89004-2. [DOI] [PubMed] [Google Scholar]
- 75.Arnhold IJ, Lofrano-Porto A, Latronico AC. Inactivating mutations of luteinizing hormone beta-subunit or luteinizing hormone receptor cause oligo-amenorrhea and infertility in women. Horm Res. 2009;71:75–82. doi: 10.1159/000183895. [DOI] [PubMed] [Google Scholar]
- 76.Yariz KO, Walsh T, Uzak A, et al. Inherited mutation of the luteinizing hormone/choriogonadotropin receptor (LHCGR) in empty follicle syndrome. Fertil Steril. 2011;96:e125–30. doi: 10.1016/j.fertnstert.2011.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brokken LJ, Lundberg-Giwercman Y, De-Meyts ER, et al. Association of polymorphisms in genes encoding hormone receptors ESR1, ESR2 and LHCGR with the risk and clinical features of testicular germ cell cancer. Mol Cell Endocrinol. 2012;351:279–85. doi: 10.1016/j.mce.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 78.Elkins DA, Yokomizo A, Thibodeau SN, et al. Luteinizing hormone beta polymorphism and risk of familial and sporadic prostate cancer. Prostate. 2003;56:30–6. doi: 10.1002/pros.10220. [DOI] [PubMed] [Google Scholar]
- 79.Piersma D, Berns EM, Verhoef-Post M, et al. A common polymorphism renders the luteinizing hormone receptor protein more active by improving signal peptide function and predicts adverse outcome in breast cancer patients. J Clin Endocrinol Metab. 2006;91:1470–6. doi: 10.1210/jc.2005-2156. [DOI] [PubMed] [Google Scholar]
- 80.Piersma D, Themmen AP, Look MP, et al. GnRH and LHR gene variants predict adverse outcome in premenopausal breast cancer patients. Breast Cancer Res. 2007;9:R51. doi: 10.1186/bcr1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rull K, Nagirnaja L, Ulander VM, et al. Chorionic gonadotropin beta-gene variants are associated with recurrent miscarriage in two European populations. J Clin Endocrinol Metab. 2008;93:4697–706. doi: 10.1210/jc.2008-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haavisto AM, Pettersson K, Bergendahl M, et al. Occurrence and biological properties of a common genetic variant of luteinizing hormone. J Clin Endocrinol Metab. 1995;80:1257–63. doi: 10.1210/jcem.80.4.7714098. [DOI] [PubMed] [Google Scholar]
- 83.Nilsson C, Pettersson K, Millar RP, et al. Worldwide frequency of a common genetic variant of luteinizing hormone: an international collaborative research. International Collaborative Research Group. Fertil Steril. 1997;67:998–1004. doi: 10.1016/s0015-0282(97)81430-6. [DOI] [PubMed] [Google Scholar]
- 84.Boot AM, Lumbroso S, Verhoef-Post M, et al. Mutation analysis of the LH receptor gene in Leydig cell adenoma and hyperplasia and functional and biochemical studies of activating mutations of the LH receptor gene. J Clin Endocrinol Metab. 2011;96:E1197–205. doi: 10.1210/jc.2010-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bentov Y, Kenigsberg S, Casper RF. A novel luteinizing hormone/chorionic gonadotropin receptor mutation associated with amenorrhea, low oocyte yield, and recurrent pregnancy loss. Fertil Steril. 2012;97:1165–8. doi: 10.1016/j.fertnstert.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 86.Gromoll J, Eiholzer U, Nieschlag E, Simoni M. Male hypogonadism caused by homozygous deletion of exon 10 of the luteinizing hormone (LH) receptor: differential action of human chorionic gonadotropin and LH. J Clin Endocrinol Metab. 2000;85:2281–6. doi: 10.1210/jcem.85.6.6636. [DOI] [PubMed] [Google Scholar]
- 87.Muller T, Gromoll J, Simoni M. Absence of exon 10 of the human luteinizing hormone (LH) receptor impairs LH, but not human chorionic gonadotropin action. J Clin Endocrinol Metab. 2003;88:2242–9. doi: 10.1210/jc.2002-021946. [DOI] [PubMed] [Google Scholar]
- 88.Wang Z, Li T, Zhang W, et al. Variants in DENND1A and LHCGR are associated with endometrioid adenocarcinoma. Gynecol Oncol. 2012;127:403–5. doi: 10.1016/j.ygyno.2012.08.007. [DOI] [PubMed] [Google Scholar]