Abstract
The cancer stem cell (CSC) paradigm presumes the existence of self-renewing cancer cells capable of regenerating all tumor compartments and exhibiting stem cell-associated phenotypes. Recent interpretations of the CSC hypothesis envision stemness as a dynamic trait of tumor-initiating cells rather than a defined and unique cell type. Bidirectional crosstalk between the tumor microenvironment and the cancer bulk is well described in the literature and the tumor-associated stroma, vasculature and immune infiltrate have all been implicated as direct contributors to tumor development. These non-neoplastic cell types have also been shown to organize specific niches within the tumor bulk where they can control the intra-tumor CSC content and alter the fate of CSCs and tumor progenitors during tumorigenesis to acquire phenotypic features for invasion, metastasis and dormancy. Despite the complexity of the tumor-stroma interactome, novel therapeutic approaches envision combining tumor-ablative treatment with manipulation of the tumor microenvironment. We will review the currently available literature that provides clues about the complex cellular network that regulate the CSC phenotype and its niches during tumor progression.
Keywords: Cancer stem cells, tumor-initiating cells, tumor microenvironment, mesenchymal stem/stromal cells, tumor progression
Introduction
Most cancers are characterized by marked phenotypic and functional heterogeneity within the tumor bulk that can result from the accumulation of intrinsic (genetic and epigenetic) insults and extrinsic signals from the microenvironment [1]. Despite the absence of comprehensive organization among all cancer types, several mechanisms have been postulated to model the acquisition of intratumor cellular heterogeneity, including the clonal evolution theory [2] and the cancer stem cell (CSC) hypothesis [3]. The latter has become increasingly popular after the identification of defined tumor subsets endowed with tumorigenic activity and exhibiting phenotypic features of normal stem cells [4]. Although the existence of tumor cells displaying CSC features has been well described in the literature for a number of cancers, no single CSC phenotype can be generalized to all cancers and several distinct populations within a unique tumor may display CSC features [5]. In tumors that incorporate cells having a CSC phenotype, the CSC compartment concentrates most of the tumor-initiating activity and has also been implicated in tumor progression, invasion, and metastasis [5]. Due to their propensity to exhibit metabolic and transport activities usually associated with normal stem cells, CSCs represent an attractive culprit for the augmented radio- and chemotherapy resistance that plagues cancer recurrence. However, the evolution of CSC phenotype accompanying distinct steps of tumor progression has not been clearly established. Acquisition of CSC features by non-CSC subsets has been described in a number of studies, mostly involving cancer cell lines. Dedifferentiation has been especially proposed to be a possible feature of metastasis and relapse [6]. Metastatic CSCs display distinct properties that separate them from CSCs detected in primary tumors, including long-term self-renewal [7] or heightened chemoresistance [8] and expression of CXCR4 has also been used to differentiate pancreatic CSCs having metastatic potential [9]. The contribution of microenvironmental cues to cancer progression is well described in the literature [10] and the identification of several niches within the tumor microenvironment revealed interactions between stromal, vascular or immune populations and CSCs that influence the fate of the CSC compartment during tumor progression (Figure 1). Here, we will review the recent literature pertaining to the interactions between CSCs and niche-resident stromal cells and we will discuss their complex crosstalk as well as its incidence for possible therapeutics.
Figure 1. Evolving Cancer Stem Cell niche interactions during tumor progression.
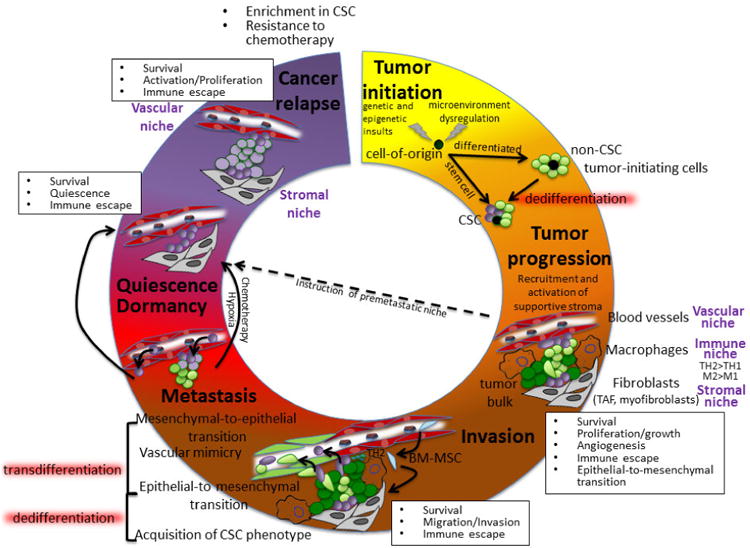
The tumor cell-of origin may initially display CSC features or CSC may appear during tumor progression. Complex interactions between all components of the microenvironment and CSCs organize distinct niches that govern tumor proliferation, immune escape, invasion, metastasis, dormancy and cancer relapse. CSCs have been located in close relationship with two distinct niches: the stroma and the vasculature. Both niches have been shown to play a critical role in regulating CSC phenotypes and initiate invasive, metastatic or dormant behaviors. The immune infiltrate plays also critical roles, modulating these niches or directly interacting with CSC. Circulating BM-MSCs can be recruited at the primary site of tumor, where they can contribute both directly and indirectly to the primary tumor niche or can participate in the establishment of the metastatic niche. CSC transdifferentiation has been suggested based on CSC acquisition of endothelial (vascular mimicry) or mesenchymal (epithelial-to-mesenchymal/mesenchymal-to-epithelial transitions) traits, to support tumor growth or invasiveness. The involvement of the microenvironment in the possible dedifferentiation of non-CSCs to a CSC phenotype has also been suggested. Acquisition of CSC features is often partially reminiscent of embryonic phenotypes and possible dedifferentiation process may involve signaling routes exploited by induced pluripotency. The metastatic process is highly inefficient, but instruction of a premetastatic niche by the primary tumor and acquisition of a CSC phenotype by invasive cells may favor survival and engraftment of circulating cancer cells in secondary niches. While the osteoblast niche seems to regulate the fate of leukemic CSCs for cancer relapse, the vascular niche has been involved in establishment and exit of breast cancer dormancy.
Experimental designs to study CSC-stroma interactions
The tumor microenvironment is heterogeneous (including stroma, vasculature and inflammatory cells) and recruited cells often display an activated phenotype upon interactions with tumor cells to augment their pro-tumorigenic activities. Thus, the study of interactions between putative CSCs and the stromal microenvironment remains challenging, due to high heterogeneity and variability in both cellular compartments. In vivo recapitulation of interactions between human CSCs and their niche is typically attempted using immunodeficient rodent models [4]. Modulation of the microenvironment can be achieved using transgenic animals, orthotopic transplantation, or co-injection of stromal cells and engineered niches. Alteration of the medullar hematopoietic niche using transgenic immunodeficient animal strains was used to evaluate the effects of unbalanced hematopoietic cytokines on the fate of CD34+ leukemic CSCs [11]. Co-injection of basement membrane matrix protein has been shown to support tumor initiation and growth of putative CSCs [12]. The site of injection has also been reported to be of critical importance and can modify the frequency of tumor-initiating cells that can be measured in these assays. Mammary fat pads were the most reliable site of injection to study tumor-initiation potential of ovarian tumors [13], and represents an orthotopic injection site for breast CSC studies [14]. Orthotopic transplantation assays via coinjection of human tumor and stromal cells to humanized microenvironment is gaining acceptance [4], but most models involve a single component, either endothelial [15, 16] or stromal [17]. Finally, current CSC sorting strategies from primary isolates often do not distinguish CSC subsets or CSCs from non-CSC tumor-initiating progenitors. Putative breast CSCs were originally identified by their CD44+CD24-phenotype. Further refinement of the breast tumorigenic compartment can be achieved using additional markers such as CD90 [18] or by including CSC-associated phenotypes such as multidrug resistance (MDR) transporter expression and activity [19] or cellular size via light scatter properties [14, 18]. A small resting CSC-like phenotype was associated with a tumorigenic activity at low dose without requirement for stromal support, whereas larger progenitor-like cells either required injection of larger number of cells or the presence of supportive stroma to retain tumor-initiating activity [14].
Current approach to the cancer stem cell paradigm
The modern interpretation of the cancer stem cell (CSC) hypothesis was formulated over a decade ago following studies on acute myeloid leukemia [5]. Other putative CSC subsets were subsequently identified in a variety of solid tumors [5]. The identification of rare self-renewing cancer cell subsets capable of serially generating heterogeneous tumors in xenograft models and the convergence of signaling pathways dysregulated during oncogenesis and governing the self-renewal/differentiation fate of normal stem cells has led to the hypothesis that CSCs in primary tumors may arise from the transformation of normal stem cells or alternatively via acquisition of stem cell features (i.e. self-renewal) by more differentiated cells harboring genetic or epigenetic insults (Figure 1). The initial concept of a clonogenic tumor-initiating CSC atop heterogeneous cancer cell progenies was extrapolated from the hierarchical differentiation model of the hematopoietic system, a unidirectional differentiation scheme in which self-renewing multipotent stem cells give rise to pools of proliferating intermediate progenitor cells and ultimately all mature cell types. Most recent iterations to define CSCs have emphasized the inherent complexity and fluctuation of the CSC compartment within a unique tumor and have embraced a definition of the CSC phenotype as a dynamic cell state rather than a distinct cell type [1, 5, 6, 20]. Beyond the clonogenic properties of CSC (self-renewal and differentiation/tumorigenicity), numerous analogies have been made between putative CSCs and their normal counterpart to identify them within the heterogeneous tumor bulk, including surface marker expression, cell cycle state, migratory properties, immune escape, or metabolic and transporters activities [5, 6]. Recent studies have exposed that CSCs may also arise from dedifferentiation of more differentiated tumor cells [21], after receiving specific signals from the local microenvironment (Figure 1). Data obtained in the Donnenberg laboratory using clinical isolates seems to support such a scenario. Unlike small CSC-like MDR+ breast cancer-initiating cells, high light scatter MDR-CD90+ breast cancer progenitors exhibit tumorigenic activity only when injected at high cell numbers [19] or when coinjected with adipose derived stromal cells [14]. Yet these progenitors were shown to be able to generate tumors that recapitulated the heterogeneity of original patient tumors, including the rare small resting CSC population. These observations support the plasticity of tumor initiating cells and highlight the link between tumorigenicity, expression of mesenchymal associated markers, and stromal interactions.
Tumor microenvironment cellular components
The tumor microenvironment consists of an extra-cellular matrix (ECM) and multiple cell types. The tumor ECM mainly results from extravasation of plasma proteins and dense deposits of collagen delivered by the fibrotic component. The cellular components include a substantial inflammatory infiltrate (i.e. macrophages, dentritic cells and T-cells), which is reminiscent of chronic inflammatory and fibrotic lesions and has now become an attractive target for the development of anti-cancer immunomodulating therapies [22]. Tumor-associated macrophages (TAMs) often accumulate in hypoxic areas and can support angiogenesis via release of proangiogenic factors (e.g. vascular endothelial growth factor (VEGF)) sequestered in the ECM, or can facilitate revascularization via release of metalloproteinases [23]. TAMs have been shown to interact with CSCs in several cancers, including breast, hepatocellular and colon carcinomas and gliomas [24-27]. Under hypoxic conditions, glioma CSCs can inhibit TAM phagocytosis, as well as T-cell proliferation and activation via STAT3 signaling [28]. Although the tumor microenvironment is often considered to promote tumorigenicity by inhibition of the innate and adaptive responses [22], including dendritic cell maturation and subsequent antigen presentation [29], immune cells such as follicular dendritic cells can directly support the maintenance of the tumorigenic CSC state, as occurs in some cases of therapy-resistant follicular lymphoma [30]. The microenvironment also incorporates tumor-associated fibroblasts (TAF), vascular cells and local or recruited progenitors (bone marrow-derived mesenchymal stem cells (BM-MSC), endothelial progenitors (EPC)). TAFs display an activated phenotype that often resembles myofibroblasts and secrete a battery of growth factors and cytokines at the primary site of tumor to support both cancer cell proliferation and survival [31]. TAFs not only regulate directly tumor growth, but can also support local angiogenesis via recruitment of endothelial progenitors [32]. Possible crosstalk between CSCs and myofibroblasts is supported by their close localization at the invasive front of epithelial tumors [18, 33]. Interactions between CSCs and vascular lineages are particularly prominent in highly vascularized brain tumors [34, 35], but have also been reported to govern metastatic activities of dormant breast cancer cells [36]. The perivascular niche of glioblastoma tumors can self-regulate its growth in a loop fashion in which CSCs stimulate local angiogenesis by releasing paracrine factors and endothelial cells control migratory and tumorigenic CSC activities [35]. The effects of BM-MSC on tumor cells have been reviewed in [37, 38]. Large numbers of BM-derived MSC can be mobilized and recruited to the local microenvironment via release of endocrine and paracrine signals during tumor development. Both pro- and anti-tumorigenic activities of BM-MSC have been acknowledged in the literature [37, 38]. BM-MSCs interact with all other stroma-resident populations. They can replenish intra-tumor TAFs via differentiation, regulate local angiogenesis and modulate innate immunity via interactions with macrophages [37, 38]. Several studies have suggested that MSCs can contribute to the acquisition of a CSC phenotype by non-CSC tumor cells or support epithelial-to-mesenchymal transition (EMT) leading to invasion and metastasis [37, 39].
The cancer stem cell niche
Stem cells reside in a specific microenvironment (or niche) that can regulate their self-renewal and differentiation. A similar niche concept has been extrapolated to cancer in which microenvironmental cues regulate the CSC fate during tumor development [40].
Quiescent hematopoietic stem cells (HSC) reside in an osteoblastic niche, although HSC can also occupy a vascular niche within the sinusoidal endothelium [41]. Assuming leukemia CSCs occupy similar niches in the bone marrow, several studies have investigated the effects of microenvironment modulation on the fate of cancer cells. The injection of leukemia CSCs into transgenic animals revealed an instructive role of the microenvironment [11]. Adhesion signals involving the glycoprotein CD44 seem to play a critical role in leukemia CSC-niche interactions as disruption of CD44 signaling altered the myeloproliferative and homing activities of chronic myeloid leukemia (CML) [42] and acute myeloid leukemia (AML) [43] CSCs. Disturbance of the osteoblastic niche has direct repercussions on leukemia CSCs. Activation of osteoblasts via Dicer1 deletion can induce myelodysplasia and secondary leukemia [44]. Similarly, modulation of the osteoblastic niche via parathyroid hormone signaling can alter the myeloproliferative activity of leukemia CSCs [45], although in the latter, bone remodeling resulted in increased TGF-β release by medullar osteoblasts, which was detrimental to myeloproliferative neoplasia and engraftment of CML cells. The same approach improved engraftment and tumorigenicity of AML CSCs [45]. TGF-β signaling had been previously implicated in the maintenance of a CSC phenotype in CML cells [46]. Yet, in this study, in vitro inhibition of TGF-β actually impaired the colony-forming ability of CML cells and combination of TGF-β, SCF and Foxo3a inhibition depleted the CML in vivo. While the results obtained in these studies are contradictory, they confirm a pivotal role of TGF-β signaling in the regulation of the leukemia CSC niche. The diverging effects of TGF-β signaling on CSC activities probably reflect the complexity of the interplay between CSCs and their cellular partners in the niche.
In highly vascularized brain tumors such as gliomas, CSCs are tightly regulated by the tumor endothelium [34]. Brain CSCs are chemoattracted towards endothelial cells in vitro and their tumorigenicity in animal models can be enhanced via co-injection of endothelial cells [34]. Niche-glioma CSC interactions have been shown to be bidirectional [35, 47]. Other CSC niches can be observed at the invasive front of epithelial tumors [18, 33] where stromal cells are suspected to control tumor invasion [6, 48]. The high density of myofibroblasts at the tumor-stroma interface in colon cancer coincides with an enrichment of tumor cells with high nuclear expression of β-catenin which is mediated by myofibroblast secretion of hepatocyte growth factor (HGF) [33]. Similarly, a population of CD44+CD90+ CSCs has been shown to reside in direct contact with a layer of CD90+ stromal cells at the periphery of invasive nests and trabeculae observed throughout breast tumors [18], supporting possible regulation of the invasive phenotype of breast CSCs by the adjacent stroma.
Tumor initiation and CSC pool regulation
The acquisition of CSC features by tumor cells upon interaction with the microenvironment has been reported for a variety of cancers. Restricted leukemia progenitors can reexpress a CSC phenotype via reactivation of self-renewal programs [49]. Hedgehog (Hh) signaling seems to be essential for maintenance of leukemia-CSCs [50, 51], possibly involving activation of β-catenin signaling [52]. Recently, stromal cells have been shown to modulate Hh signaling and proliferation in myeloid neoplasms via expression of the Hh-interacting protein [53]. Induction of Hh signaling in epithelial cancers upon interaction with TAMs has also been reported [25].
Stroma-mediated regulation of the CSC phenotype is well described in epithelial tumors. Cancer-associated MSCs were shown to rely on altered BMP production to regulate ovarian CSCs and their tumorigenesis [54]. Similarly, pancreatic stromal cells can enhance the CSC phenotype in pancreatic cancer cells [55] and promote their self-renewal and invasiveness [56]. Infiltrating immune cells also exert control over the CSC pool. Secretion of interleukin-6 (IL-6) by innate immune cells stimulates the proliferation of colon CSCs [57]. IL-6 was also found to enhance conversion of breast cancer progenitors to a CSC phenotype via a positive feedback loop involving NFκb, Lin28 and Let7miRNA [58] and was identified among other TAM-secreted cytokines as an inducer of tumor-initiating capacity and chemotherapy resistance in colon and lung cancer cells [25].
TAFs were shown to promote a CSC phenotype in colorectal carcinoma cells via production of collagen type I [59], but can also induce a CSC phenotype in non-tumorigenic cancer cells via reactivation of the Wnt pathway and HGF signaling [33]. Wnt signaling has also been proposed to be essential to maintain a CSC phenotype in epidermal tumors [60]. Wnt activation by the surrounding microenvironment and HGF signaling seem to be redundant mechanisms to promote tumor activation [33, 52, 61-63]. HGF promoted a CSC phenotype in various cell lines [64, 65] and is implicated in the acquisition of an invasive phenotype via EMT [66]. Gastric TAFs were suggested to exploit another EMT-related growth factor (i.e. transforming growth factor beta (TGF-β) to regulate CSC content [67].
Low physiological oxygen favors acquisition of CSC features in various cancer cells including glioblastoma [68] and ovarian cancer cell lines [69]. Hypoxic conditions increased expression of the ABC transporter ABCG2 in ovarian CSCs [61] and acquisition of pro-inflammatory phenotype by breast CSCs via Wnt signaling [62]. Hypoxic and acidic microenvironments potentiate the maintenance or the acquisition of CSC features via induction of hypoxia inducible factor 2α (HIF2α) [35, 47]. HIF2α expression promoted local release of angiogenic factors [35] and acquisition of a CSC phenotype [47, 70, 71] that was marked by an upregulation of stem cell associated networks such as the pluripotency-associated factors Oct4, Nanog or c-Myc. Similar acquisition of human embryonic stem cell (ESC) markers in various cancer lines following hypoxia was recently reported [72]. Tumorigenicity shares many features with pluripotency and induced pluripotency, exploiting factors that are known oncogenes (MYC) or are commonly detected in tumors (NANOG, SOX2, OCT4) [73]. Non-tumorigenic mammary cells and differentiated populations of luminal-like breast cancer cells can acquire a CSC phenotype using cellular reprogramming [74, 75]. Interestingly, hypoxia also enhances efficiency of induced pluripotent stem cells (iPSC) generation from mouse embryonic fibroblast and human dermal fibroblasts [76], suggesting that it may play a critical role for dedifferentiation. The Zambidis laboratory has derived human iPSC lines using a methodology involving both low oxygen and micro-environmental stroma-priming that dramatically enhanced cellular reprogramming of myeloid progenitors to pluripotency [77]. BM-MSC-secreted factors active during progenitor reprogramming included known MSC-released cytokines such as platelet-derived growth factor (PDGF), CCL2 and IL-6, which have already been implicated in the acquisition of CSC features. For example, CCL2 has been shown to mediate crosstalk between cancer cells and stromal fibroblasts that augments the CSC phenotype and self-renewal of breast cancer cell lines [78]. BM-MSC secretion of IL-6 has also been suggested to modulate the CSC content of breast cancer [79]. In another study, BM-MSC secretion of IL-6, CCL5 and IL-8 resulted in activation of β-catenin/WNT signaling in various cancer cell lines and promotion of a CSC phenotype [80].
Vascular regulation
Bidirectional crosstalk between CSCs and vascular cells has been demonstrated in the perivascular niche of highly vascularized tumors (i.e. glioblastoma). Local endothelial cells support retention of the stem cell phenotype and tumorigenicity by CSCs [34], while glioma CSCs closely promote local angiogenesis through the release of VEGF and stromal-derived factor 1 [34, 81-84]. Glioma CSC self-renewal has been shown to be mediated by activation of the Notch pathway following release of nitric oxide by endothelial cells [85]. Glioma CSCs not only promote recruitment and expansion of the local vascular network by releasing VEGF [81, 86], but also protect vascular cells from hypoxia and irradiation-induced apoptosis [87, 88]. Skin carcinoma CSCs have also been shown to populate a vascular niche [89]. Both niches seem to resolve upon an autocrine VEGF loop that regulates both CSC and niche self-renewal [89, 90]. Glioblastoma CSCs can also transdifferentiate into vascular cells and contribute to the microvasculature via a process termed vascular mimicry (VM) [91, 92]. Vascular mimicry (VM) is a new pattern for tumor vascularization involving the formation by tumor cells of highly patterned vascular channels (Figure 1) that has been observed primarily in aggressive types of cancer [93]. Although these tumor tubes are deprived of endothelial cells, they include a basement membrane and have been demonstrated to anastomose to the vasculature [93].
Invasion and metastasis regulation
Numerous components of the tumor microenvironment have been implicated in local and distal dissemination of tumor cells [48]. Stroma-tumor interactions have been shown to promote acquisition by CSCs of an invasive phenotype in various cancers including pancreatic [94] and bladder [95] carcinomas. A population of CSCs is observed at the invasive front of epithelial cancers [18, 33]. The breach of the basement membrane by carcinoma cells facilitates tumor-stroma interactions and recruitment of circulating stromal components (i.e. immune cells, EPCs, MSCs). TAMs can participate to the acquisition of an invasive phenotype by CSCs. While bidirectional interactions between carcinoma CSCs and macrophages can modulate metastasis [26], TAMs and resident microglia can also regulate the invasive phenotype of glioma CSCs via TGF-β signaling [27]. Invasion is often accompanied by a transdifferentiation process (Figure 1), termed epithelial-to-mesenchymal-transition (EMT). Epithelial cancer cells that undergo EMT exhibit mesenchymal features (loss of polarized epithelial morphology and acquisition of spindle shape) that favor motility, invasiveness and survival [96]. Carcinogenic EMT is a critical step for invasion/metastasis that is partially reminiscent to embryonic developmental programs [39]. EMT is often associated with dedifferentiation and acquisition of CSC features (Figure 1). Induction of EMT in normal immortalized human mammary epithelial cells led to augmented expression of CSC markers, self-renewal capacity and tumor-initiating activity [97].
Cancer EMT can be triggered by various factors including HGF, PDGF, and TGF-β [66]. Hypoxia can regulate expression of EMT-associated genes [98] and promote acquisition of an invasive phenotype via activation of Wnt signaling in breast, colon, hepatic and pancreatic cancer cell lines [99]. HGF secretion by myofibroblasts at the invasive front in colorectal cancer can induce a CSC phenotype in non-tumorigenic cancer cells [33]. In lung adenocarcinoma, putative CSCs expressing cytokeratin and the EMT-associated markers CD44 and CD90 are rare in primary tumors, but prevalent in metastatic pleural effusions [100].
Occurrence of metastasis does not occur randomly and recent studies suggest that primary tumors can instruct the microenvironment of distant organs to develop premetastatic niches (Figure 1) [101]. Pre-metastatic plasma and BM-MSCs of advanced breast cancer patients facilitate transendothelial migration of breast cancer cell lines and may participate to remodeling of the bone marrow prior to colonization by cancer cells [102]. Other cell types, including tumor-associated T-cells can participate to the organization of the premetastatic niche [103]. A reciprocal role of the metastatic niche in the control of CSC content and invasive phenotype at the primary site of tumor has also been suggested. Peritoneal mesothelial cells contributed to the acquisition of a CSC phenotype and invasive phenotype by ovarian cancer cells via SDF1-CXCR4 signaling [104]. Periostin, a component of tumor ECM, was found to be critical for CSC colonization of their metastatic niche [105]. Periostin deposition by stromal fibroblasts was induced in the secondary target organ upon interactions with infiltrating CSCs [105]. Local stroma-resident populations can also accompany metastatic cancer cells to facilitate their engraftment at distal niches. Pancreatic stellate cells were shown to intravasate/extravasate to and from blood vessels and accompany metastatic pancreatic cells to distant metastatic nodules where they stimulate angiogenesis [106].
Metastatic cancer CSCs specifically migrate and incorporate into a suitable niche [101] where they can potentially lay dormant until reactivation by niche signals (Figure 1). Although cancer dormancy is not a defining hallmark of CSCs, significant phenotypic overlap between dormant cancer cells and CSCs (quiescence, radio- and chemotherapy resistance, immune escape, response to angiogenic factors) suggest at least an overlap of both phenotypes [107]. The local microenvironment has been proposed to play a critical role in the establishment and maintenance of cancer dormancy [108]. Activation of osteoblasts can disrupt the local niche and induce myelodysplasia and secondary leukemia [44]. The perivascular niche has recently been implicated in the regulation of breast tumor dormancy [36]. Using engineered microvascular niches, the authors determined the factors critical to maintaining dormancy or promoting reactivation of disseminated tumorigenic cells. Secretion of thrombospondin-1 by the vasculature was critical to sustain cell quiescence. Inversely, active angiogenesis resulted in a sprouting neovasculature and release of TGF-β and periostin, leading to micrometastatic outgrowth. A vascularized “inhibitory niche” was also reconstructed ex vivo using stromal cell lines and umbilical cord vascular endothelial cells and shown to support a resting state in breast cancer cell lines [109].
4. Conclusions: Therapeutic perspectives
Many of the aspects of tumor progression and resistance to treatment result from the interplay between the neoplastic cells and the surrounding non-malignant stroma [110]. The tumor stroma has become an attractive target for anti-cancer therapies due to its global contribution to tumorigenesis and direct interactions with therapy-refractory CSCs. Tumors in which interactions between CSC and vascular cells are closely regulated are possible targets for anti-angiogenesis strategies. For example, anti-angiogenic treatment was shown to decrease the glioblastoma CSC content resulting in reduced overall tumor growth and higher sensitivity to cytotoxic agents [81, 111]. In another study, the use of inhibitors of VEGFR2 and PDGFR-β targeted both endothelium and pericytes, resulting in diminished vascular supply of glioma tumors [31]. Disruption of Notch signaling in glioblastoma CSCs resulted in higher sensitivity to radiotherapy by disrupting the attachment of CSCs to their vascular niche [112]. Yet, inhibition of local angiogenesis in other models elicited increased invasiveness and metastasis [113] and created a local hypoxic niche, which could also result in expansion of a radioresistant CSC phenotype [70]. In colon cancer, CSCs have been shown to display resistance to antiangiogenic therapy [114]. Other therapies envision augmenting the local vasculature to facilitate delivery of chemotherapeutic agents. Coadministration of gemcitabine and IPI-926, a drug that depletes tumor-associated stromal cells via inhibition of Hedgehog signaling, produced a transient increase in intratumor vascular density and the intratumor concentration of gemcitabine, leading to temporary stabilization of the disease [115]. Targeted strategies against non-endothelial contributors of the tumor microenvironment have also been investigated. TAF depletion via T-cell mediated killing was shown to lead to a significant reduction of tumor growth and metastasis in colon cancer, as well as improving chemotherapy efficacy [116]. Anti-HGF signaling treatments (anti-MET antibodies) prevented colon cancer tumor growth in vivo [117]. A number of strategies have been targeted to microenvironmental support to invasion and metastasis. Disruption of the CXCR4-CXCL12 axis has proven to affect migratory properties of leukemia [118-120], follicular lymphoma [30] and colon [121] CSCs. Combined blockade of CXCR4 and dacarbazine treatment efficiently inhibited tumor growth and metastasis in a chemoresistant melanoma CSC model by modifying the lymphatic microenvironment [122]. MT1-MMP and MMP9 targeting has been proposed to reduce CSC content and invasive phenotype in medulloblastoma [123]. CCL2 targeting disrupted CSC-TAF interactions and reduced tumorigenesis [78]. Metformin has been shown to specifically target CSCs in several cancers including glioblastoma [124] and ovarian carcinoma [125]. Combination therapy including metformin and a stroma-targeting smoothened inhibitor was able to reduce pancreatic tumor CSC content and affect their proinvasion phenotype [126]. Finally, some approaches rely on microenvironment manipulation to disrupt dormancy and target chemoresistant CSCs. The induction of oxidative stress disrupted quiescence of leukemia CSCs, leading to their entry into cycle and significant sensitivity to cytosine arabinoside and apopotosis [127]. Overall, an improved understanding of the interactions between CSCs and their specific niches during tumor progression has the potential to reveal new ways in which to target radio and chemoresistant CSC populations that are often selected during cancer recurrence.
Acknowledgments
Ludovic Zimmerlin was supported by a grant from the Maryland Stem Cell Research Fund 2013-MSCRF-114936. Albert D. Donnenberg and Vera S. Donnenberg were supported by grants BC032981 and BC044784 from the Department of Defense, grant R01CA 114246 from the National Institutes of Health (NIH), grant R01-HL-085819 from the National Heart, Lung, and Blood Institute, the Hillman Foundation, the Glimmer of Hope Foundation, the Commonwealth of Pennsylvania, through the McGowan Institute of Regenerative Medicine, the NHLBI (Production Assistance for Cellular Therapy (PACT) N01-HB-37165), and the Department of Defense Biomedical Translational Initiative (W911QY-09-C-0209). Drs. Donnenberg would also like to thank Diana Napper from The Glimmer of Hope Foundation for her support. Elias T. Zambidis and Tea Soon Park were supported by grants from NIH 1U01HL099775 and U01HL100397 (Elias T. Zambidis) and the Maryland Stem Cell Research Fund: 2011-MS CRF II-0008-00 and 2007-MSCRF II-0379-00 (Elias T. Zambidis), and the Maryland Stem Cell Research Fund (MSCFR) Postdoctoral Fellowship grant 2009-MSCRF III-106570 (Tea Soon Park).
Abbreviations
- AML
acute myeloid leukemia
- BM-MSC
bone marrow-derived mesenchymal stem cells
- CML
chronic myeloid leukemia
- CSC
cancer stem cells
- ECM
extracellular matrix
- EMT
epithelial-to-mesenchymal transition
- ESC
embryonic stem cells
- Hh
hedgehog
- HIF
hypoxia-inducible factor
- HGF
hepatocyte growth factor
- HSC
hematopoietic stem cells
- iPSC
induced pluripotent stem cell
- MSC
mesenchymal stem/stromal cells
- PDGF
platelet-derived growth factor
- PDGFR
platelet derived growth factor-receptor
- TAF
tumor-associated fibroblast
- TAM
tumor-associated macrophage
- VEGF
vascular endothelial growth factor
- VEGFR2
vascular endothelial growth fact receptor 2
- VM
vascular mimicry
Footnotes
Compliance with Ethics Guidelines: Conflict of Interest: Tea Soon Park, Vera S. Donnenberg, Albert D. Donnenberg, Elias T. Zambidis, and Ludovic Zimmerlin declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21:283–296. doi: 10.1016/j.ccr.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 3.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 4.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 5•.Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717–728. doi: 10.1016/j.stem.2012.05.007. An excellent review of the current interpretation of the CSC paradigm. [DOI] [PubMed] [Google Scholar]
- 6.Donnenberg AD, Hicks JB, Wigler M, Donnenberg VS. The cancer stem cell: cell type or cell state? Cytometry A. 2013;83:5–7. doi: 10.1002/cyto.a.22208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu DY, Wu FG, Zhen ZM, Lu TR, Wu HY, Che JY, Xu B. Different spontaneous pulmonary metastasis inhibitions against lewis lung carcinoma in mice by bisdioxopiperazine compounds of different treatment schedules. Sci Pharm. 2010;78:13–20. doi: 10.3797/scipharm.0910-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pang R, Law WL, Chu AC, Poon JT, Lam CS, Chow AK, Ng L, Cheung LW, Lan XR, Lan HY, et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell. 2010;6:603–615. doi: 10.1016/j.stem.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Wei J, Wunderlich M, Fox C, Alvarez S, Cigudosa JC, Wilhelm JS, Zheng Y, Cancelas JA, Gu Y, Jansen M, et al. Microenvironment determines lineage fate in a human model of MLL-AF9 leukemia. Cancer Cell. 2008;13:483–495. doi: 10.1016/j.ccr.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fridman R, Benton G, Aranoutova I, Kleinman HK, Bonfil RD. Increased initiation and growth of tumor cell lines, cancer stem cells and biopsy material in mice using basement membrane matrix protein (Cultrex or Matrigel) co-injection. Nat Protoc. 2012;7:1138–1144. doi: 10.1038/nprot.2012.053. [DOI] [PubMed] [Google Scholar]
- 13.Stewart JM, Shaw PA, Gedye C, Bernardini MQ, Neel BG, Ailles LE. Phenotypic heterogeneity and instability of human ovarian tumor-initiating cells. Proc Natl Acad Sci U S A. 2011;108:6468–6473. doi: 10.1073/pnas.1005529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Zimmerlin L, Donnenberg AD, Rubin JP, Basse P, Landreneau RJ, Donnenberg VS. Regenerative therapy and cancer: in vitro and in vivo studies of the interaction between adipose-derived stem cells and breast cancer cells from clinical isolates. Tissue Eng Part A. 2011;17:93–106. doi: 10.1089/ten.tea.2010.0248. Distinct effects of stromal cells on the tumorigenicity of putative breast CSCs and progenitors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu D, Martin V, Fueyo J, Lee OH, Xu J, Cortes-Santiago N, Alonso MM, Aldape K, Colman H, Gomez-Manzano C. Tie2/TEK modulates the interaction of glioma and brain tumor stem cells with endothelial cells and promotes an invasive phenotype. Oncotarget. 2010;1:700–709. doi: 10.18632/oncotarget.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu TS, Costello MA, Talsma CE, Flack CG, Crowley JG, Hamm LL, He X, Hervey-Jumper SL, Heth JA, Muraszko KM, et al. Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer Res. 2011;71:6061–6072. doi: 10.1158/0008-5472.CAN-10-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmerlin L, Donnenberg VS, Donnenberg AD. Rare event detection and analysis in flow cytometry: bone marrow mesenchymal stem cells, breast cancer stem/progenitor cells in malignant effusions, and pericytes in disaggregated adipose tissue. Methods Mol Biol. 2011;699:251–273. doi: 10.1007/978-1-61737-950-5_12. [DOI] [PubMed] [Google Scholar]
- 18.Donnenberg VS, Donnenberg AD, Zimmerlin L, Landreneau RJ, Bhargava R, Wetzel RA, Basse P, Brufsky AM. Localization of CD44 and CD90 positive cells to the invasive front of breast tumors. Cytometry B Clin Cytom. 2010;78:287–301. doi: 10.1002/cyto.b.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donnenberg VS, Luketich JD, Landreneau RJ, DeLoia JA, Basse P, Donnenberg AD. Tumorigenic epithelial stem cells and their normal counterparts. Ernst Schering Found Symp Proc. 2006:245–263. doi: 10.1007/2789_2007_054. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Laterra J. Cancer stem cells: distinct entities or dynamically regulated phenotypes? Cancer Res. 2012;72:576–580. doi: 10.1158/0008-5472.CAN-11-3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, Brooks M, Reinhardt F, Su Y, Polyak K, et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci U S A. 2011;108:7950–7955. doi: 10.1073/pnas.1102454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carmans S, Hendriks JJ, Thewissen K, Van den Eynden J, Stinissen P, Rigo JM, Hellings N. The inhibitory neurotransmitter glycine modulates macrophage activity by activation of neutral amino acid transporters. J Neurosci Res. 2010;88:2420–2430. doi: 10.1002/jnr.22395. [DOI] [PubMed] [Google Scholar]
- 24.Mantovani A, Locati M. Tumor-associated macrophages as a paradigm of macrophage plasticity, diversity, and polarization: lessons and open questions. Arterioscler Thromb Vasc Biol. 2013;33:1478–1483. doi: 10.1161/ATVBAHA.113.300168. [DOI] [PubMed] [Google Scholar]
- 25.Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, Yagita H, Takaoka A, Tahara H. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci U S A. 2011;108:12425–12430. doi: 10.1073/pnas.1106645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muramatsu S, Tanaka S, Mogushi K, Adikrisna R, Aihara A, Ban D, Ochiai T, Irie T, Kudo A, Nakamura N, et al. Visualization of stem cell features in human hepatocellular carcinoma reveals in vivo significance of tumor-host interaction and clinical course. Hepatology. 2013;58:218–228. doi: 10.1002/hep.26345. [DOI] [PubMed] [Google Scholar]
- 27.Ye XZ, Xu SL, Xin YH, Yu SC, Ping YF, Chen L, Xiao HL, Wang B, Yi L, Wang QL, et al. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-beta1 signaling pathway. J Immunol. 2012;189:444–453. doi: 10.4049/jimmunol.1103248. [DOI] [PubMed] [Google Scholar]
- 28.Wei J, Wu A, Kong LY, Wang Y, Fuller G, Fokt I, Melillo G, Priebe W, Heimberger AB. Hypoxia potentiates glioma-mediated immunosuppression. PLoS One. 2011;6:e16195. doi: 10.1371/journal.pone.0016195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee CG, Das B, Lin TL, Grimes C, Zhang X, Lavezzi T, Huang L, Cole J, Yau L, Li L. A rare fraction of drug-resistant follicular lymphoma cancer stem cells interacts with follicular dendritic cells to maintain tumourigenic potential. Br J Haematol. 2012;158:79–90. doi: 10.1111/j.1365-2141.2012.09123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castells M, Thibault B, Delord JP, Couderc B. Implication of tumor microenvironment in chemoresistance: tumor-associated stromal cells protect tumor cells from cell death. Int J Mol Sci. 2012;13:9545–9571. doi: 10.3390/ijms13089545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 33•.Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. HGF-mediated interactions between myofibroblasts and colon cancer cells at the invasive front promote a CSC phenotype. [DOI] [PubMed] [Google Scholar]
- 34.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 35.Hjelmeland AB, Wu Q, Heddleston JM, Choudhary GS, MacSwords J, Lathia JD, McLendon R, Lindner D, Sloan A, Rich JN. Acidic stress promotes a glioma stem cell phenotype. Cell Death Differ. 2011;18:829–840. doi: 10.1038/cdd.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15:807–817. doi: 10.1038/ncb2767. Engineered vascular niches can reversably switch breast cancer cells from dormancy to an active state. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmerlin L, Park TS, Zambidis ET, Donnenberg VS, Donnenberg AD. Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie. 2013;95:2235–2245. doi: 10.1016/j.biochi.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barcellos-de-Souza P, Gori V, Bambi F, Chiarugi P. Tumor microenvironment: Bone marrow-mesenchymal stem cells as key players. Biochim Biophys Acta. 2013;1836:321–335. doi: 10.1016/j.bbcan.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Iwasaki H, Suda T. Cancer stem cells and their niche. Cancer Sci. 2009;100:1166–1172. doi: 10.1111/j.1349-7006.2009.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•.Ema H, Suda T. Two anatomically distinct niches regulate stem cell activity. Blood. 2012;120:2174–2181. doi: 10.1182/blood-2012-04-424507. Distinct niches (osteoblast vs endothelial) regulate the fate of hematopoietic stem cells. Similar niches have been suggested to regulate leukemia CSCs and metastatic breast CSCs in the bone marrow. [DOI] [PubMed] [Google Scholar]
- 42.Krause DS, Lazarides K, von Andrian UH, Van Etten RA. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med. 2006;12:1175–1180. doi: 10.1038/nm1489. [DOI] [PubMed] [Google Scholar]
- 43.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 44.Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464:852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Krause DS, Fulzele K, Catic A, Sun CC, Dombkowski D, Hurley MP, Lezeau S, Attar E, Wu JY, Lin HY, et al. Differential regulation of myeloid leukemias by the bone marrow microenvironment. Nat Med. 2013;19:1513–1517. doi: 10.1038/nm.3364. In vivo modulation of the osteoblastic niche affects the fate of distinct leukemia CSCs in opposing ways: possible target for CML, but opposing effects on AML. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naka K, Hoshii T, Muraguchi T, Tadokoro Y, Ooshio T, Kondo Y, Nakao S, Motoyama N, Hirao A. TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature. 2010;463:676–680. doi: 10.1038/nature08734. [DOI] [PubMed] [Google Scholar]
- 47.Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8:3274–3284. doi: 10.4161/cc.8.20.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calorini L, Bianchini F. Environmental control of invasiveness and metastatic dissemination of tumor cells: the role of tumor cell-host cell interactions. Cell Commun Signal. 2010;8:24. doi: 10.1186/1478-811X-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J, Levine JE, Wang J, Hahn WC, Gilliland DG, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- 50.Dierks C, Beigi R, Guo GR, Zirlik K, Stegert MR, Manley P, Trussell C, Schmitt-Graeff A, Landwerlin K, Veelken H, et al. Expansion of Bcr-Abl-positive leukemic stem cells is dependent on Hedgehog pathway activation. Cancer Cell. 2008;14:238–249. doi: 10.1016/j.ccr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Zhao C, Chen A, Jamieson CH, Fereshteh M, Abrahamsson A, Blum J, Kwon HY, Kim J, Chute JP, Rizzieri D, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–779. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Su W, Meng F, Huang L, Zheng M, Liu W, Sun H. Sonic hedgehog maintains survival and growth of chronic myeloid leukemia progenitor cells through beta-catenin signaling. Exp Hematol. 2012;40:418–427. doi: 10.1016/j.exphem.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Kobune M, Iyama S, Kikuchi S, Horiguchi H, Sato T, Murase K, Kawano Y, Takada K, Ono K, Kamihara Y, et al. Stromal cells expressing hedgehog-interacting protein regulate the proliferation of myeloid neoplasms. Blood Cancer J. 2012;2:e87. doi: 10.1038/bcj.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McLean K, Gong Y, Choi Y, Deng N, Yang K, Bai S, Cabrera L, Keller E, McCauley L, Cho KR, et al. Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest. 2011;121:3206–3219. doi: 10.1172/JCI45273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamada S, Masamune A, Takikawa T, Suzuki N, Kikuta K, Hirota M, Hamada H, Kobune M, Satoh K, Shimosegawa T. Pancreatic stellate cells enhance stem cell-like phenotypes in pancreatic cancer cells. Biochem Biophys Res Commun. 2012;421:349–354. doi: 10.1016/j.bbrc.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 56.Lonardo E, Frias-Aldeguer J, Hermann PC, Heeschen C. Pancreatic stellate cells form a niche for cancer stem cells and promote their self-renewal and invasiveness. Cell Cycle. 2012;11:1282–1290. doi: 10.4161/cc.19679. [DOI] [PubMed] [Google Scholar]
- 57.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kirkland SC. Type I collagen inhibits differentiation and promotes a stem cell-like phenotype in human colorectal carcinoma cells. Br J Cancer. 2009;101:320–326. doi: 10.1038/sj.bjc.6605143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P, Huber M, Hohl D, Cano A, Birchmeier W, et al. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature. 2008;452:650–653. doi: 10.1038/nature06835. [DOI] [PubMed] [Google Scholar]
- 61.Chau WK, Ip CK, Mak AS, Lai HC, Wong AS. c-Kit mediates chemoresistance and tumor-initiating capacity of ovarian cancer cells through activation of Wnt/beta-catenin-ATP-binding cassette G2 signaling. Oncogene. 2013;32:2767–2781. doi: 10.1038/onc.2012.290. [DOI] [PubMed] [Google Scholar]
- 62.Storci G, Bertoni S, De Carolis S, Papi A, Nati M, Ceccarelli C, Pirazzini C, Garagnani P, Ferrarini A, Buson G, et al. Slug/beta-Catenin-Dependent Proinflammatory Phenotype in Hypoxic Breast Cancer Stem Cells. Am J Pathol. 2013;183:1688–1697. doi: 10.1016/j.ajpath.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 63.De Bacco F, Casanova E, Medico E, Pellegatta S, Orzan F, Albano R, Luraghi P, Reato G, D'Ambrosio A, Porrati P, et al. The MET oncogene is a functional marker of a glioblastoma stem cell subtype. Cancer Res. 2012;72:4537–4550. doi: 10.1158/0008-5472.CAN-11-3490. [DOI] [PubMed] [Google Scholar]
- 64.van Leenders GJ, Sookhlall R, Teubel WJ, de Ridder CM, Reneman S, Sacchetti A, Vissers KJ, van Weerden W, Jenster G. Activation of c-MET induces a stem-like phenotype in human prostate cancer. PLoS One. 2011;6:e26753. doi: 10.1371/journal.pone.0026753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y, Li A, Glas M, Lal B, Ying M, Sang Y, Xia S, Trageser D, Guerrero-Cazares H, Eberhart CG, et al. c-Met signaling induces a reprogramming network and supports the glioblastoma stem-like phenotype. Proc Natl Acad Sci U S A. 2011;108:9951–9956. doi: 10.1073/pnas.1016912108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yao D, Dai C, Peng S. Mechanism of the mesenchymal-epithelial transition and its relationship with metastatic tumor formation. Mol Cancer Res. 2011;9:1608–1620. doi: 10.1158/1541-7786.MCR-10-0568. [DOI] [PubMed] [Google Scholar]
- 67.Hasegawa T, Yashiro M, Nishii T, Matsuoka J, Fuyuhiro Y, Morisaki T, Fukuoka T, Shimizu K, Shimizu T, Miwa A, et al. Cancer-associated fibroblasts might sustain the stemness of scirrhous gastric cancer cells via transforming growth factor-beta signaling. Int J Cancer. 2013 doi: 10.1002/ijc.28520. [DOI] [PubMed] [Google Scholar]
- 68.Kolenda J, Jensen SS, Aaberg-Jessen C, Christensen K, Andersen C, Brunner N, Kristensen BW. Effects of hypoxia on expression of a panel of stem cell and chemoresistance markers in glioblastoma-derived spheroids. J Neurooncol. 2011;103:43–58. doi: 10.1007/s11060-010-0357-8. [DOI] [PubMed] [Google Scholar]
- 69.Liang D, Ma Y, Liu J, Trope CG, Holm R, Nesland JM, Suo Z. The hypoxic microenvironment upgrades stem-like properties of ovarian cancer cells. BMC Cancer. 2012;12:201. doi: 10.1186/1471-2407-12-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blazek ER, Foutch JL, Maki G. Daoy medulloblastoma cells that express CD133 are radioresistant relative to CD133- cells, and the CD133+ sector is enlarged by hypoxia. Int J Radiat Oncol Biol Phys. 2007;67:1–5. doi: 10.1016/j.ijrobp.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 71.Das B, Tsuchida R, Malkin D, Koren G, Baruchel S, Yeger H. Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells. 2008;26:1818–1830. doi: 10.1634/stemcells.2007-0724. [DOI] [PubMed] [Google Scholar]
- 72.Mathieu J, Zhang Z, Zhou W, Wang AJ, Heddleston JM, Pinna CM, Hubaud A, Stadler B, Choi M, Bar M, et al. HIF induces human embryonic stem cell markers in cancer cells. Cancer Res. 2011;71:4640–4652. doi: 10.1158/0008-5472.CAN-10-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Knoepfler PS. Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells. 2009;27:1050–1056. doi: 10.1002/stem.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74•.Nishi M, Sakai Y, Akutsu H, Nagashima Y, Quinn G, Masui S, Kimura H, Perrem K, Umezawa A, Yamamoto N, et al. Induction of cells with cancer stem cell properties from nontumorigenic human mammary epithelial cells by defined reprogramming factors. Oncogene. 2013 doi: 10.1038/onc.2012.614. Induced pluripotency and intra-tumor dedifferentiation/EMT share many features IPSC methods may unravel epigenetic mechanisms for acquisition of CSC features. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75•.Corominas-Faja B, Cufi S, Oliveras-Ferraros C, Cuyas E, Lopez-Bonet E, Lupu R, Alarcon T, Vellon L, Iglesias JM, Leis O, et al. Nuclear reprogramming of luminal-like breast cancer cells generates Sox2-overexpressing cancer stem-like cellular states harboring transcriptional activation of the mTOR pathway. Cell Cycle. 2013;12:3109–3124. doi: 10.4161/cc.26173. Another recent study exploiting cellular reprogramming to investigate the acquisition of CSC features by progenitor populations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5:237–241. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 77•.Park TS, Huo JS, Peters A, Talbot CC, Jr, Verma K, Zimmerlin L, Kaplan IM, Zambidis ET. Growth factor-activated stem cell circuits and stromal signals cooperatively accelerate non-integrated iPSC reprogramming of human myeloid progenitors. PLoS One. 2012;7:e42838. doi: 10.1371/journal.pone.0042838. Hypoxia and stromal/inflammatory signals dramatically enhance cellular reprogramming (dedifferentiation) of hematopoietic progenitors to reprogramming. The elucidation of the mechanisms invovled during iPSC reprogramming may offer new insights in CSC emergence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsuyada A, Chow A, Wu J, Somlo G, Chu P, Loera S, Luu T, Li AX, Wu X, Ye W, et al. CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Res. 2012;72:2768–2779. doi: 10.1158/0008-5472.CAN-11-3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, Korkaya H, Heath A, Dutcher J, Kleer CG, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71:614–624. doi: 10.1158/0008-5472.CAN-10-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li HJ, Reinhardt F, Herschman HR, Weinberg RA. Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signaling. Cancer Discov. 2012;2:840–855. doi: 10.1158/2159-8290.CD-12-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 82.Folkins C, Shaked Y, Man S, Tang T, Lee CR, Zhu Z, Hoffman RM, Kerbel RS. Glioma tumor stem-like cells promote tumor angiogenesis and vasculogenesis via vascular endothelial growth factor and stromal-derived factor 1. Cancer Res. 2009;69:7243–7251. doi: 10.1158/0008-5472.CAN-09-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gilbertson RJ, Rich JN. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 84.Lathia JD, Gallagher J, Heddleston JM, Wang J, Eyler CE, Macswords J, Wu Q, Vasanji A, McLendon RE, Hjelmeland AB, et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6:421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Charles N, Ozawa T, Squatrito M, Bleau AM, Brennan CW, Hambardzumyan D, Holland EC. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 2010;6:141–152. doi: 10.1016/j.stem.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yao XH, Ping YF, Chen JH, Xu CP, Chen DL, Zhang R, Wang JM, Bian XW. Glioblastoma stem cells produce vascular endothelial growth factor by activation of a G-protein coupled formylpeptide receptor FPR. J Pathol. 2008;215:369–376. doi: 10.1002/path.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ezhilarasan R, Mohanam I, Govindarajan K, Mohanam S. Glioma cells suppress hypoxia-induced endothelial cell apoptosis and promote the angiogenic process. Int J Oncol. 2007;30:701–707. [PMC free article] [PubMed] [Google Scholar]
- 88.Brown CK, Khodarev NN, Yu J, Moo-Young T, Labay E, Darga TE, Posner MC, Weichselbaum RR, Mauceri HJ. Glioblastoma cells block radiation-induced programmed cell death of endothelial cells. FEBS Lett. 2004;565:167–170. doi: 10.1016/j.febslet.2004.03.099. [DOI] [PubMed] [Google Scholar]
- 89•.Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A, Sotiropoulou PA, Loges S, Lapouge G, Candi A, et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478:399–403. doi: 10.1038/nature10525. Self-supporting vascular niche of epidermal CSCs. [DOI] [PubMed] [Google Scholar]
- 90•.Hamerlik P, Lathia JD, Rasmussen R, Wu Q, Bartkova J, Lee M, Moudry P, Bartek J, Jr, Fischer W, Lukas J, et al. Autocrine VEGF-VEGFR2-Neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J Exp Med. 2012;209:507–520. doi: 10.1084/jem.20111424. Self-supporting vascular niche of glioma CSCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G, Larocca LM, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 92.Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C, Tabar V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 93.Fan YL, Zheng M, Tang YL, Liang XH. A new perspective of vasculogenic mimicry: EMT and cancer stem cells (Review) Oncol Lett. 2013;6:1174–1180. doi: 10.3892/ol.2013.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB, Logsdon CD. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peek EM, Li DR, Zhang H, Kim HP, Zhang B, Garraway IP, Chin AI. Stromal modulation of bladder cancer-initiating cells in a subcutaneous tumor model. Am J Cancer Res. 2012;2:745–751. [PMC free article] [PubMed] [Google Scholar]
- 96.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 97.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hung JJ, Yang MH, Hsu HS, Hsu WH, Liu JS, Wu KJ. Prognostic significance of hypoxia-inducible factor-1alpha, TWIST1 and Snail expression in resectable non-small cell lung cancer. Thorax. 2009;64:1082–1089. doi: 10.1136/thx.2009.115691. [DOI] [PubMed] [Google Scholar]
- 99.Cannito S, Novo E, Compagnone A, Valfre di Bonzo L, Busletta C, Zamara E, Paternostro C, Povero D, Bandino A, Bozzo F, et al. Redox mechanisms switch on hypoxia-dependent epithelial-mesenchymal transition in cancer cells. Carcinogenesis. 2008;29:2267–2278. doi: 10.1093/carcin/bgn216. [DOI] [PubMed] [Google Scholar]
- 100.Donnenberg AD, Zimmerlin L, Landreneau RJ, Luketich JD, Donnenberg VS. KIT (CD117) expression in a subset of non-small cell lung carcinoma (NSCLC) patients. PLoS One. 2012;7:1–11. doi: 10.1371/journal.pone.0052885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van de Stolpe A. On the origin and destination of cancer stem cells: a conceptual evaluation. Am J Cancer Res. 2013;3:107–116. [PMC free article] [PubMed] [Google Scholar]
- 102.Martinez LM, Vallone VB, Labovsky V, Choi H, Hofer EL, Feldman L, Bordenave RH, Batagelj E, Dimase F, Villafane AR, et al. Changes in the peripheral blood and bone marrow from untreated advanced breast cancer patients that are associated with the establishment of bone metastases. Clin Exp Metastasis. 2013 doi: 10.1007/s10585-013-9622-5. [DOI] [PubMed] [Google Scholar]
- 103.Monteiro AC, Leal AC, Goncalves-Silva T, Mercadante AC, Kestelman F, Chaves SB, Azevedo RB, Monteiro JP, Bonomo A. T cells induce pre-metastatic osteolytic disease and help bone metastases establishment in a mouse model of metastatic breast cancer. PLoS One. 2013;8:e68171. doi: 10.1371/journal.pone.0068171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mitsui H, Shibata K, Suzuki S, Umezu T, Mizuno M, Kajiyama H, Kikkawa F. Functional interaction between peritoneal mesothelial cells and stem cells of ovarian yolk sac tumor (SC-OYST) in peritoneal dissemination. Gynecol Oncol. 2012;124:303–310. doi: 10.1016/j.ygyno.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 105•.Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–89. doi: 10.1038/nature10694. Stroma-CSC bidirectional crosstalk governs establishment of the metastatic niche. [DOI] [PubMed] [Google Scholar]
- 106.Xu Z, Vonlaufen A, Phillips PA, Fiala-Beer E, Zhang X, Yang L, Biankin AV, Goldstein D, Pirola RC, Wilson JS, et al. Role of pancreatic stellate cells in pancreatic cancer metastasis. Am J Pathol. 2010;177:2585–2596. doi: 10.2353/ajpath.2010.090899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kleffel S, Schatton T. Tumor dormancy and cancer stem cells: two sides of the same coin? Adv Exp Med Biol. 2013;734:145–179. doi: 10.1007/978-1-4614-1445-2_8. [DOI] [PubMed] [Google Scholar]
- 108.Bragado P, Sosa MS, Keely P, Condeelis J, Aguirre-Ghiso JA. Microenvironments dictating tumor cell dormancy. Recent Results Cancer Res. 2012;195:25–39. doi: 10.1007/978-3-642-28160-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marlow R, Honeth G, Lombardi S, Cariati M, Hessey S, Pipili A, Mariotti V, Buchupalli B, Foster K, Bonnet D, et al. A Novel Model of Dormancy for Bone Metastatic Breast Cancer Cells. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-13-0991. [DOI] [PubMed] [Google Scholar]
- 110.Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99:1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 111.Folkins C, Man S, Xu P, Shaked Y, Hicklin DJ, Kerbel RS. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67:3560–3564. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
- 112.Hovinga KE, Shimizu F, Wang R, Panagiotakos G, Van Der Heijden M, Moayedpardazi H, Correia AS, Soulet D, Major T, Menon J, et al. Inhibition of notch signaling in glioblastoma targets cancer stem cells via an endothelial cell intermediate. Stem Cells. 2010;28:1019–1029. doi: 10.1002/stem.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin SP, Lee YT, Yang SH, Miller SA, Chiou SH, Hung MC, Hung SC. Colon cancer stem cells resist antiangiogenesis therapy-induced apoptosis. Cancer Lett. 2013;328:226–234. doi: 10.1016/j.canlet.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 115.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Loeffler M, Kruger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest. 2006;116:1955–1962. doi: 10.1172/JCI26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van der Horst EH, Chinn L, Wang M, Velilla T, Tran H, Madrona Y, Lam A, Ji M, Hoey TC, Sato AK. Discovery of fully human anti-MET monoclonal antibodies with antitumor activity against colon cancer tumor models in vivo. Neoplasia. 2009;11:355–364. doi: 10.1593/neo.81536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kawaguchi A, Orba Y, Kimura T, Iha H, Ogata M, Tsuji T, Ainai A, Sata T, Okamoto T, Hall WW, et al. Inhibition of the SDF-1alpha-CXCR4 axis by the CXCR4 antagonist AMD3100 suppresses the migration of cultured cells from ATL patients and murine lymphoblastoid cells from HTLV-I Tax transgenic mice. Blood. 2009;114:2961–2968. doi: 10.1182/blood-2008-11-189308. [DOI] [PubMed] [Google Scholar]
- 119.O'Callaghan K, Lee L, Nguyen N, Hsieh MY, Kaneider NC, Klein AK, Sprague K, Van Etten RA, Kuliopulos A, Covic L. Targeting CXCR4 with cell-penetrating pepducins in lymphoma and lymphocytic leukemia. Blood. 2012;119:1717–1725. doi: 10.1182/blood-2011-04-347518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang C, Cui GH, Liu F, Wu QL, Chen Y. Inhibitory effect of triptolide on lymph node metastasis in patients with non-Hodgkin lymphoma by regulating SDF-1/CXCR4 axis in vitro. Acta Pharmacol Sin. 2006;27:1438–1446. doi: 10.1111/j.1745-7254.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 121.Margolin DA, Silinsky J, Grimes C, Spencer N, Aycock M, Green H, Cordova J, Davis NK, Driscoll T, Li L. Lymph node stromal cells enhance drug-resistant colon cancer cell tumor formation through SDF-1alpha/CXCR4 paracrine signaling. Neoplasia. 2011;13:874–886. doi: 10.1593/neo.11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim M, Koh YJ, Kim KE, Koh BI, Nam DH, Alitalo K, Kim I, Koh GY. CXCR4 signaling regulates metastasis of chemoresistant melanoma cells by a lymphatic metastatic niche. Cancer Res. 2010;70:10411–10421. doi: 10.1158/0008-5472.CAN-10-2591. [DOI] [PubMed] [Google Scholar]
- 123.Annabi B, Rojas-Sutterlin S, Laflamme C, Lachambre MP, Rolland Y, Sartelet H, Beliveau R. Tumor environment dictates medulloblastoma cancer stem cell expression and invasive phenotype. Mol Cancer Res. 2008;6:907–916. doi: 10.1158/1541-7786.MCR-07-2184. [DOI] [PubMed] [Google Scholar]
- 124.Wurth R, Pattarozzi A, Gatti M, Bajetto A, Corsaro A, Parodi A, Sirito R, Massollo M, Marini C, Zona G, et al. Metformin selectively affects human glioblastoma tumor-initiating cell viability: A role for metformin-induced inhibition of Akt. Cell Cycle. 2013;12:145–156. doi: 10.4161/cc.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shank JJ, Yang K, Ghannam J, Cabrera L, Johnston CJ, Reynolds RK, Buckanovich RJ. Metformin targets ovarian cancer stem cells in vitro and in vivo. Gynecol Oncol. 2012;127:390–397. doi: 10.1016/j.ygyno.2012.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lonardo E, Cioffi M, Sancho P, Sanchez-Ripoll Y, Trabulo SM, Dorado J, Balic A, Hidalgo M, Heeschen C. Metformin targets the metabolic achilles heel of human pancreatic cancer stem cells. PLoS One. 2013;8:e76518. doi: 10.1371/journal.pone.0076518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]


