Abstract
Background
Selective serotonin reuptake inhibitors (SSRIs) are often prescribed in patients with postural tachycardia syndrome (POTS), and act at synaptic terminals to increase monoamine neurotransmitters. We hypothesized that they act to increase blood pressure (BP) and attenuate reflex tachycardia, thereby improving symptoms. Acute hemodynamic profiles after SSRI administration in POTS patients have not previously been reported.
Methods
Patients with POTS (n=39; F=37, 39 ±9 years) underwent a randomized crossover trial with sertraline 50mg and placebo. Heart rate (HR), systolic, diastolic, and mean BP were measured with the patient seated and standing for 10 minutes prior to drug or placebo administration, and then hourly for 4 hours. The primary endpoint was standing HR at 4 hours.
Results
At 4 hours, standing HR and systolic BP were not significantly different between sertraline and placebo. Seated systolic (106±12 mmHg vs. 101±8 mmHg; P=0.041), diastolic (72±8 mmHg vs. 69±8 mmHg; P=0.022), and mean BP (86±9 mmHg vs. 81±9 mmHg; P=0.007) were significantly higher after sertraline administration than placebo. At 4 hours, symptoms were worse with sertraline than placebo.
Conclusions
Sertraline had a modest pressor effect in POTS patients, but this did not translate into a reduced HR or improved symptoms.
Keywords/MeSH: SSRI, sertraline, postural tachycardia syndrome, blood pressure, heart rate
Introduction
Postural tachycardia syndrome (POTS) is a chronic dysautonomia syndrome comprising symptoms of inadequate cerebral perfusion, dizziness, palpitations, altered mentation, fatigue, and sometimes syncope, when transitioning to an upright position (Robertson, 1999). The hallmark physiological feature is excessive orthostatic tachycardia. This condition is estimated to affect roughly half a million Americans, targeting mostly young women with a 4:1 ratio (Raj, 2006; Robertson, 1999).
Selective serotonin reuptake inhibitors (SSRIs) are drugs that are widely used for a number of psychiatric disorders. They work by inhibiting presynaptic monoamine reabsorption, thereby increasing monoamine neurotransmitters at the synaptic cleft, with resultant increased neurotransmission. While SSRIs primarily affect serotonin, other monoamine neurotransmitters such as norepinephrine and dopamine are also affected (Wong et al., 1993). Sertraline is a rapidly absorbed SSRI that has been shown in randomized placebo-controlled studies to be safe and efficacious in treating several psychiatric conditions, including major depressive disorder (Keller et al., 1998), obsessive compulsive disorder (Kronig et al., 1999), panic disorder (Londborg et al., 1998), post-traumatic stress disorder (Davidson et al., 2001), and premenstrual dysphoric disorder (Yonkers et al., 1997).
Sertraline has also been shown to be useful in the treatment of vasovagal syncope (Grubb et al., 1994). Perhaps due to the overlapping symptoms between vasovagal syncope and POTS, some experts have also recommended the use of sertraline for the treatment of POTS (Grubb, 2008; Kanjwal et al., 2011). The mechanism through which sertraline might benefit POTS patients is unclear. One possibility is that SSRIs could increase standing blood pressure (BP) and consequently decrease reflex tachycardia through inhibition of catecholamine reuptake (Goodnick and Goldstein, 1998a). Chronically administered sertraline did not produce significant hemodynamic changes in depressed patients (Scalco et al., 2009). There have been no studies that have evaluated the acute hemodynamic effects in patients with POTS. Conversely, short-term sertraline administration acutely suppressed sympathetic activity in healthy subjects (Stewart and Weldon, 2000). We tested the hypothesis that sertraline will induce a pressor response in patients with POTS, resulting in increased blood pressure, reduced reflex tachycardia and improved patient symptoms.
Methods
Subjects
Patients with POTS referred to the Vanderbilt University Autonomic Dysfunction Center between September 2007 and August 2011 were candidates for inclusion in this study. Patients met criteria for POTS in that they developed symptoms of orthostatic intolerance accompanied by a HR rise of >30 bpm (beats per minute) within 10 minutes of standing in the absence of orthostatic hypotension (a fall in blood pressure BP of >20/10 mmHg) (Raj et al., 2005a; Raj, 2006; Schondorf and Low, 1993). All patients had at least a 6-month history of symptoms in the absence of an additional chronic disorder known to cause orthostatic intolerance and in the absence of prolonged bed rest. All patients were at least 18 years old. The Vanderbilt University Investigational Review Board approved this study. Written informed consent was obtained from each subject before initiating the study. The data reported are a part of “The Treatment of Orthostatic Intolerance” study, which is registered with http://www.clinicaltrials.gov (NCT00262470).
Study Diet and Posture Study
Study investigations were performed in the Elliot V. Newman Clinical Research Center at Vanderbilt University. For at least 3 days before testing, subjects consumed a caffeine-free diet containing 150 mEq/day sodium and 60–80 mEq/day potassium. Long-term medications were discontinued 5 half-life periods before the study. Heart rate (HR), systolic BP (SBP), diastolic BP (DBP), and fractionated plasma catecholamines were measured after overnight rest with the patient in the supine position and again after standing for up to 30 minutes (as tolerated) as part of the posture study. For catecholamine measurements, blood was collected in plastic syringes, immediately transferred to chilled vacuum tubes with sodium heparin (BD, Franklin Lakes, NJ), and placed on ice. Plasma was separated by centrifugation at −4°C and stored at −70°C in collection tubes with 6% reduced glutathione (Sigma-Aldrich Inc, St Louis, Mo) until the assay was performed. Concentrations of norepinephrine and epinephrine were measured by batch alumina extraction followed by high-performance liquid chromatography for separation with electrochemical detection and quantification (Jacob et al., 1997).
Medication Trials
These “proof-of-concept” drug trials were started in the morning at least 2 hours after an early, light breakfast (to avoid acute hemodynamic effects from eating) in a post-void state. Sertraline is a rapidly absorbed SSRI with peak concentrations at 4 hours with a 50mg dose (Park et al., 2011). In this trial, patients with POTS were given sertraline hydrochloride 50mg (American Health Packaging, Columbus, OH) and placebo (“Cebocaps”; Forest Pharmaceuticals, New York, NY) in a randomized crossover fashion on separate days. The patients were seated in a chair during the data collection except during prescribed periods of standing. Seated rather than supine position was used in this study as this is the more clinically relevant posture for the patient. BP and HR were measured with an automated arm cuff vital signs monitor (Dinamap Vital Signs Monitor; Critikon Company, Tampa, FL) and digitally acquired into a custom-designed database (Microsoft Access, Microsoft Corporation, Redmond, WA). At time zero and hourly for 4 hours after study drug administration, each patient was asked to stand for 10 minutes while standing HR and BP were recorded. The study was double-blinded, with the patient and the principal investigator blinded. Only the nurse administering the study drug was aware of its contents.
Symptoms
Patients were asked to self-report their symptom burden immediately before and at 2 and 4 hours after study drug administration by using the Vanderbilt Orthostatic Symptom Score (Raj et al., 2005b). The patients were asked to rate the severity of 9 symptoms on a scale of 0 to 10 (with 0 reflecting an absence of symptoms). The sum of the scores at each time point was used as a measure of symptom burden (lower score reflects reduced symptom burden). The 9 symptoms were mental clouding, blurred vision, shortness of breath, rapid heartbeat, tremulousness, chest discomfort, headache, lightheadedness, and nausea. This symptom score has been used previously by our center (Coffin et al., 2012; Raj et al., 2005b; Raj et al., 2009a). These symptoms were chosen because they reflect common complaints of patients with POTS.
Statistical Analysis
Our primary end point was the standing HR 4 hours after study drug administration. The 4-hour time point was chosen because the peak plasma concentration of sertraline occurs 4 hours after dosing (Park et al., 2011). The null hypothesis was that the standing HR would not be statistically different between sertraline and placebo. The primary statistical analysis involved a paired t-test that compared the standing HR at 4 hours after study drug administration between sertraline and placebo. Secondary analyses were performed with paired t-tests to compare the standing SBP and standing HR at the other hourly time points after study drug administration, the seated HR, delta HR (standing minus sitting), and BP values at each time point. Repeated-measures analysis of variance (ANOVA) was used to compare HR, SBP, and symptom score over time for the sertraline and placebo days; the Greenhouse-Geisser correction to the degrees of freedom from these analyses was used to adjust for failures of the sphericity assumption. Subgroup analysis of the primary outcome was performed based on posture study standing HR ≥ 120 bpm or not and standing norepinephrine levels ≥ 600 pg/mL. (3.54 nmol/L) or not. Our study had a power of 80% to detect a decrease in standing HR of 6.5 bpm with a standard deviation for the difference of 14, based on conservative estimates from our previous studies in our main study population (Raj et al., 2009a).
Values are reported as means and standard deviations unless otherwise noted. Probability values ≤ 0.05 were considered statistically significant for the ANOVA, but a threshold of ≤ 0.25 was used for individual 2h and 4h paired tests due to the multiple comparisons. All tests were 2-tailed. Statistical analyses were performed with SPSS for Windows (version 20.0, IBM Corporation, Armonk, NY). Prism for Windows 5 (version 5.02, GraphPad Software, Inc, La Jolla, CA) was used for graphical presentation.
Results
Baseline Data and Demographics
Study inclusion criteria were met by 39 patients with POTS (37 female; age 31±9 years). The data from the demographics and posture study are presented in Table 1. Upon standing from a supine position, the HR increased significantly from 74±11 bpm to 118±26 bpm (P<0.001), without a significant decrease in BP, consistent with the diagnosis of POTS. The mean supine plasma norepinephrine and epinephrine values were within the normal range (norepinephrine <475 nmol/mL and epinephrine <75 nmol/mL). Both plasma norepinephrine (1.46±1.53 nmol/ml vs. 4.57±3.09 nmol/mL; P<0.001) and plasma epinephrine (0.10±0.08 nmol/ml vs. 0.41±0.41 nmol/ml; P=0.040) increased significantly with standing. There were 19 patients with an excessively high standing HR (≥120 bpm), and there were 19 patients with standing norepinephrine ≥600 pg/ml.
Table 1.
Demographics and Posture Study.
| Total Subjects (n) | 39 |
| Woman, (n) | 37 (95%) |
| Age (years) | 39 ± 9 |
| Supine | |
| Heart Rate (beats/min) | 74 ± 11 |
| Systolic Blood Pressure (mmHg) | 106 ± 12 |
| Diastolic Blood Pressure (mmHg) | 65 ± 8 |
| Norepinephrine (nmol/mL) | 1.46 ± 1.53 |
| Epinephrine (nmol/mL) | 0.10 ± 0.08 |
| Standing | |
| Heart Rate (beats/min) | 118 ± 26 * |
| Systolic Blood Pressure (mmHg) | 103 ± 22 |
| Diastolic Blood Pressure (mmHg) | 69 ± 16 |
| Norepinephrine (nmol/mL) | 4.57 ± 3.09 * |
| Epinephrine (nmol/mL) | 0.41 ± 0.41 * |
| Change from Supine to Standing | |
| Heart Rate (beats/min) | 45 ± 23 |
| Systolic Blood Pressure (mmHg) | −3 ± 22 |
| Diastolic Blood Pressure (mmHg) | 4 ± 15 |
| Norepinephrine (nmol/mL) | 3.08 ± 2.92 |
| Epinephrine (nmol/mL) | 0.32 ± 0.43 |
Data are presented as mean ± standard deviation. Reported P values are for paired t-test comparing supine and upright parameters.
P < 0.001
Seated and Standing Heart Rate
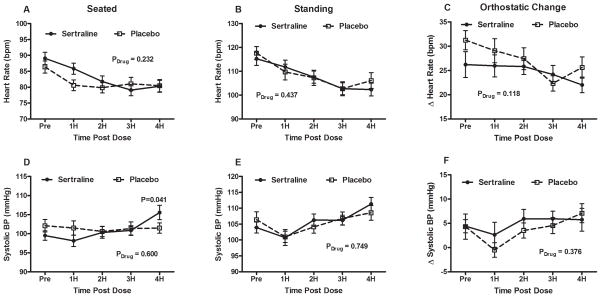
All 39 subjects underwent the paired administration of placebo and sertraline on different random days. The data for the drug trials are presented in Table 2. Immediately before administration of the study drug, there was no difference in seated HR between sertraline and placebo (89±12 bpm vs. 86±12 bpm; P=0.170). As seen in Figure 1(a), the seated HR decreased over time for both drugs, but sertraline did not decrease the seated HR significantly more than placebo over time (Pdrug= 0.232).
Table 2.
Orthostatic hemodynamics and symptoms with sertraline 50mg and placebo in patients with Postural Tachycardia Syndrome (n=39).
| Pre | 2 hour | 4 Hours | |
|---|---|---|---|
| Seated HR (beats/min) | |||
| Sertraline | 89±12 | 82±11 | 80±12 |
| Placebo | 86±12 | 80±10 | 80±12 |
| P value (between drugs) | 0.165 | 0.166 | 0.912 |
| Standing HR (beats/min) | |||
| Sertraline | 115±17 | 108±16 | 102±17 |
| Placebo | 117±17 | 107±20 | 106±21 |
| P value (between drugs) | 0.312 | 0.913 | 0.167 |
| Delta (Standing-Seated) HR (beats/min) | |||
| Sertraline | 26±17 | 26±10 | 22±10 |
| Placebo | 31±12 | 27±14 | 25±14 |
| P value (between drugs) | 0.076 | 0.443 | 0.13 |
| Seated SBP (mmHg) | |||
| Sertraline | 100±8 | 100±10 | 106±12 |
| Placebo | 102±10 | 101±8 | 101±8 |
| P value (between drugs) | 0.086 | 0.87 | *0.041 |
| Standing SBP (mmHg) | |||
| Sertraline | 104±11 | 106±12 | 111±13 |
| Placebo | 106±15 | 104±12 | 109±15 |
| P value (between drugs) | 0.397 | 0.277 | 0.244 |
| Delta (Standing-Seated) SBP (mmHg) | |||
| Sertraline | 4±9 | 6±12 | 6±15 |
| Placebo | 4±16 | 4±10 | 7±12 |
| P value (between drugs) | 0.076 | 0.443 | 0.13 |
| Symptom Score (au) [n=35] | |||
| Sertraline | 16±13 | 15±14 | 16±15 |
| Placebo | 20±18 | 14±13 | 13±11 |
| P value (between drugs) | 0.052 | 0.965 | 0.188 |
Repeated - measures analysis of variance (RM ANOVA) was used to determine the p value for the overall change between sertraline and placebo. The P values are reported for the drug effect (Pdrug; sertraline vs. placebo). P-values were calculated using a 2-tailed paired t-test. Delta values were calculated as the standing – seated values. Data are presented as mean ± standard deviation.
P<0.05 was considered significant.
HR: heart rate; SBP: systolic blood pressure; au: arbitrary unit.
Figure 1. Heart rate and systolic blood pressure profiles of placebo and sertraline groups.
(A) Seated, (B) standing, and (C) orthostatic ΔHR in profiles for placebo vs. sertraline are presented with HR measurements at baseline and at hour intervals for 4 hours after administration. Solid circles represent sertraline values while open boxes represent placebo values. (D) Seated, (E) standing, and (F) orthostatic changes in SBP profiles for placebo vs. sertraline are also presented with BP measurements at baseline and at hour intervals for 4 hours after administration. Repeated measures analysis of variance P values are also presented for the overall effect of the study drug over time (Pdrug).
BPM: beats per minute; ΔHR changes in heart rate; SBP: systolic blood pressure; BP: blood pressure.
The standing HR (Figure 1(b)) before study drug administration was not significantly different between sertraline and placebo (115±18 bpm vs. 117±17 bpm; P=0.310). There was a significant decrease in standing HR over the 4 hour period across both groups (Ptime < 0.001), but no difference between sertraline and placebo over time (ANOVA Pdrug = 0.437). The standing HR at 4 hours post administration was no different between the sertraline group and the placebo group (102±17 bpm vs. 106±20 bpm; P=0.167).
The orthostatic tachycardia (Figure 1(c)), which is a cardinal feature of POTS, was similar at baseline between the sertraline and placebo groups (26±17 bpm vs. 31±12 bpm; P=0.076), and was not different after 4 hours (22±10 bpm vs. 26±14 bpm; P=0.130).
Seated and Standing Blood Pressure
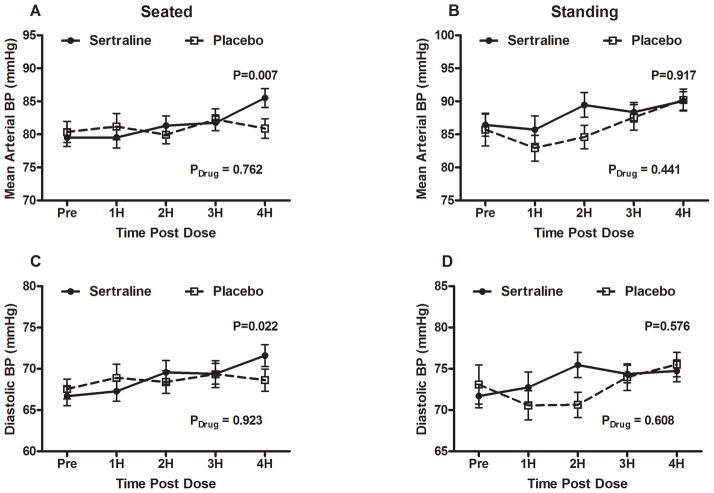
There were no differences between the sertraline and placebo days in seated SBP, seated DBP, or seated mean arterial BP (MAP) at baseline prior to the study. By 4 hours, there was a trend toward higher BP in the sertraline group than placebo for seated MAP (86±9 mmHg vs. 81±9 mmHg; P=0.030; Figure 2(a)), seated SBP (106±12 mmHg vs. 101±8 mmHg; P=0.041; Figure 1(d)), and seated DBP (72±8 mmHg vs. 69±8 mmHg; P=0.022; Figure 2(d)). The seated SBP over time was not significantly different between sertraline and placebo (Pdrug=0.600).
Figure 2. Mean arterial blood pressure and diastolic blood pressure profiles of placebo and sertraline groups.
(A) Seated MAP, (B) standing MAP, (C) seated DBP, and (D) standing DBP profiles for placebo vs. sertraline 50mg are presented with BP measurements at baseline and at hour intervals for 4 hours after administration. Solid circles represent sertraline values while open boxes represent placebo values. P-value of paired samples t-test (2 tailed) for blood pressures at 4 hours between placebo and sertraline are shown. Repeated measures analysis of variance P values are also presented for the overall effect of the study drug over time (Pdrug).
MAP: mean arterial pressure; DBP: diastolic blood pressure
Baseline standing BP parameters were similar for sertraline and placebo. At 4 hours post study drug, BP parameters were not different between the two groups. At 2 hours, however, the standing MAP (111±13 mmHg vs. 89±11 mmHg; P=0.022) and the standing DBP (75±9 mmHg vs. 70±10 mmHg; P=0.003) was significantly higher on the sertraline day compared to the placebo day.
Orthostatic SBP between the sertraline and placebo groups was not significantly different at baseline (4±9 mmHg vs. 4±16 mmHg; P=0.080), or at 4 hours, (6±15 mmHg vs. 7±12 mmHg; P=0.130).
Subgroup Analysis
There were no significant differences in the standing SBP, DBP, and MAP, standing HR, or the orthostatic tachycardia between sertraline and placebo in any of the subgroups of patients (based on standing HR above or below 120 bpm or standing norepinephrine levels above or below 600 pg/ml.
Symptoms
The symptom scores were completed for both placebo and sertraline days by 34 patients with POTS. The change in symptom score from baseline to 4 hours was significantly different between the 2 days (Figure 3), with little change in the sertraline group while the placebo group reported improved symptoms (0.25 ± 13 AU vs. −7.69 ± 12 AU; P=0.010).
Figure 3. Symptom profiles at baseline between placebo and sertraline groups.
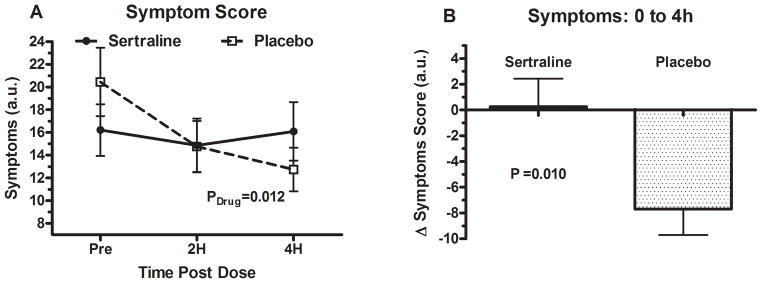
Panel (A) shows the symptom scores at baseline and then at 2 and 4 hours post administration of drug in the placebo and sertraline group. Panel (B) shows changes in symptom scores at 4 hours between placebo and sertraline. The P value was generated by using a paired t-test (2 tailed). Symptoms were recorded using the Vanderbilt Orthostatic Symptom Score (in arbitrary units). Lower scores correlate with lesser disease burden while higher scores correlate with greater disease burden.
AU: arbitrary units
Discussion
Acute Hemodynamics Effects of Sertraline
Sertraline was associated with a trend toward increase in seated BP at 4 hours compared to placebo, suggesting a sertraline induced pressor response, but this did not achieve statistical significance. Sertraline did not significantly elevate the standing SBP 4 hours after its administration compared to placebo, and subsequently, the standing HR at 4 hours was also unchanged (which was our primary study endpoint). Unfortunately, sertraline did not improve symptoms acutely, and may have made symptoms worse.
The dose of sertraline used in this study was 50mg, but doses as high as 200mg/day are commonly used in clinical practice to treat some psychiatric disorders (Goodnick and Goldstein, 1998b). It is possible that higher doses of sertraline could affect beneficial hemodynamic changes in POTS patients. These potential benefits would have to be balanced against the potential increase in adverse effects associated with higher doses of sertraline, and requires further study.
The mechanism by which sertraline produces this pressor effect is unclear. In a study of depressed young males, the administration of serotonin chronically up to six weeks did not increase blood pressure significantly, but it did increase the serum levels of aldosterone and plasma renin activity (Ahmed et al., 2011). As we have shown earlier, individuals with POTS have paradoxically low aldosterone levels given their markedly reduced plasma volumes (Raj et al., 2005a). Therefore, it is plausible that sertraline is exerting its pressor response through the renin-aldosterone pathway, and this effect is more apparent in patients who have a baseline marked reduction in aldosterone levels, such as individuals with POTS.
POTS Symptoms with Sertraline
Standing HR decreased in both groups over time, which can be explained by diurnal variability, with an early morning peak in orthostatic and standing tachycardia (Brewster et al., 2012). Symptoms improved in the placebo group, but did not improve in the sertraline group. Possible explanations for the improvement in symptoms with placebo include diurnal variability or “placebo effect”, but the latter would not explain the less favorable response with sertraline.
Despite a potential pressor response, sertraline does not provide any immediate clinical improvement and may, in fact, worsen symptoms acutely. The reasons for this interesting finding are unclear, but could relate to the side effect profile of this drug class which is known to cause nausea, tremor, and dizziness (Beasley, Jr. et al., 2000).
The patients in our study were exposed to sertraline acutely for four hours. In this acute setting, sertraline does not appear to improve the symptoms of POTS; however the overall clinical benefit of using sertraline long-term in POTS patients is unknown, especially given that many POTS patients have symptoms that resemble anxiety (Raj et al., 2009b). The full therapeutic benefits of SSRIs for psychiatric disorders are not fully realized until after several weeks of daily administration. It has been reported that the neurotransmission of monoamines is further potentiated about 14 days after the initiation of treatment, when desensitization of pre-synaptic serotonin autoreceptors takes place (Goodnick and Goldstein, 1998a). Also, it has been shown that long-term administration of sertraline can blunt the effects of an overactive sympathetic nervous system (Scalco et al., 2009), which is a cardinal feature of POTS (Raj, 2006). Alternatively, there could be delayed potentiation of monoamine transmission with chronic SSRI administration. While the long-term administration of sertraline in a limited number of patients with major depressive disorder has not been shown to change BP (Scalco et al., 2009), the long term administration of sertraline in a small group of hemodialysis patients (n=9) over 6 weeks at doses of 50–100mg per day did improve the MAP (Dheenan et al., 1998). Similar long-term studies have not been performed in POTS patients (Scalco et al., 2009).
Norepinephrine transporter inhibition
Although sertraline selectively blocks serotonin reuptake at nerve terminals, it is also a weak inhibitor of the norepinephrine transporter. The short-term administration of sertraline has been shown to decrease circulating levels of norepinephrine in healthy patients (Shores et al., 2001). In addition, the long-term administration of sertraline has been shown to decrease muscle sympathetic nervous activity in depressed patients without POTS (Scalco et al., 2009). These two observations could explain why the standing BP failed to increase significantly with sertraline. Sertraline has marked affinity for the dopamine transporter (Goodnick and Goldstein, 1998a), and the observed elevation of seated BP might result from increased dopaminergic neurotransmission of adequate magnitude to affect seated but not standing BP.
Selective Serotonin-Norepinephrine vs. Selective Serotonin Reuptake Inhibitors
Serotonin/norepinephrine reuptake inhibitors (SNRIs) are sometimes used in the management of POTS as second-line agents when more conventional medications such as fludrocortisone (Freitas et al., 2000), propranolol (Raj et al., 2009a), or midodrine (Jacob et al., 1997) either fail or cannot be tolerated. High-dose duloxetine can cause healthy volunteers to develop a POTS-like phenotype with increased orthostatic tachycardia after chronic administration that is likely related to norepinephrine transporter inhibition (Vincent et al., 2004). A more selective serotonin reuptake inhibitor like sertraline, with less affinity for the norepinephrine transporter, might be better tolerated by POTS patients. Further studies need to be conducted to determine the utility of sertraline as a long-term agent for POTS.
Limitations
This study has several limitations. The sample size was relatively small, and therefore a small effect, albeit one of questionable clinical significance, may have been missed. Importantly, sertraline did not show a trend to improved symptoms, and was actually worse than placebo. This study used sertraline 50mg, and it is possible that larger doses might have yielded different results. Our patients were studied after a single administration of sertraline and then observed over 4 hours. It is possible that with more prolonged administration, the pressor effects of sertraline would be accentuated and the increase in standing blood pressure would be more evident. Typically, the psychiatric benefits of sertraline take several weeks to emerge. We did not find significant differences in any of our analyzed subgroups, although these analysis were not properly powered, and an effect cannot be excluded without a larger study. The overall clinical benefit of sertraline in POTS cannot be fully elucidated from this acute study focusing primarily on hemodynamic effects. This requires further longer duration studies, and these should ideally incorporate psychological measures.
Conclusion
The acute use of sertraline may be associated with a seated pressor response but did not increase standing BP or significantly reduce HR in POTS patients. Acute sertraline did not improve symptoms in POTS patients. Longer duration studies are needed to exclude a benefit of chronic sertraline therapy in this population.
Acknowledgments
Funding
Supported in part by NIH grants [R01HL102387], [R01 HL071784], [P01 HL56693], and the Clinical and Translational Science Award [UL1 TR000445].
We would like to thank our patients who participated in this project and to recognize the highly professional care provided by the staff of the Elliot V. Newman Clinical Research Center.
Footnotes
Clinical Trials Registration: NCT00262470.
Conflict of Interest Statement
The authors declare that there is no conflict of interest.
References
- Ahmed AH, Calvird M, Gordon RD, Taylor PJ, Ward G, Pimenta E, et al. Effects of two selective serotonin reuptake inhibitor antidepressants, sertraline and escitalopram, on aldosterone/renin ratio in normotensive depressed male patients. J Clin Endocrinol Metab. 2011;96:1039–1045. doi: 10.1210/jc.2010-2603. [DOI] [PubMed] [Google Scholar]
- Beasley CM, Jr, Koke SC, Nilsson ME, Gonzales JS. Adverse events and treatment discontinuations in clinical trials of fluoxetine in major depressive disorder: an updated meta-analysis. Clin Ther. 2000;22:1319–1330. doi: 10.1016/s0149-2918(00)83028-3. [DOI] [PubMed] [Google Scholar]
- Brewster JA, Garland EM, Biaggioni I, Black BK, Ling JF, Shibao CA, et al. Diurnal variability in orthostatic tachycardia: implications for the postural tachycardia syndrome. Clin Sci (Lond) 2012;122:25–31. doi: 10.1042/CS20110077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin ST, Black BK, Biaggioni I, Paranjape SY, Orozco C, Black PW, et al. Desmopressin acutely decreases tachycardia and improves symptoms in the postural tachycardia syndrome. Heart Rhythm. 2012;9:1484–1490. doi: 10.1016/j.hrthm.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JR, Rothbaum BO, van der Kolk BA, Sikes CR, Farfel GM. Multicenter, double-blind comparison of sertraline and placebo in the treatment of posttraumatic stress disorder. Arch Gen Psychiatry. 2001;58:485–492. doi: 10.1001/archpsyc.58.5.485. [DOI] [PubMed] [Google Scholar]
- Dheenan S, Venkatesan J, Grubb BP, Henrich WL. Effect of sertraline hydrochloride on dialysis hypotension. Am J Kidney Dis. 1998;31:624–630. doi: 10.1053/ajkd.1998.v31.pm9531178. [DOI] [PubMed] [Google Scholar]
- Freitas J, Santos R, Azevedo E, Costa O, Carvalho M, de Freitas AF. Clinical improvement in patients with orthostatic intolerance after treatment with bisoprolol and fludrocortisone. Clin Auton Res. 2000;10:293–299. doi: 10.1007/BF02281112. [DOI] [PubMed] [Google Scholar]
- Goodnick PJ, Goldstein BJ. Selective serotonin reuptake inhibitors in affective disorders--I. Basic pharmacology. J Psychopharmacol. 1998a;12:S5–20. doi: 10.1177/0269881198012003021. [DOI] [PubMed] [Google Scholar]
- Goodnick PJ, Goldstein BJ. Selective serotonin reuptake inhibitors in affective disorders--II. Efficacy and quality of life. J Psychopharmacol. 1998b;12:S21–S54. doi: 10.1177/0269881198012003031. [DOI] [PubMed] [Google Scholar]
- Grubb BP. Postural tachycardia syndrome. Circulation. 2008;117:2814–2817. doi: 10.1161/CIRCULATIONAHA.107.761643. [DOI] [PubMed] [Google Scholar]
- Grubb BP, Samoil D, Kosinski D, Kip K, Brewster P. Use of sertraline hydrochloride in the treatment of refractory neurocardiogenic syncope in children and adolescents. J Am Coll Cardiol. 1994;24:490–494. doi: 10.1016/0735-1097(94)90308-5. [DOI] [PubMed] [Google Scholar]
- Jacob G, Shannon JR, Black B, Biaggioni I, Mosqueda-Garcia R, Robertson RM, et al. Effects of volume loading and pressor agents in idiopathic orthostatic tachycardia. Circulation. 1997;96:575–580. doi: 10.1161/01.cir.96.2.575. [DOI] [PubMed] [Google Scholar]
- Kanjwal K, Sheikh M, Karabin B, Kanjwal Y, Grubb BP. Neurocardiogenic syncope coexisting with postural orthostatic tachycardia syndrome in patients suffering from orthostatic intolerance: a combined form of autonomic dysfunction. Pacing Clin Electrophysiol. 2011;34:549–554. doi: 10.1111/j.1540-8159.2010.02994.x. [DOI] [PubMed] [Google Scholar]
- Keller MB, Kocsis JH, Thase ME, Gelenberg AJ, Rush AJ, Koran L, et al. Maintenance phase efficacy of sertraline for chronic depression: a randomized controlled trial. JAMA. 1998;280:1665–1672. doi: 10.1001/jama.280.19.1665. [DOI] [PubMed] [Google Scholar]
- Kronig MH, Apter J, Asnis G, Bystritsky A, Curtis G, Ferguson J, et al. Placebo-controlled, multicenter study of sertraline treatment for obsessive-compulsive disorder. J Clin Psychopharmacol. 1999;19:172–176. doi: 10.1097/00004714-199904000-00013. [DOI] [PubMed] [Google Scholar]
- Londborg PD, Wolkow R, Smith WT, DuBoff E, England D, Ferguson J, et al. Sertraline in the treatment of panic disorder. A multi-site, double-blind, placebo-controlled, fixed-dose investigation. Br J Psychiatry. 1998;173:54–60. doi: 10.1192/bjp.173.1.54. [DOI] [PubMed] [Google Scholar]
- Park MK, Shin KH, Kim KP, Kim TE, Yoon SH, Cho JY, et al. Open label, three period, single sequence, study of 5, 25, 50 mg sertraline pharmacokinetics in healthy male Korean volunteers. Int J Clin Pharmacol Ther. 2011;49:672–678. doi: 10.5414/cp201578. [DOI] [PubMed] [Google Scholar]
- Raj SR. The Postural Tachycardia Syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J. 2006;6:84–99. [PMC free article] [PubMed] [Google Scholar]
- Raj SR, Biaggioni I, Yamhure PC, Black BK, Paranjape SY, Byrne DW, et al. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005a;111:1574–1582. doi: 10.1161/01.CIR.0000160356.97313.5D. [DOI] [PubMed] [Google Scholar]
- Raj SR, Black BK, Biaggioni I, Harris PA, Robertson D. Acetylcholinesterase inhibition improves tachycardia in postural tachycardia syndrome. Circulation. 2005b;111:2734–2740. doi: 10.1161/CIRCULATIONAHA.104.497594. [DOI] [PubMed] [Google Scholar]
- Raj SR, Black BK, Biaggioni I, Paranjape SY, Ramirez M, Dupont WD, et al. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: less is more. Circulation. 2009a;120:725–734. doi: 10.1161/CIRCULATIONAHA.108.846501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj V, Haman KL, Raj SR, Byrne D, Blakely RD, Biaggioni I, et al. Psychiatric profile and attention deficits in postural tachycardia syndrome. J Neurol Neurosurg Psychiatry. 2009b;80:339–344. doi: 10.1136/jnnp.2008.144360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci. 1999;317:75–77. doi: 10.1097/00000441-199902000-00001. [DOI] [PubMed] [Google Scholar]
- Scalco AZ, Rondon MU, Trombetta IC, Laterza MC, Azul JB, Pullenayegum EM, et al. Muscle sympathetic nervous activity in depressed patients before and after treatment with sertraline. J Hypertens. 2009;27:2429–2436. doi: 10.1097/HJH.0b013e3283310ece. [DOI] [PubMed] [Google Scholar]
- Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–137. doi: 10.1212/wnl.43.1_part_1.132. [DOI] [PubMed] [Google Scholar]
- Shores MM, Pascualy M, Lewis NL, Flatness D, Veith RC. Short-term sertraline treatment suppresses sympathetic nervous system activity in healthy human subjects. Psychoneuroendocrinology. 2001;26:433–439. doi: 10.1016/s0306-4530(01)00002-6. [DOI] [PubMed] [Google Scholar]
- Stewart JM, Weldon A. Vascular perturbations in the chronic orthostatic intolerance of the postural orthostatic tachycardia syndrome. J Appl Physiol. 2000;89:1505–1512. doi: 10.1152/jappl.2000.89.4.1505. [DOI] [PubMed] [Google Scholar]
- Vincent S, Bieck PR, Garland EM, Loghin C, Bymaster FP, Black BK, et al. Clinical assessment of norepinephrine transporter blockade through biochemical and pharmacological profiles. Circulation. 2004;109:3202–3207. doi: 10.1161/01.CIR.0000130847.18666.39. [DOI] [PubMed] [Google Scholar]
- Wong DT, Bymaster FP, Reid LR, Mayle DA, Krushinski JH, Robertson DW. Norfluoxetine enantiomers as inhibitors of serotonin uptake in rat brain. Neuropsychopharmacology. 1993;8:337–344. doi: 10.1038/npp.1993.33. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Halbreich U, Freeman E, Brown C, Endicott J, Frank E, et al. Symptomatic improvement of premenstrual dysphoric disorder with sertraline treatment. A randomized controlled trial. Sertraline Premenstrual Dysphoric Collaborative Study Group. JAMA. 1997;278:983–988. [PubMed] [Google Scholar]