Abstract
Irbesartan, a partial agonist of peroxisome proliferators activated receptor-γ (PPARγ), has been reported to improve insulin resistance and lipid profile in patients with diabetes mellitus or metabolic syndrome (MS). However, the down effectors of PPARγ have yet to be elucidated. Thus, in this study, we focused on the role of the hepatocyte growth factor (HGF) in the anti-metabolic effects of irbesartan, using apolipoprotein E (ApoE) knockout (KO) mice. ApoE KO mice placed on a high-fat diet (HFD) for 12 weeks were divided into four groups: i) the control (HFD only), ii) the HFD + irbesartan (5 mg/kg/day), iii) the HFD + irbesartan + GW9662, a PPARγ antagonist (0.5 mg/kg/day) and iv) the HFD + irbesartan + anti-HGF neutralizing antibody (200 μg/week). The liver and epididymal adipose tissues were evaluated histologically. Serum adiponectin and HGF levels were also measured by ELISA. Fatty liver (as detected by oil-red O staining) and macrophage infiltration were markedly reduced by irbesartan. Irbesartan treatment also reduced macrophage infiltration into epididymal adipose tissue and hypertrophy of adipocytes. However, these effects of irbesartan were attenuated by GW9662 as well as by anti-HGF neutralizing antibody. Serum and hepatic HGF levels were also markedly increased by irbesartan, whereas GW9662 decreased the HGF level. In conclusion, irbesartan, an angiotensin (Ang) receptor blocker (ARB) and partial agonist of PPARγ (metabosartan), demonstrated a reduction in fatty liver and chronic inflammation, such as macrophage infiltration, beyond its blood pressure-lowering effect. These favorable characteristics of irbesartan might be due to local HGF activation through its partial PPARγ agonistic action, in addition to Ang II blockade. Upregulation of local HGF by irbesartan might provide a novel advantage in a strategy for the prevention and treatment of cardiovascular diseases (CVDs).
Keywords: irbesartan, proliferator-activated receptor, metabolic syndrome, angiotensin receptor blocker
Introduction
Metabolic syndrome (MS), an extremely frequent condition characterized by dyslipidemia, insulin resistance, abdominal obesity and hypertension, is associated with an elevated risk of cardiovascular events. Of the risk factors for MS, high blood pressure is the most significant, since hypertension is closely associated with insulin resistance and dyslipidemia. Angiotensin (Ang) type I receptor (AT1R) blockers (ARBs) have been widely used in the treatment of hypertension and hypertension-related cardiovascular end-organ damage (1,2), since treatment with ARBs is known to improve the clinical manifestations of MS. However, new antihypertensive drugs should be designed to affect cell and biochemical mechanisms contributing to increased blood pressure and to also address disordered lipid metabolism in a more favorable manner.
To this end, peroxisome proliferator-activated receptor-γ (PPARγ) is in the center of interest, since ligands for PPARγ improve insulin sensitivity, reduce triglyceride levels and decrease the risk for atherosclerosis (3–5). Notably, among the approved ARBs, irbesartan and telmisartan were demonstrated to constitute a unique subset of ARBs that are also capable of activating PPARγ (2,6). Findings by Schupp et al(2) demonstrated that irbesartan and telmisartan could be considered partial and selective PPAR modulators (SPPARMs) (2,7). The SPPARM approach has also been suggested to be a method to avoid unwanted complications of PPARγ ligands, such as obesity and edema (7). In patients with MS, irbesartan markedly reduced blood pressure, and was associated with a reduction in cardiovascular risk factors, such as high-density lipoprotein (HDL) cholesterol, serum triglyceride, fasting blood glucose and waist circumference (8). Beneficial therapeutic effects of irbesartan in hypertensive patients with MS might be mediated via the AT1 receptor antagonistic and partial PPARγ agonistic actions. Based upon these observations, ARBs with partial PPARγ agonistic activity, such as irbesartan are now termed ‘metabosartans’. Hepatocyte growth factor (HGF) was previously shown to be a downstream effector of PPARγ agonists (9). Thus, in this study, we investigated the effects of a metabosartan, irbesartan, on fatty liver and the hypertrophy of adipocytes in apolipoprotein E (ApoE) knockout (KO) mice.
Materials and methods
Animals, diets and drug treatment
ApoE KO mice were used as a murine model exhibiting fatty liver and hyperlipidemia, which are typical phenotypes of MS. The experiments carried out in animals were performed in accordance with guidelines laid down by the Ethics Committee for Animal Experiments of the Osaka University Graduate School of Medicine (Osaka, Japan). Male ApoE KO mice with a C57/BL6 background were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Irbesartan was donated by Shionogi Pharma, Inc. (Osaka, Japan). HGF neutralizing antibody was purchased from Kringle Pharma, Inc. (Osaka, Japan). GW9662 was purchased from Cayman Chemical Company (Ann Arbor, MI, USA).
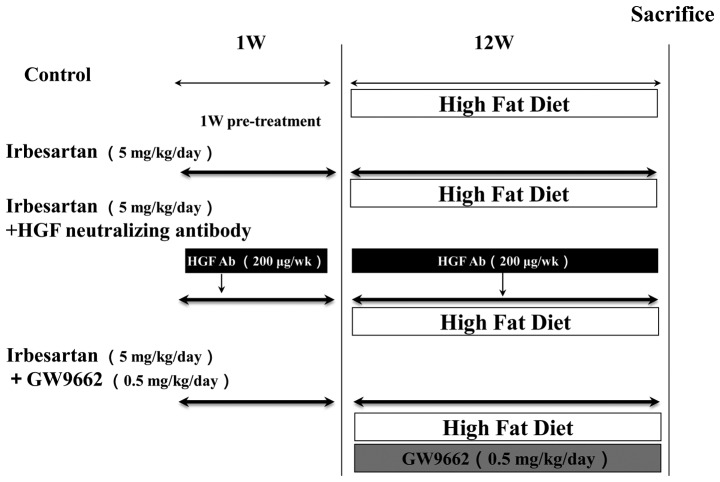
Ten-week old mice (n=6 per group) were fed a high-fat diet (HFD) (MF plus 0.5% (wt/wt) cholesterol and 10% yashi oil; Oriental Yeast Co., Ltd., Tokyo, Japan). Mice were divided into four groups: i) the control group, ApoE KO mice with HFD only; ii) the irbesartan group, ApoE KO mice with HFD and 5 mg/kg/day irbesartan; iii) the HGF antibody group, ApoE KO mice with HFD, 5 mg/kg/day irbesartan and 200 μg/week HGF neutralizing antibody and iv) the GW9662 group, ApoE KO mice with HFD, 5 mg/kg/day irbesartan and 0.5 mg/kg/day GW9662 (Fig. 1). Drugs were dissolved in water and administered ad libitum. There were 6 mice/group, which were housed in the animal facilities of the Osaka University (Osaka, Japan). The mice had free access to water and food during the experimental period. Following three months of drug and HFD treatment, the mice were sacrificed. Samples of epididymal adipose tissue and liver were evaluated.
Figure 1.
Experimental protocol is shown. ApoE KO mice placed on a high-fat diet (HFD) for 12 weeks were divided into four groups: i) control (HFD only); ii) HFD + irbesartan (5 mg/kg/day); iii) HFD + irbesartan + GW9662, a PPARγ antagonist (0.5 mg/kg/day) and iv) HFD + irbesartan + neutralizing antibody against HGF (200 μg/week). ApoE, apolipoprotein E; KO, knockout; PPARγ; peroxisome proliferators activated receptor-γ.
Measurement of HGF and adiponectin
HGF concentration was measured by an enzyme-linked immunosorbent assay (ELISA), using an IMMUNIS mouse HGF ELISA kit (Institute of Immunology Co., Ltd., Tokyo, Japan). Mouse liver samples were disintegrated with IMMUNIS HGF extraction buffer, using a Multi-beads shocker (Yasui Kikai, Osaka, Japan) at 2000 × g for 15 sec. Homogenates were centrifuged at 14,000 × g for 30 min. The supernatant was used for the HGF assay, according to the manufacturer’s instructions. Serum adiponectin level was also measured using an ELISA kit (Otsuka Pharmaceuticals, Co., Ltd., Tokyo, Japan).
Immunohistochemical staining
Tissues fixed with 4% formalin and embedded in paraffin were subjected to immunohistochemical staining, as described previously (10). Immunostaining of F4/80 was performed using anti-mouse F4/80 antibody (ab6640; Abcam, Cambridge, UK). Immunostained images were quantified using the NIH Image J software (http://rsb.info.nih.gov/ij/) and then analyzed visually under a light microscope by two investigators blinded to treatment.
Statistical analysis
Data were expressed as the mean ± standard error of the mean (SEM). Comparisons were made using analysis of variance (ANOVA) followed by Tukey’s simultaneous multiple comparisons. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of irbesartan on adipose tissue in ApoE KO mice
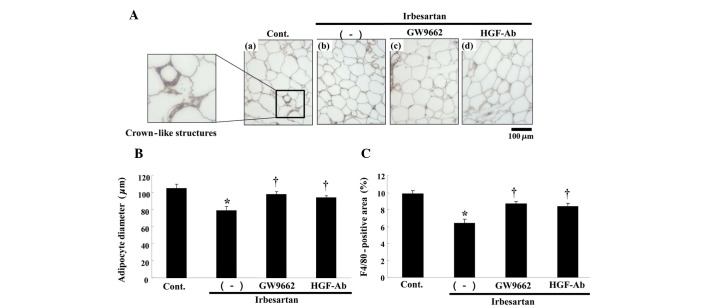
Administration of irbesartan at 5 mg/kg/day for 12 weeks lowered the epididymal adipose tissue weight. Histological examination showed a smaller adipocyte size and crown-like structures (formed by the gathering of macrophages), as detected by F4/80 (a macrophage marker)-positive areas in the irbesartan-treated group (Fig. 2A). Consistent with the histo-logical findings, adipocyte diameter was markedly decreased by irbesartan (Fig. 2B) and the F4/80-positive areas were also markedly decreased by irbesartan (Fig. 2C). These beneficial effects of irbesartan were attenuated by treatment with anti-HGF antibody or an inhibitor of PPARγ, GW9662.
Figure 2.
Effects of irbesartan, GW9662 and hepatocyte growth factor neutralizing antibody (HGF-Ab) on F4/80 staining and adipocyte diameter in adipose tissue. (A) Typical micrographs of immunostaining for F4/80 in periodic cross-sections of epididymal adipose tissue after 12 weeks of high-fat diet (HFD). Brown-stained area shows F4/80 protein-positive area; (a) control (HFD only), (b) HFD + irbesartan (5 mg/kg/day), (c) HFD + irbesartan + GW9662, a PPARγ antagonist (0.5 mg/kg/day) and (d) HFD + irbesartan + neutralizing antibody against HGF (200 μg/week); bar, 100 μm. (B) Adipocyte diameter (μm). (C) Quantitative data of immunohistochemical staining for macrophages (F4/80) (%). Control, HFD only; (−), HFD + irbesartan (5 mg/kg/day); GW9662, HFD + irbesartan + GW9662, a PPARγ antagonist (0.5 mg/kg/day); HGF-Ab, HFD + irbesartan + neutralizing antibody against HGF (200 μg/week). *P<0.01 vs. control, †P<0.05 vs. irbesartan only. Data are shown as the mean ± standard error of the mean. PPARγ; peroxisome proliferators activated receptor-γ.
Irbesartan also reduced the weight of epididymal adipose tissue (Fig. 3A). This effect was also attenuated by anti-HGF antibody or GW9662 treatment (Fig. 3A). Notably, irbesartan significantly increased the serum adiponectin level, while anti-HGF antibody or GW9662 treatment decreased the serum adiponectin level (Fig. 3B).
Figure 3.
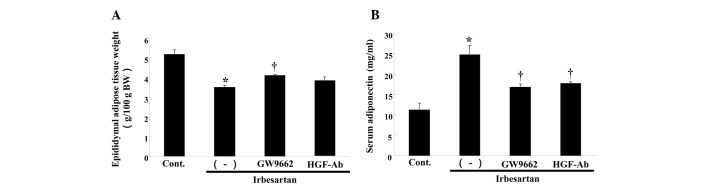
Effects of irbesartan, GW9662 and hepatocyte growth factor neutralizing antibody (HGF-Ab) on serum adiponectin and epididymal adipose tissue weight. (A) Serum concentration of adiponectin (mg/ml). (B) Epididymal adipose tissue weight [g/100 g of body weight (BW)] after 12 weeks of high-fat diet (HFD). Control, HFD only; (−), HFD + irbesartan (5 mg/kg/day); GW9662, HFD + irbesartan + GW9662, a PPARγ antagonist (0.5 mg/kg/day); HGF-Ab, HFD + irbesartan + neutralizing antibody against HGF (200 μg/week). *P<0.01 vs. control, †P<0.05 vs. irbesartan only. PPARγ; peroxisome proliferators activated receptor-γ.
Effect of irbesartan on fatty liver in ApoE KO mice
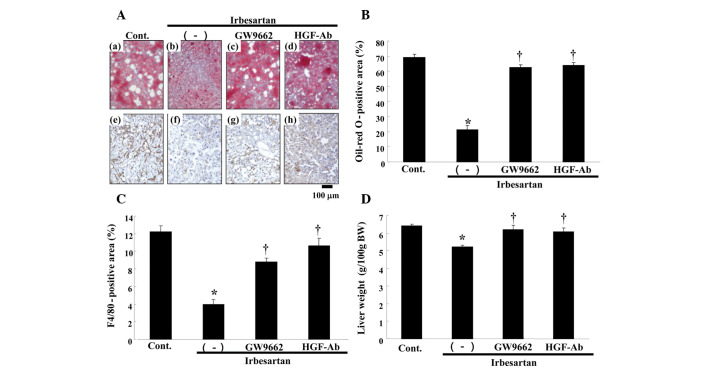
In this model, the control group demonstrated findings of severe fatty liver, such as marked deposition of lipid, as assessed by oil-red O staining (Fig. 4A). Notably, irbesartan markedly reduced lipid accumulation, while anti-HGF antibody or GW9662 treatment reversed the beneficial effect of irbesartan (Fig. 4A and B). Irbesartan also decreased serum aspartate transaminase (AST) as compared to the control (Table I), whereas anti-HGF antibody or GW9662 treatment attenuated the changes induced by irbesartan. Although no significant changes were detected in the low-density lipoprotein (LDL) cholesterol and total cholesterol levels, the free fatty acid (FFA) level was markedly decreased and HDL cholesterol level was markedly increased by irbesartan (Table I). The changes were also reversed by anti-HGF antibody or GW9662 treatment. No statistically significant changes were observed in body weight (BW), blood pressure or food intake in the groups (Table II). Therefore, this beneficial effect of irbesartan was not due to blood pressure lowering or a decrease in food intake.
Figure 4.
Effects of irbesartan, GW9662 and hepatocyte growth factor neutralizing antibody (HGF-Ab) on liver. (A) Upper panels: typical micro-graphs of periodic cross-sections of liver with oil-red O staining after 12 weeks of high-fat diet (HFD). (a) Control (HFD only), (b) HFD + irbesartan (5 mg/kg/day), (c) HFD + irbesartan + GW9662, a PPARγ antagonist (0.5 mg/kg/day) and (d) HFD + irbesartan + neutralizing antibody against HGF (200 μg/week); bar, 100 μm. Lower panels: typical micrographs of immunostaining for macrophages (F4/80) in periodic cross-sections of liver tissue after 12 weeks of HFD. Brown-stained area shows F4/80 protein-positive area; (e) control (HFD only), (f) HFD + irbesartan (5 mg/kg/day), (g) HFD + irbesartan + GW9662, a PPARγ antagonist (0.5 mg/kg/day) and (h) HFD + irbesartan + neutralizing antibody against HGF (200 μg/week); bar, 100 μm. (B) Quantitative data for percentage of oil deposits (oil-red O-positive area) in liver. (C) Quantitative data of immunohistochemical staining for macrophages (F4/80) in liver (%). (D) Liver weight [g/100g of body weight (BW)]. Control, HFD only; (−), HFD + irbesartan (5 mg/kg/day); GW9662, HFD + irbesartan + GW9662, a PPARγ antagonist (0.5 mg/kg/day); HGF-Ab, HFD + irbesartan + neutralizing antibody against HGF (200 μg/week). *P<0.01 vs. control, †P<0.05 vs. irbesartan only. PPARγ; peroxisome proliferators activated receptor-γ.
Table I.
Serum parameters in each group.
| Parameter | Irbesartan
|
|||
|---|---|---|---|---|
| Control | (−) | GW9662 | HGF-Ab | |
| AST (U/l) | 182.0±10.3 | 133.3±5.7a | 168.8±5.7b | 161.5±4.8b |
| ALT (U/l) | 30.5±2.3 | 29.6±1.7 | 30.9±2.5 | 31.4±2.9 |
| LDL-chol (mg/dl) | 1034±56 | 925±16 | 991±73 | 1006±29 |
| T-chol (mg/dl) | 1248±21 | 1069±48 | 1095±89 | 1194±47 |
| FFA (mEq/l) | 1.58±0.19 | 1.13±0.02a | 1.29±0.05 | 1.43±0.08b |
| HDL-chol (mg/dl) | 62.2±3.7 | 88.2±4.4a | 58.0±5.7a | 66.3±6.6 |
P<0.05 vs. control.
P<0.05 vs. irbesartan only. Data are shown as the mean ± standard error of the mean. HGF-Ab, hepatocyte growth factor neutralizing antibody; AST, aspartate transaminase; ALT, alanine aminotransferase; LDL-chol, low-density lipoprotein cholesterol; T-chol, total cholesterol; FFA, free fatty acid; HDL-chol, high-density lipoprotein cholesterol.
Table II.
Body weight, blood pressure and food intake in each group.
| Irbesartan
|
||||
|---|---|---|---|---|
| Control | (−) | GW9662 | HGF-Ab | |
| Body weight (g) | ||||
| PreHFD | 27.6±0.9 | 27.8±0.9 | 27.4±0.6 | 27.6±0.7 |
| PostHFD | 35.7±1.6 | 31.1±0.8 | 33.3±1.6 | 33.2±1.3 |
| Systolic BP (mmHg) | ||||
| PreHFD | 103.8±2.0 | 104.3±2.2 | 108.2±0.9 | 105.5±3.4 |
| PostHFD | 104.7±2.1 | 101.3±2.3 | 103.5±2.1 | 107.7±3.1 |
| Diastolic BP (mmHg) | ||||
| PreHFD | 70.3±1.6 | 68.5±1.9 | 69.8±2.0 | 68.7±3.3 |
| PostHFD | 67.3±2.9 | 65.0±2.3 | 67.5±1.5 | 66.5±2.1 |
| Food intake (g/day) | 2.06±0.11 | 2.05±0.10 | 2.09±0.11 | 2.08±0.09 |
HGF-Ab, hepatocyte growth factor neutralizing antibody; HFD, high-fat diet; BP, blood pressure.
As resident macrophages are crucially involved in the progression of fatty liver 11, immunostaining with F4/80 was performed. As shown in Fig. 4C, irbesartan markedly reduced the infiltration of macrophages in the liver, as detected by F4/80 staining. Irbesartan also reduced the liver weight (Fig. 4D). Consistently, these effects of irbesartan were also reversed by anti-HGF antibody or GW9662 treatment (Fig. 4C and D).
Effect of irbesartan on HGF protein levels in serum and liver
To confirm the role of HGF in the reduction of fatty liver by irbesartan, we measured the tissue HGF protein level in the liver. As shown in Fig. 5A, irbesartan markedly increased hepatic HGF protein as compared to the control, while GW9662 treatment markedly inhibited the increase in the HGF level induced by irbesartan. Similar changes were observed in the serum HGF level. As shown in Fig. 5B, the serum HGF level was markedly increased by irbesartan, while GW9662 treatment reduced the increase.
Figure 5.
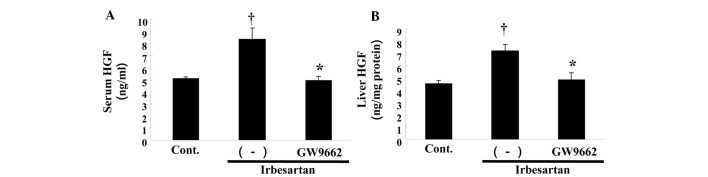
Effect of irbesartan and GW9662 on hepatocyte growth factor (HGF) protein levels in serum and liver. (A) Serum HGF protein concentration (ng/ml). (B) HGF protein concentration in liver (ng/mg tissue protein). Control, HFD only, irbesartan, HFD + irbesartan (5 mg/kg/day); irbesartan + GW9662, HFD + irbesartan + GW9662, a PPARγ antagonist (0.5 mg/kg/day). *P<0.01 vs. control, †P<0.01 vs. irbesartan only. HFD, high-fat diet; PPARγ; peroxisome proliferators activated receptor-γ.
Discussion
The present study has demonstrated that irbesartan significantly reduced fatty liver and chronic inflammation, such as macrophage infiltration through the PPARγ-HGF pathway, beyond its blood pressure-lowering effect. In general, adiponectin is a well-known downstream effector of PPARγ (12,13). The present study demonstrated that irbesartan markedly increased adiponectin expression. However, another downstream effector of PPARγ, HGF, is also likely to act as a ‘guardian’ in MS, in addition to adiponectin. Previous studies suggested that HGF mediates multiple various biological effects in various cells including anti-fibrotic, anti-inflammatory and anti-apoptotic activities (14). The present study clearly demonstrated that the anti-metabolic effects of irbesartan were largely mediated by the PPARγ-HGF pathway, with the exception of Ang II blockade.
Recent data have suggested that the presence of NAFLD in type 2 diabetes mellitus is linked to an increased risk of cardiovascular disease (CVD) independent of MS. In addition, the prevalence of carotid plaques has been reported to be higher in patients with NAFLD compared to the normal controls, regardless of classical cardiovascular risk factors (15). Therefore, NAFLD should be considered an independent risk factor for CVD. However, currently there are limited therapeutic options to treat NAFLD. To this end, the present findings suggesting that irbesartan reduced fatty liver and adipocyte hypertrophy may provide a new therapeutic option to treat NAFLD.
The manner in which PPARγ activates local HGF has yet to be elucidated. PPARγ has been reported to bind to the putative peroxisome proliferator response element (PPRE) in the promoter region of the HGF gene, resulting in an increase in HGF gene transcription, mRNA expression and protein secretion (9). Our group of investigators have reported that telmisartan (another PPARγ agonistic ARB), but not losartan (a classical ARB), improved endothelial dysfunction and fatty liver, due to an increased tissue HGF level (16). More directly, using AT1R KO mice, telmisartan, but not losartan, exhibited renal protective effects in a unilateral ureteral obstruction model (10). The partial PPARγ agonistic effect of irbesartan might provide an additional advantage as a strategy for the prevention and treatment of CVD, beyond its blood pressure-lowering effect through Ang II blockade. Notably, although irbesartan as well as telmisartan selectively induced PPARγ target genes, distinctive gene expression profiles have also been reported in 3T3-L1 adipocytes (2). The X-ray crystal structure exhibited various binding modes to PPARγ between irbesartan and telmisartan (6). In addition, irbesartan is also known to activate PPARα. Irbesartan has been reported to improve hepatic steatosis and hepatic fibrosis by activating PPARα, in NASH model FLS ob/ob mice (17). Another study also demonstrated that irbesartan upregulated PPARα in obese Koletsky (fak/fak) rats and improved their metabolic disorders (18). In our model, PPARα activation may also act to improve the pathological characteristics of the MS.
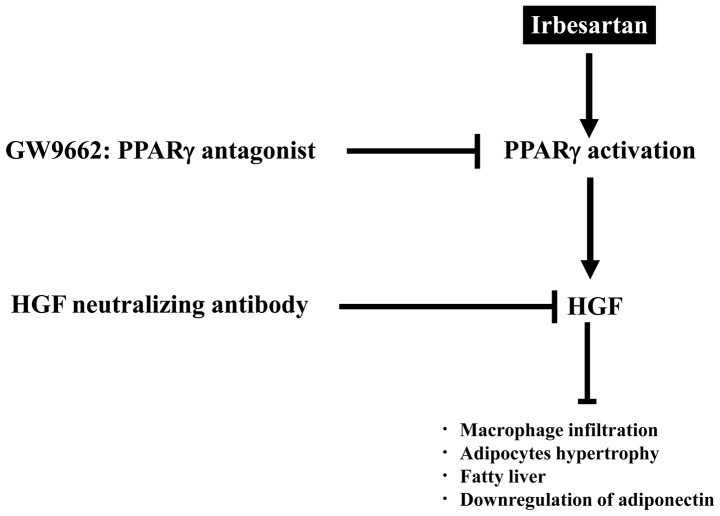
Overall, irbesartan, an ARB with partial PPARγ agonistic activity (metabosartan), demonstrated a reduction in fatty liver and chronic inflammation, such as macrophage infiltration, beyond its blood pressure-lowering effect (Fig. 6). These favorable characteristics of irbesartan might be due to local HGF activation through partial PPARγ agonistic activity, in addition to Ang II blockade. Upregulation of local HGF by irbesartan might provide a novel advantage in a strategy for the prevention and treatment of CVDs.
Figure 6.
Summary of findings. First, irbesartan activates PPARγ and then hepatocyte growth factor (HGF) is upregulated and exerts favorable effects against pathological characteristics of metabolic syndrome (MS). When this signaling is blocked, these beneficial effects are reduced. PPARγ; peroxisome proliferators activated receptor-γ.
Acknowledgments
This study was partially supported by a Grant-in-Aid from the Organization for Pharmaceutical Safety and Research, a Grant-in-Aid from the Ministry of Public Health and Welfare, a Grant-in-Aid from the Japan Promotion of Science and by special coordination funds of the Ministry of Education, Culture, Sports, Science and Technology of the Japanese Government.
References
- 1.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- 2.Schupp M, Janke J, Clasen R, Unger T, Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activity. Circulation. 2004;109:2054–2057. doi: 10.1161/01.CIR.0000127955.36250.65. [DOI] [PubMed] [Google Scholar]
- 3.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 4.Rosen ED, Spiegelman BM. PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276:37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 5.Picard F, Auwerx J. PPAR(gamma) and glucose homeostasis. Annu Rev Nutr. 2002;22:167–197. doi: 10.1146/annurev.nutr.22.010402.102808. [DOI] [PubMed] [Google Scholar]
- 6.Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA, Kurtz TW. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension. 2004;43:993–1002. doi: 10.1161/01.HYP.0000123072.34629.57. [DOI] [PubMed] [Google Scholar]
- 7.Schupp M, Clemenz M, Gineste R, Witt H, Janke J, Helleboid S, Hennuyer N, Ruiz P, Unger T, Staels B, Kintscher U. Molecular characterization of new selective peroxisome proliferator-activated receptor gamma modulators with angiotensin receptor blocking activity. Diabetes. 2005;54:3442–3452. doi: 10.2337/diabetes.54.12.3442. [DOI] [PubMed] [Google Scholar]
- 8.Parhofer KG, Münzel F, Krekler M. Effect of the angiotensin receptor blocker irbesartan on metabolic parameters in clinical practice: the DO-IT prospective observational study. Cardiovasc Diabetol. 2007;6:36. doi: 10.1186/1475-2840-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Wen X, Spataro BC, Hu K, Dai C, Liu Y. Hepatocyte growth factor is a downstream effector that mediates the antifibrotic action of peroxisome proliferator-activated receptor-gamma agonists. J Am Soc Nephrol. 2006;17:54–65. doi: 10.1681/ASN.2005030257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kusunoki H, Taniyama Y, Azuma J, Iekushi K, Sanada F, Otsu R, Iwabayashi M, Okayama K, Rakugi H, Morishita R. Telmisartan exerts renoprotective actions via peroxisome proliferator-activated receptor-γ/hepatocyte growth factor pathway independent of angiotensin II type 1 receptor blockade. Hypertension. 2012;59:308–316. doi: 10.1161/HYPERTENSIONAHA.111.176263. [DOI] [PubMed] [Google Scholar]
- 11.Bieghs V, Rensen PC, Hofker MH, Shiri-Sverdlov R. NASH and atherosclerosis are two aspects of a shared disease: central role for macrophages. Atherosclerosis. 2012;220:287–293. doi: 10.1016/j.atherosclerosis.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 12.Janke J, Schupp M, Engeli S, Gorzelniak K, Boschmann M, Sauma L, Nystrom FH, Jordan J, Luft FC, Sharma AM. Angiotensin type 1 receptor antagonists induce human in-vitro adipogenesis through peroxisome proliferator-activated receptor-gamma activation. J Hypertens. 2006;24:1809–1816. doi: 10.1097/01.hjh.0000242405.68461.84. [DOI] [PubMed] [Google Scholar]
- 13.Clasen R, Schupp M, Foryst-Ludwig A, Sprang C, Clemenz M, Krikov M, Thöne-Reineke C, Unger T, Kintscher U. PPARgamma-activating angiotensin type-1 receptor blockers induce adiponectin. Hypertension. 2005;46:137–143. doi: 10.1161/01.HYP.0000168046.19884.6a. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura T, Mizuno S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:588–610. doi: 10.2183/pjab.86.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi SY, Kim D, Kang JH, Park MJ, Kim YS, Lim SH, Kim CH, Lee HS. Nonalcoholic fatty liver disease as a risk factor of cardiovascular disease: relation of non-alcoholic fatty liver disease to carotid atherosclerosis. Korean J Hepatol. 2008;14:77–88. doi: 10.3350/kjhep.2008.14.1.77. (In Korean). [DOI] [PubMed] [Google Scholar]
- 16.Nakagami H, Osako MK, Takami Y, Hanayama R, Koriyama H, Mori M, Hayashi H, Shimizu H, Morishita R. Differential response of vascular hepatocyte growth factor concentration and lipid accumulation between telmisartan and losartan in ApoE-deficient mice. Mol Med Rep. 2008;1:657–661. doi: 10.3892/mmr_00000008. [DOI] [PubMed] [Google Scholar]
- 17.Kato J, Koda M, Kishina M, Tokunaga S, Matono T, Sugihara T, Ueki M, Murawaki Y. Therapeutic effects of angiotensin II type 1 receptor blocker, irbesartan, on non-alcoholic steatohepatitis using FLS-ob/ob male mice. Int J Mol Med. 2012;30:107–113. doi: 10.3892/ijmm.2012.958. [DOI] [PubMed] [Google Scholar]
- 18.Rong X, Li Y, Ebihara K, Zhao M, Kusakabe T, Tomita T, Murray M, Nakao K. Irbesartan treatment up-regulates hepatic expression of PPARalpha and its target genes in obese Koletsky (fa(k)/fa(k)) rats: a link to amelioration of hypertriglyceridaemia. Br J Pharmacol. 2010;160:1796–1807. doi: 10.1111/j.1476-5381.2010.00835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]