Abstract
Today, acute kidney injury (AKI) and congenital anomalies of the kidney and urinary tract (CAKUT) represent major issues in healthcare. Both AKI and CAKUT can lead to end stage renal disease (ESRD) that requires life-long medical care with renal replacement therapy. Renal replacement by dialysis is intensive, and kidney transplantation is restricted by organ availability. These limitations, along with the growing epidemic of patients affected by kidney disease, highlight the significant need to identify alternative ways to treat renal injury and birth defects. Drug discovery is one promising avenue of current research. Here, we discuss zebrafish chemical genetics and its latent potency as a method to rapidly identify small molecule therapeutics to accelerate recovery after AKI. Specifically, we review two groundbreaking studies that have recently provided a template to screen for compounds that expand the renal progenitor field in development that were capable of treating AKI in both the zebrafish and the mouse. These new findings demonstrate that drug discovery using zebrafish can be used for relevant translational research to identify clinical interventions for renal conditions in humans.
Keywords: Kidney, CAKUT, AKI, Zebrafish, Chemical genetics, Histone deacetylase, HDAC inhibitor
Introduction
The kidney is critical for a number of important bodily functions, including metabolic waste excretion and electrolyte homeostasis. Among vertebrates, the kidney is composed of functional units called nephrons that are generally made up of a renal corpuscle (a blood filter), a tubule, and a duct (Figure 1) [1,2]. Kidney disease can be a product of conditions such as birth defects or acute injury. Congenital anomalies of the kidney and urinary tract (CAKUT) represent 20–30% of all prenatal anomalies, and occur in 1 in 500 live births [3]. CAKUT occurs when there is a disruption in the normal development of either the kidney or urinary tract and can result in absent kidneys, diminished kidney size or incorrectly formed nephrons [3,4]. Acute kidney injury (AKI) accounts for 2–7% of inpatient hospital admissions and afflicts 5–6% of patients who become critically ill, with a 60% mortality rate [5]. AKI can be initiated from toxins, ischemia or sepsis, and is defined by an immediate loss of kidney function [6]. Both CAKUT and AKI can lead to end stage renal disease (ESRD), a state of kidney failure in which renal replacement therapy is necessary to maintain life.
Figure 1.
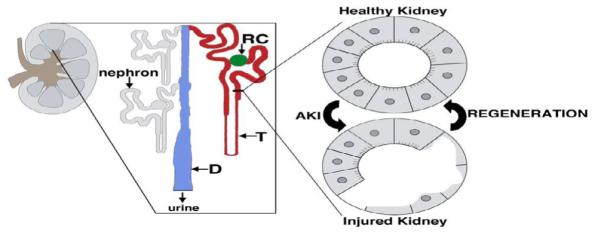
The kidney is comprised of nephron functional units that have some capacity to regenerate after epithelial injury. (Left) Schematic of the adult mammalian kidney, with (enlargement) depicting individual nephrons attached to the collecting duct drainage system. Each nephron is comprised of a renal corpuscle (RC) (green), tubule (T) (red) and attaches to the collecting duct (D) (blue).
Although dialysis is effective as a renal replacement therapy for patients suffering from kidney failure, it can be a demanding and exhaustive regimen over time, and it is an expensive treatment. Further, dialysis is the only medically available option to patients on ever lengthening transplant wait-lists [6]. Unfortunately, patients that do receive kidney transplants often face a multitude of health issues, with 40% of recipients either dying or losing graft function within ten years [7]. Thus, there is a dire need to identify new preventative and curative treatments for defects in renal development and adult kidney disease. One appealing avenue is the stimulation of more robust renal regeneration following injury, while other possibilities include the generation of cell-based therapies using stem cells [8].
In recent years, the zebrafish (Danio rerio) has received more and more attention as a prominent and unique model organism in the field of nephrology [9]. Zebrafish are freshwater vertebrates that share both major organs and important developmental pathways with mammals, including humans [10,11]. The large degree of gene conservation between zebrafish and mammals is only one of the many reasons why zebrafish are fast becoming widely used in translational settings [12]. There are a growing number of zebrafish mutants and transgenics that enable intricate questions to be investigated and answered. Adult zebrafish generate large clutches of embryos that develop externally, making them ideal for high-throughput studies. Furthermore, zebrafish embryos are transparent, which allow organ development and gene expression to be simply and easily observed. In particular, zebrafish are an attractive model to study the kidney because of the conserved nature of their nephron composition with mammals [13,14]. They share similarities in the composition of the renal corpuscle as well as the nephron, which is comprised of a series of proximal and distal tubule segments that reabsorb and secrete metabolites and assorted ions [13,14]. These reasons, coupled with the ability to regenerate nephrons as adults [15–18], make zebrafish an exceptional model to study kidney development and disease [19,20].
Chemical genetics screening is the use of chemical assays to detect biological repercussions in living systems [21,22]. This method allows molecules to be tested en masse to rescue a mutant phenotype. In addition, chemical genetics allows for a temporal dimension, such that the investigator control the time of drug exposure-which is a level of experimental typically not found in traditional (i.e. classical) genetics. In zebrafish, this method of screening is exceptionally useful, as compounds from chemical libraries can simply be added and removed to the zebrafish embryo media during a time window of interest for the organ or tissue under investigation (Figure 2). In the past decade, chemical genetics in zebrafish has recruited increasing attention as proof of concept experiments have emerged that demonstrate its translational value [23,24].
Figure 2.
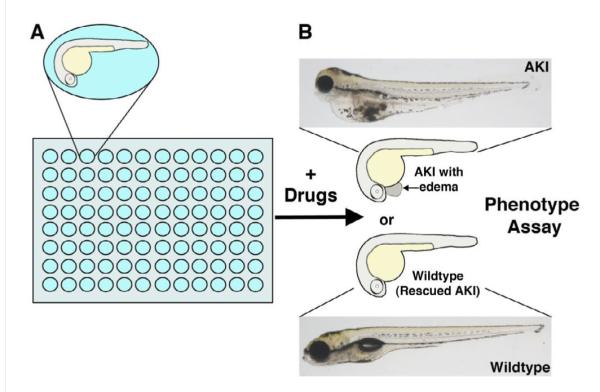
Experimental strategy for using chemical genetics with the zebrafish embryo to screen for AKI-relevant therapeutics. (A) Zebrafish embryos are arrayed in 96-well plates (or other multi-well plates) and exposed to drugs in a temporally-controlled fashion during development, in which the researcher adds the drug at the time of interest. (B) Embryos are examined for subsequent phenotypic alterations, such as the rescue of edema associated with AKI. Zebrafish embryos are optically transparent, and develop minimal pigmentation by 3–5 days of development, which facilitates the visualization of internal organs. Compared to a wild type embryo (bottom), an embryo exposed to gentamicin (top) develops AKI symptoms that include pericardial edema, seen as a swelling around the heart adjacent to the yolk sac.
The benefits of using zebrafish to screen for therapeutics initially became apparent after Peterson et al. described a small molecule screen to investigate developmental processes in the zebrafish embryo [25]. They treated zebrafish embryos with a library consisting of 1100 small molecules and observed an array of phenotype defects [25]. Of particular note is a subsequent screen they performed on the hey2 (also known as gridlock) mutant that develops an aortic coarctation [26]. This mutant results in a narrowing of the aorta and hence, atypical blood flow. Therefore, Peterson et al. [26] screened for a return in regular blood flow to the tail after chemical treatment to flag for hits. Using this method, they were able to identify compounds that upregulated vascular endothelial growth factor (VEGF). This study set the stage for zebrafish chemical genetics and the innovative translational research to follow. Here, we review two cornerstone studies that have now specifically highlighted the singular advantage and translational aspect of using zebrafish chemical genetics to screen for therapeutically pertinent drugs to study kidney development and treat AKI.
Insights into Renal Progenitor Biology with Chemical Genetics
Adult stem cell and progenitor populations have garnered much attention in the past decade, heralded by many as the solution for a multitude of conditions, including AKI [8]. It has been speculated that increasing these populations in adults could be key for regenerative medicine [8]. Regeneration in some organs, including the kidney, recapitulates the gene expression seen during organ formation [20,27]. Therefore, investigating organogenesis processes and progenitors in developing embryos could help to define methods of facilitating regeneration in the adult, which would be of exceptional value for organs such as the kidney.
The seminal study of this sort in the zebrafish kidney was a chemical screen by de Groh et al. [28] who interrogated a diverse library of small molecules to discover a series of compounds that expanded the renal progenitor cell population in embryos. The renal progenitor population is comprised of two stripes of cells that give rise to a pair of nephrons that serve as the functioning embryonic kidney, known as the pronephros [29]. This embryonic kidney has been credited as being an excellent model for study, as it only contains two nephrons, yet is composed of the complex cell types found in higher vertebrates [13]. de Groh et al. [28] screened specifically for molecules that caused pericardial edema in zebrafish embryos, an excess of fluid in the pericardial chamber that is indicative of kidney failure. This phenotype-based search coupled with large ex utero transparent clutches allowed for rapid screening with a focus on kidney altercation. From this screen, they identified 4-(phenylthio) butanoic acid (PTBA) as a positive hit, which generated pericardial edema and axis curvature at 72 hours post fertilization (hpf).
From here, they proceeded to determine if PTBA affected the kidney field by performing a pax2 in situ hybridization, which labels the renal progenitors [12]. As they suspected, there was a marked expansion in pax2a expression upon PTBA treatment. This led them to ask the question if PTBA also increased the expression of other renal progenitor markers, namely lhx1a, pax2a, and pax8 [12]. Strikingly, they observed an increase in transcript expression of all three markers compared to control embryos. Importantly, the increase in lhx1a expression was observed in the bilateral stripes of the intermediate mesoderm that gives rise to the pronephros.
The observed increase in lhx1a expression, while significant, did not speak to the notion that the number of renal progenitors were increased, and could be an artifact of increased mRNA expression per cell. To this end, they used the transgenic line Tg (lhx1a: EGFP) pt303 to visualize renal progenitor cells. PTBA-treated embryos revealed a 2.4-fold increase in renal progenitor cell number compared to controls.
Following this, de Groh et al. [28] asked the question if the PTBA-mediated increase in kidney field size stemmed from a fate transformation event of non-renal cells or a proliferation of renal progenitor cells. In situ hybridizations of two mesodermal tissue markers myod1 and fli1a, which label the somites and vasculature respectively, illustrated that PTBA caused no significant change in expression. Embryos were then treated with hydroxyurea and aphidicolin (HUA), which inhibits cell proliferation without affecting tissue specification, either alone or in combination with PTBA. Here, they saw that HUA had little effect on lhx1a expansion by itself, yet when combined with PTBA there was a significant decline in expansion of lhx1a compared to embryos treated with only PTBA. This suggested that PTBA functioned to increase renal progenitor cell expansion through proliferation rather than redirection of tissue fate. Finally, de Groh et al. [28] used a structure activity study to show that PTBA may act as a histone deacetylase inhibitor (HDACi). Indeed, upon further interrogation, other HDACi compounds appeared to have similar effects on kidney field expansion. Further complementing the notion that PTBA acts as an HDACi was an in vitro fluorescent assay, showing that PTBA blocked the ability of HDACs to deacetylate a HeLa cell nuclear extract. Interestingly, two other HDACis, Trichostatin A (TSA) and valproic acid, have both been shown to prevent cyst formation in a zebrafish model of polycystic kidney disease (PKD) and attenuate progression of cyst generation in a mouse model of PKD [30]. These findings suggested other possible roles of PTBA and HDACis in kidney disease. Taken together, these results highlight the benefits of zebrafish chemical genetics to identify compounds that can increase renal progenitor populations with potentially therapeutic functions. This work created an important precedence for using zebrafish as a model for kidney organogenesis-based chemical genetics.
Insights into AKI from Chemical Genetics
The field of nephrology has been stagnant concerning the treatment of AKI, and this coupled with the growing socio-economic stresses resulting from AKI, demands that innovative and translational research forge ahead for potential cures [6]. One promising source is the stimulation of endogenous renal progenitors to aid in patient recovery [4]. In a recently published report, Cosentino et al. have constructed a template utilizing zebrafish chemical genetics to find suitable drug candidates that both expand developmental renal progenitor populations and accelerate regeneration after AKI [31]. Following the previously discussed de Groh et al. study who identified that PTBA was a small molecule HDACi that expanded the renal progenitor field, Cosentino et al. discovered that an esterfied analog of PTBA, methyl-4-(phenylthio) butanoate (m4PTB), that was more active than PTBA, helped to facilitate recovery from AKI in zebrafish and mice [31].
Consentino et al. reasoned that since m4PTB expanded renal progenitor populations in development, it may also induce renal tubular epithelial cells (RTECs) to proliferate, which are primarily responsible for tubule repair after AKI [4]. They first used a zebrafish model of AKI to analyze the effects of m4PTB treatment. To do this, they administered injections of gentamicin, an aminoglycoside antibiotic, into 2 dpf zebrafish, which caused edema at 2 days post injection (dpi). In mammalian AKI, there is a loss of epithelial cells and a denigration of parts of the basement membrane [4]. To compare their model of AKI, Cosentino et al. used the transgenic reporter Tg(PT:EGFP), where enhanced green fluorescence protein (GFP) is expressed in the proximal tubule (PT), which is the segment of the nephron tubule damaged most acutely by gentamicin exposure. Gentamicin injected zebrafish had flattened RTECs and an enlarged tubular lumen compared to controls. Histological sections showed that nephrons had undergone morphology changes including flattening of epithelial cells and thinning of the brush border, accompanied by tubular distention and lumenal debris. Further, whole-mount immunofluorescence demonstrated that gentamicin injected fish had a disruption of cell polarity, evidenced by diffusion of anti-Na+/K+ ATPase α-subunit (α6F) in the cytoplasm, which is typically concentrated in the basolateral membrane of proximal tubular cells. Taken together, these data demonstrated the many similarities between gentamicin-induced pronephric injury in zebrafish and mammalian AKI, consistent with prior observations [32–34].
Cosentino et al. next treated gentamicin and vehicle injected zebrafish with m4PTB. Strikingly, they observed a 21% increase in survival of m4PTB treated fish compared to controls by 5 dpi. To delineate if this increase in survival was a result of proliferation, they subjected fish larvae to 5-ethynyl-2'-deoxyuridine (Edu), which colocalizes with the 3G8 antibody that stains the apical brush border of the PT. The PT Edu index increased from 3.5% to 7.1% after m4PTB treatment in uninjected fish and likewise, increased from 7.3% to 10.7% in gentamicin injected fish. This suggested that m4PTB-mediated renal recovery acts, at least in part, through an increase in tubular cell proliferation.
Cosentino et al. [31] then endeavored to observe the effects of m4PTB on AKI recovery in mice. They initially induced moderate ischemia reperfusion-induced AKI (IR-AKI) in BALB/c mice as an AKI model by conducting a unilateral nephrectomy and 26 minutes of renal pedicle clamping. Following injury, they treated mice with intraperitoneal injections of 100 mg/kg m4PTB or a vehicle control 24 hours after injury and daily for 6 days. A decrease in serum creatine and BUN was observed in m4PTB treated mice compared to controls at 3 days post injury. This indicated that the decrease in serum creatine levels was not due to a reduction in creatine synthesis. Although this suggests an improvement in renal function, there was no difference in tubular injury score or Kim1 mRNA expression, a renal tubular injury marker, 3 days post injury. However, at 14 days post injury there was a reduction in Kim1 mRNA expression and a reduction in two of the five renal fibrosis markers, Collagen 1 and LoxL2.
To further define the effects of m4PTB, Consentino et al. generated a severe IR-AKI model in mice. Here, they performed unilateral renal pedicle clamping for 30 minutes in BALB/c mice and subsequent contralateral nephrectomy 8 days post injury. Mice were treated with m4PTB in the same manner described before for moderate IR-AKI. M4PTB mice exhibited a decrease in serum creatine and BUN 9 days post injury and interestingly, by day 28 there was a dramatically lower tubular injury score in m4PTB treated mice compared to controls, as well as a marked reduction in Kim 1 mRNA expression. Additionally, there was less fibrosis in the outer medulla (OM) of m4PTB treated mice, depicted by the sirius red marker for collagen deposition, along with reduced mRNA expression of four out of the five renal fibrosis markers: Collagen 1, Lox, LoxL2, and α-smooth muscle actin. These results suggested that m4PTB enhances renal tubular recovery and lessens post-injury fibrosis after severe IR-AKI.
In mammals, RTECs dedifferentiate and proliferate to regenerate the tubular epithelial, yet after AKI, RTECs undergo G2/M cell cycle arrest [9]. Cosentino et al. evaluated the changes in gene expression after m4PTB treatment by harvesting mice kidneys 12 hours after m4PTB treatment (100 mg/kg), which was administered 24 hours after kidney injury. These expression profiles revealed that m4PTB upregulated genes involved with regulating the cell cycle, DNA, and microtubule-based processes. This indicated that m4PTB facilitates renal recovery through a cell cycle dependent mechanism. To test this, Cosentino et al. looked at the proliferation of RTECs 72 hours after moderate IR-AKI and 48 hours after m4PTB or vehicle treatment. While they did not see an increase in the total number or proportion of proliferating RTECs or RTECs in S phase, visualized by the Ki67 and PCNA markers respectively, they did see a reduction in the proportion of RTECs in the G2 and M phases (pH3-positive cells) and an increase in RTECs in the G1 phase after m4PTB treatment compared to controls. A similar treatment in severe IR-AKI mice showed a reduction of RTECs in the G2 phase and an increase of RTECs in the S phase, while there was no change in the proportion of RTECs in the M phase and a reduction of RTECs in the G1 phase. Taken together, these findings suggest that m4PTB enhances renal recovery by reducing cell G2/M cell cycle arrest after IR-AKI.
Conclusions and Future Perspectives
As discussed throughout the previous sections, the de Groh et al. [28] and Cosentino et al. [31] zebrafish AKI studies have demonstrated that the zebrafish gentamicin-injury model represents a rapid way to find therapeutics to treat AKI. This series of reports have now demonstrated that there are drugs that can facilitate a regenerative response after injury has already occurred in the kidney. These breakthroughs represent a template to discover therapeutics that can treat kidney disease after insult has already occurred, which would be valuable in a clinical setting.
Zebrafish are a powerful addition to the animal models used to study AKI [35] and may lead to new clinical trials or impactful findings helpful in evaluating ongoing clinical trials [36]. Further, continued advancements in automated imaging and analysis are redefining high-throughput methodologies for zebrafish embryos, with systems that eliminate time in manipulating samples and enable the collection of data through artificial intelligence data processing [37,38]. However, it is also likely that adult-based screens will have utility in identifying factors and/or signaling pathways that are relevant, or even specific to, the adult environment and physiology. The adult zebrafish kidney is easy to isolate and can be analyzed with standard cellular and molecular assays [39,40]. Given the remarkable regenerative capacities of the adult kidney [15–18], future research centered on the drugs that modulate regeneration is another promising venue for future research. In conclusion, zebrafish chemical genetics represent a powerful translational tool that can be used to dissect and delineate the regulation of renal progenitors, which is relevant to identifying methods to treat kidney developmental defects, and has already begun to identify exciting potential therapeutics for AKI.
Acknowledgements
The Wingert laboratory is supported by: NIH-NIDDK grant K01DK083512, NIH New Innovator award grant DP2OD008470, the March of Dimes Basil O'Connor Starter Scholar grant award #5-FY12-75, start up funds from the University of Notre Dame College of Science, and a generous gift from the Gallagher family. We thank the staffs of the Department of Biological Sciences and Center for Zebrafish Research at Notre Dame for their dedication and assistance. We thank Kristen K. McCampbell for providing the live zebrafish embryo images for Figure 2. Finally, we thank the members of our research lab for their comments, discussions and support.
References
- 1.Reily RF, Bulger RE, Kriz W. Chapter 1: Structural-functional relationships in the kidney. 8thedn Lippincott Williams & Wilkins; Philadelphia: 2007. Diseases of the Kidney and Urinary Tract. [Google Scholar]
- 2.Wingert RA, Davidson AJ. The zebrafish pronephros: a model to study nephron segmentation. Kidney Int. 2008;73:1120–1127. doi: 10.1038/ki.2008.37. [DOI] [PubMed] [Google Scholar]
- 3.Nakanishi K, Yoshikawa N. Genetic disorders of human congenital anomalies of the kidney and urinary tract (CAKUT) Pediatr Int. 2003;45:610–616. doi: 10.1046/j.1442-200x.2003.01779.x. [DOI] [PubMed] [Google Scholar]
- 4.McCampbell KK, Wingert RA. Renal stem cells: fact or science fiction? Biochem J. 2012;444:153–168. doi: 10.1042/BJ20120176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munshi R, Hsu C, Himmelfarb J. Advances in understanding ischemic acute kidney injury. BMC Med. 2011;9:11. doi: 10.1186/1741-7015-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zarjou A, Sanders PW, Mehta RL, Agarwal A. Enabling innovative translational research in acute kidney injury. Clin Transl Sci. 2012;5:93–101. doi: 10.1111/j.1752-8062.2011.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, et al. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19:646–651. doi: 10.1038/nm.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Wingert RA. Regenerative medicine for the kidney: stem cell prospects & challenges. Clin Transl Med. 2013;2:11. doi: 10.1186/2001-1326-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swanhart LM, Cosentino CC, Diep CQ, Davidson AJ, de Caestecker M, et al. Zebrafish kindey development: basic science to translational research. Birth Defects Res C Embryo Today. 2011;93:141–156. doi: 10.1002/bdrc.20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- 11.Santoriello C, Zon LI. Hooked! Modeling human disease in zebrafish. J Clin Invest. 2012;122:2337–2343. doi: 10.1172/JCI60434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamplin OJ, White RM, Jing L, Kaufman CK, Lacadie SA, et al. Small molecule screening in zebrafish: swimming in potential drug therapies. Wiley Interdiscip Rev Dev Biol. 2012;1:459–468. doi: 10.1002/wdev.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wingert RA, Selleck R, Yu J, Song HD, Chen Z, et al. The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet. 2007;3:1922–1938. doi: 10.1371/journal.pgen.0030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerlach GF, Wingert RA. Kidney organogenesis in the zebrafish: insights into vertebrate nephrogenesis and regeneration. WIRES Dev Biol. 2012 doi: 10.1002/wdev.92. doi: 10/1002/wdev.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou W, Boucher RC, Bollig F, Englert C, Hildebrandt F. Characterization of mesonephric development and regeneration using transgenic zebrafish. Am J Physiol Renal Physiol. 2010;299:F1040–1047. doi: 10.1152/ajprenal.00394.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diep CQ, Ma D, Deo RC, Holm TM, Naylor RW, et al. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature. 2011;470:95–100. doi: 10.1038/nature09669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou W, Hildebrandt F. Inducible podocyte injury and proteinuria in transgenic zebrafish. J Am Soc Nephrol. 2012;23:1039–1047. doi: 10.1681/ASN.2011080776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J, McKee M, Huang HD, Xiang A, Davidson AJ, et al. A zebrafish model of conditional targeted podocyte ablation and regeneration. Kidney Int. 2013;83:1193–1200. doi: 10.1038/ki.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebarasi L, Oddsson A, Hultenby K, Betsholtz C, Tryggvason K. Zebrafish: a model system for the study of vertebrate renal development, function, and pathophysiology. Curr Opin Nephrol Hypertens. 2011;20:416–424. doi: 10.1097/MNH.0b013e3283477797. [DOI] [PubMed] [Google Scholar]
- 20.Romagnani P, Lasagni L, Remuzzi G. Renal progenitors: an evolutionary conserved strategy for kidney regeneration. Nat Rev Nephrol. 2013;9:137–146. doi: 10.1038/nrneph.2012.290. [DOI] [PubMed] [Google Scholar]
- 21.Tsang M. Zebrafish: A tool for chemical screens. Birth Defects Res C Embryo Today. 2010;90:185–192. doi: 10.1002/bdrc.20183. [DOI] [PubMed] [Google Scholar]
- 22.Lessman CA. The developing zebrafish (Danio rerio): a vertebrate model for high-throughput screening of chemical libraries. Birth Defects Res C Embryo Today. 2011;93:268–280. doi: 10.1002/bdrc.20212. [DOI] [PubMed] [Google Scholar]
- 23.North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goessling W, Allen RS, Guan X, Jin P, Uchida N, et al. Prostaglandin E2 enhances human cord blood stem cell xenotransplants and shows long-term safety in preclinical nonhuman primate transplant models. Cell Stem Cell. 2011;8:445–458. doi: 10.1016/j.stem.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson RT, Link BA, Dowling JE, Schreiber SL. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc Natl Acad Sci U S A. 2000;97:12965–12969. doi: 10.1073/pnas.97.24.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson RT, Shaw SY, Peterson TA, Milan DJ, Zhong TP, et al. Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nat Biotechnol. 2004;22:595–599. doi: 10.1038/nbt963. [DOI] [PubMed] [Google Scholar]
- 27.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Groh ED, Swanhart LM, Cosentino CC, Jackson RL, Dai W, et al. Inhibition of histone deacetylase expands the renal progenitor cell population. J Am Soc Nephrol. 2010;21:794–802. doi: 10.1681/ASN.2009080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drummond IA, Majumdar A, Hentschel H, Elger M, Solnica-Krezel L, et al. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development. 1998;125:4655–4667. doi: 10.1242/dev.125.23.4655. [DOI] [PubMed] [Google Scholar]
- 30.Cao Y, Semanchik N, Lee SH, Somlo S, Barbano PE, et al. Chemical modifier screen identifies HDAC inhibitors as suppressors of PKD models. Proc Natl Acad Sci U SA. 2009;106:21819–21824. doi: 10.1073/pnas.0911987106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cianciolo Cosentino C, Skrypnyk NI, Brilli LL, Chiba T, Novitskaya T, et al. Histone deacetylase inhibitor enhances recovery after AKI. J Am Soc Nephrol. 2013;24:943–953. doi: 10.1681/ASN.2012111055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hentschel DM, Park KM, Cilenti L, Zervos AS, Drummond I, et al. Acute renal failure in zebrafish: a novel system to study a complex disease. Am J Physiol Renal Physiol. 2005;288:F923–929. doi: 10.1152/ajprenal.00386.2004. [DOI] [PubMed] [Google Scholar]
- 33.Cianciolo Cosentino C, Roman BL, Drummond IA, Hukriede NA. Intravenous microinjections of zebrafish larvae to study acute kidney injury. J Vis Exp. 2010 doi: 10.3791/2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rider SA, Tucker CS, del-Pozo J, Rose KN, MacRae CA, et al. Techniques for the in vivo assessment of cardio-renal function in zebrafish (Danio rerio) larvae. J Physiol. 2012;590:1803–1809. doi: 10.1113/jphysiol.2011.224352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanz AB, Sanchez-Niño MD, Martín-Cleary C, Ortiz A, Ramos AM. Progress in the development of animal models of acute kidney injury and its impact on drug discovery. Expert Opin Drug Discov. 2013;8:879–895. doi: 10.1517/17460441.2013.793667. [DOI] [PubMed] [Google Scholar]
- 36.Faubel S, Chawla LS, Chertow GM, Goldstein SL, Jaber BL, et al. Ongoing clinical trials in AKI. Clin J Am Soc Nephrol. 2012;7:861–873. doi: 10.2215/CJN.12191111. [DOI] [PubMed] [Google Scholar]
- 37.Vogt A, Cholewinski A, Shen X, Nelson SG, Lazo JS, et al. Automated image-based phenotypic analysis in zebrafish embryos. Dev Dyn. 2009;238:656–663. doi: 10.1002/dvdy.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogt A, Codore H, Day BW, Hukriede NA, Tsang M. Development of automated imaging and analysis for zebrafish chemical screens. J Vis Exp. 2010 doi: 10.3791/1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerlach GF, Schrader LN, Wingert RA. Dissection of the adult zebrafish kidney. J Vis Exp. 2011 doi: 10.3791/2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drummond IA, Davidson AJ. Zebrafish kidney development. Methods Cell Biol. 2010;100:233–260. doi: 10.1016/B978-0-12-384892-5.00009-8. [DOI] [PubMed] [Google Scholar]


