Abstract
Rationale
NMDA antagonists consistently produce social inhibition in adult animals, although effects of these manipulations on social behavior of adolescents are relatively unknown.
Objectives
The aim of this study was to assess potential age differences in the socially inhibitory effects of the non-competitive NMDA antagonist, MK-801, as well as NR2 subunit selective effects, given the regional and developmental differences that exist for the NR2 subunit during ontogeny.
Methods
In separate experiments, adolescent and adult male Sprague–Dawley rats were treated acutely with MK-801 (0, 0.05, 0.1, 0.2 mg/kg, i.p.), the NR2A antagonist, PEAQX (2.5, 5, 10, 20 mg/kg, s.c.), or the NR2B antagonist, ifenprodil (1.5, 3, 6, 12 mg/kg, i.p.), 10 min prior to a social interaction test.
Results
Adolescents required higher doses of MK-801 (0.1 and 0.2 mg/kg) to induce social suppression, whereas adults demonstrated reductions in social activity after all doses. Likewise, adolescents required higher doses of ifenprodil (6 and 12 mg/kg) to produce social inhibitory effects relative to adults (all doses). In contrast, adults were less sensitive to PEAQX than adolescents, with adults showing social inhibition after 20 mg/kg whereas adolescents showed this effect following 10 and 20 mg/kg. Although locomotor activity was generally reduced at both ages by all drugs tested, ANCOVAs using locomotor activity as a covariate revealed similar patterns of social inhibitory effects.
Conclusions
Adolescents are less sensitive than adults to the disruption of social behavior by NMDA and NR2B-selective receptor antagonism, but not by an NR2A antagonist—age differences that may be related to different subunit expression patterns during development.
Keywords: Social interactions, MK-801, PEAQX, Ifenprodil, NMDA antagonist, Rat, Adolescent
Introduction
Adolescence is associated with numerous hormonal, neural, and behavioral changes that are conserved across mammalian species (Spear 2000, 2010). These similarities have led to the use of simple animal models of certain adolescent-typical behaviors, such as the study of social behaviors in the laboratory rat (Trezza et al. 2011; Varlinskaya and Spear 2002; Varlinskaya et al. 1999). Peer-directed social interactions are of interest because these behaviors are especially prevalent during adolescence across mammalian species (Berndt 1982; Csikszentmihalyi et al. 1977; Montemayor 1982; Varlinskaya and Spear 2008; Willey et al. 2009).
The social interaction test has been used extensively for assessment of social behaviors in male rodents under normal or anxiety-provoking circumstances (File 1980; File and Hyde 1978; File and Seth 2003; Sanders and Shekhar 1995a; Siviy et al. 1995). Although the test typically consists of placing a pair of animals in an open arena and recording time spent in overall social interactions as the primary dependent measure, our laboratory has modified this test to include two sides separated by a partition with an opening to permit the test animal to freely move toward or away from its partner (Varlinskaya et al. 1999), and hence obtain an index of social motivation.
Animal models have demonstrated that numerous neural systems are involved in modulating play (Vanderschuren et al. 1997) and overall social activity (Pellow and File 1984). Among the critical contributors are the neuropeptides oxytocin and vasopressin (Carter 1998; Domes et al. 2007; Donaldson and Young 2008), the opioid receptor systems (i.e., mu and kappa opioid receptors; Trezza et al. 2011; Vanderschuren et al. 1995; Varlinskaya and Spear 2009), GABA system (Sanders and Shekhar 1995a, b), and the glutamate system (de Moura Linck et al. 2008; Deutsch et al. 2011; Jacome et al. 2011; Snigdha and Neill 2008). Of the three subtypes of receptors for glutamate, the N-methyl-D-aspartate (NMDA) receptor has been most strongly implicated in social behavior (de Moura Linck et al. 2008; Silvestre et al. 1997; Snigdha and Neill 2008). While substantial work has shown impairments of social behaviors due to NMDA receptor antagonists in adult rats and mice, only a few have examined the role of this receptor system on social behaviors in younger rodents (Moy et al. 2012; Siviy et al. 1995). In one, the non-competitive NMDA receptor antagonist, MK-801, was found to decrease social preference in adolescent C57BL/6J mice (as indexed by sniffing of a stranger mouse), whereas in another, a biphasic effect of MK-801 was evident in male Sprague–Dawley juvenile rats, with 0.025 mg/kg enhancing social play and doses of 0.1 and 0.2 mg/kg, significantly reducing this behavior. Adult comparison groups were not included in these studies and hence age-related conclusions cannot be derived.
Such age comparisons are important given well-known age differences in sensitivity to ethanol which, among its many neural consequences, acts to functionally block NMDA receptors (Eckardt et al. 1998), with strongest effects on the NR2A and NR2B subunits (Allgaier 2002; Mirshahi and Woodward 1995). Adolescent male rats and mice have been found to be relatively insensitive to a variety of ethanol’s behavioral effects when compared to adults (Anderson et al. 2010; Holstein et al. 2011; Schramm-Sapyta et al. 2010; Varlinskaya et al. 2010). Age differences in sensitivity to ethanol may be related in part to ontogenetic differences in NMDA receptor subunit sensitivity, with for instance adolescent rats requiring a higher dose of the selective NR2B antagonist, ifenprodil, to block acute tolerance to ethanol than adult rats (10 versus 5 mg/kg, respectively) (Ramirez et al. 2011). However, findings regarding age differences in sensitivity to NMDA and NR2B antagonists in male rats are mixed (Burton and Fletcher 2012; Silveri and Spear 2002) and hence more research is needed to determine the functional ontogeny of this receptor and its subunits.
Ontogenetic changes in functional consequences of NMDA receptor blockade are likely given that NMDA subunit expression undergoes considerable developmental reorganization (Haberny et al. 2002). For example, the NR2B subunit is strongly expressed at birth and generally declines throughout development whereas expression of the NR2A subunit is not detectable then, but increases dramatically by postnatal days 15–21 in rats and mice (Liu et al. 2004; Monyer et al. 1994; Sheng et al. 1994; Wenzel et al. 1997), although there are regional variations in prevalence of this NR2B to NR2A developmental shift (Lopez de Armentia and Sah 2003).
To the extent that functional blockade of the NMDA receptor systems by ethanol is critical for social suppression, adolescents would be expected to show a similar insensitivity to MK-801-induced social suppression relative to adults as they do to ethanol. Moreover, enhanced developmental expression of the NR2B subunit during adolescence (Wenzel et al. 1997) may render adolescents also relatively resistant to the social suppression resulting from blockade of this subunit. In contrast, a lower dose of the NR2A antagonist, PEAQX, may be effective for inducing social inhibition in adolescents, given developmental increases in expression of this subunit through adolescence into adulthood (Liu et al. 2004). These hypotheses were tested in three experiments examining potential ontogenetic differences in the socially inhibitory effects of MK-801 (experiment 1), PEAQX (experiment 2), and ifenprodil (experiment 3).
Methods
Subjects
A total of 240 male Sprague–Dawley rats derived from our breeding facility were used as experimental subjects, with an equal number of animals serving as test partners. On postnatal day (P) 1, all litters were culled to eight to ten pups, maintaining a sex ratio of six males to four females whenever possible. Pups were weaned at P21 and pair-housed with same-sexed littermates. Rats were given ad libitum access to food (Purina Lab chow, Lowell, MA) and water, maintained in a temperature-controlled vivarium with a 14:10-h light–dark cycle (lights on at 0700 hour), and treated in accordance with guidelines for animal care established by the National Institutes of Health under protocols approved by the Binghamton University Institutional Animal Care and Use Committee.
Experimental design
To assess potential age differences in sensitivity to the socially suppressive effects of MK-801, PEAQX, and ifenprodil (in experiments 1, 2, and 3, respectively), a two-age (adolescent, adult)×four-dose×two-test-day (baseline, test) mixed factorial was used in each experiment, with ten male subjects placed into each condition. No more than one animal from a litter served as a subject in an experimental condition. Males were utilized in the present series of experiments given that our laboratory has generally not observed sex differences in social activity (Varlinskaya and Spear 2002, 2012), allowing females to be used in other experiments. However, given that sex differences have been observed for other NMDA-related effects (e.g., analgesic responses, locomotor activity) (Frantz and Van Hartesveldt 1999; Kavaliers and Choleris 1997), future research should include females to determine the role of this receptor system on behavior.
Drugs
Both (+) MK 801(Dizocilpine) (Tocris) and PEAQX tetrasodium hydrate (Sigma Aldrich) were prepared in 0.9 % saline whereas ifenprodil hemitartrate (Tocris) was prepared in sterile water. All drugs were injected at a volume of 2 ml/kg, with MK-801 and ifenprodil injected intraperitoneally and PEAQX administered subcutaneously. The routes of administration and doses were based and modified from previous studies (Anderson and Spear 2012; Ramirez et al. 2011; Siviy et al. 1995).
Social-interaction apparatus and behavioral measures
Each age-scaled social interaction apparatus (30×20×20 cm for adolescents and 45×30×20 cm for adults) was composed of clear Plexiglas (Binghamton Plate Glass, Binghamton, NY), and was divided on the long axis into two equal sized compartments by a wall containing an aperture (measuring 7× 5 cm for adolescents and 9×7 cm for adults) that allowed animals to move between compartments. Behavioral measures scored during the 10-min videotaped sessions by an experimenter blind to the experimental condition of each animal included: play (pouncing or playful nape attack); social investigation (sniffing of any part of the partner’s body); and social contact (crawling over and under the partner and social grooming; see Varlinskaya and Spear 2002, for more details). The frequency of each behavior was scored, with a score of 1 for each time a component behavior began and stopped. For instance, an animal that playfully lunged at its partner and then pinned him would receive two frequencies for that play bout. Social behaviors were summed to get an overall social activity measure. Additionally, a preference/avoidance coefficient [Coefficient (%)=(Crossovers to the partner–Crossovers away from the partner)/(Crossovers to the partner + Crossovers away from the partner)×100] was used as an index of social motivation. An index of motor activity was calculated by summing the number of crossovers between compartments made by the experimental animal during the test.
Repeated testing procedure
Whereas previous studies examining social interactions utilized a 1-day test procedure (Morales et al. 2011; Varlinskaya and Spear 2002), a 2-day test procedure that allows each experimental animal to serve as its own control was examined and validated in experiment 1 and used in the subsequent experiments. Using this procedure, each animal received vehicle on baseline and drug challenge on test day, thereby serving as its own control. In experiment 1, experimental animals challenged with vehicle on baseline and test day demonstrated similar levels of social interactions on both days (see “Results” section). Given that repeated testing was found to have no impact on social behaviors in this test, the vehicle–vehicle group was eliminated in experiments 2 and 3, with differences in social behavior observed across days attributed to changes associated with administration of the challenge compounds rather than general changes in social behaviors due to repeated testing. Thus, the comparison of interest is between day 1 (baseline) and day 2 (drug challenge) for each drug/dose condition.
Experimental procedure
On P33 (adolescents) or P68 (adults), experimental and partner animals were individually placed into the social interaction apparatus for a 30-min habituation period to ensure that testing occurred in a familiar environment (File and Hyde 1978; Varlinskaya and Spear 2002). On the following day (day 1: baseline), each experimental animal was placed alone in a holding cage for 30 min and then injected with vehicle (experiments 1 and 2: saline; experiment 3: sterile water). Immediately post-injection, each animal was put into the social testing apparatus for 30 min, followed immediately by placement of a non-injected, non-manipulated partner of the same age and sex into the apparatus for a 10-min videotaped session. On day 2 (test: drug challenge), the same test procedure was used as on Day 1, except that experimental animals were challenged with the appropriate dose and drug (experiment 1: MK-801: 0, 0.05, 0.1, 0.2 mg/kg; experiment 2: PEAQX: 2.5, 5, 10, 20 mg/kg; experiment 3: ifenprodil: 1.5, 3, 6, 12 mg/kg). Each experimental animal was exposed to the same partner on both test days.
Data analysis
Data were subjected to an outlier test, with removal of any data points meeting the criteria of 2 standard deviations above or below the mean prior to data analysis. These tests resulted in elimination of <1 % of the total data points from each experiment. Given that expected differences in baseline levels of social activity emerged between adolescents and adults in all experiments (see “Results” section), data were analyzed separately for each age using a four-dose×two-test day analysis of variance (ANOVA). Fisher’s planned pairwise comparison test was used to explore significant effects and interactions. Given the presence of significant dose effects on locomotor activity in the ANOVAs of all drugs (see “Results” section), ANCOVAs were also conducted on data that yielded significant correlations between social behavior and locomotor activity to ensure that the social inhibitory effects of the NMDA receptor antagonists were not merely a function of reductions in general locomotor activity. In these analyses, social behavior was expressed as percentage of baseline to permit inclusion of age as a factor. Significant correlations between social behavior and locomotor activity emerged for adults in experiment 1 (r =0.63), adolescents and adults in experiment 2 (r =0.78 at each age), and adolescents and adults in experiment 3 (r =0.77 and 0.56, respectively); hence, all data were analyzed this way. Social preference/avoidance coefficient data were examined similarly, with a significant negative correlation emerging in adolescent rats from experiment 2 (i.e., PEAQX), (r = −0.63 precipitating an ANCOVA of these data). Data are expressed as mean±SEM.
Results
Experiment 1: effects of MK-801 on social interactions
Baseline levels of overall social activity
The ANOVA of overall social activity at baseline revealed a significant main effect of age [F(1,76)=19.66, p <0.0001], with adolescents displaying significantly higher levels of social activity relative to adults (117.58±4.37 and 94.20±3.03, respectively).
Total number of crosses following challenge with MK-801
In adolescents, the ANOVA of locomotor activity revealed no significant main effects or interactions. In adults, a main effect of day [F(1,36)=45.42, p <0.0001] was tempered by an interaction with dose, with motor activity being significantly suppressed after all doses of MK-801 [day×dose interaction: F(3,36)=3.75, p <0.05] (Table 1).
Table 1.
Total crossovers (mean±SEM) in adolescent and adult male rats challenged acutely with either MK-801, PEAQX, or ifenprodil
| Experiment | Adolescents
|
Adults
|
||
|---|---|---|---|---|
| Baseline | Test | Baseline | Test | |
| MK-801 | ||||
| 0 | 46.90±4.72 | 44.60±4.72 | 35.00±3.40 | 30.11±2.83 |
| 0.05 mg/kg | 45.30±3.11 | 42.60±5.01 | 37.33±2.75 | 26.33±4.40a |
| 0.10 mg/kg | 52.40±4.17 | 52.00±9.21 | 36.56±4.91 | 12.67±3.93a |
| 0.20 mg/kg | 52.60±4.96 | 42.50±7.01 | 38.10±3.63 | 17.60±4.53a |
| PEAQX | ||||
| 2.5 mg/kg | 55.80±3.77 | 49.00±3.56 | 37.90±3.26 | 35.20±3.58 |
| 5 mg/kg | 49.50±5.54 | 35.60±4.10a | 37.00±3.52 | 34.22±2.11 |
| 10 mg/kg | 56.25±2.69 | 15.50±1.76a | 35.50±1.27 | 23.10±4.30a |
| 20 mg/kg | 54.89±1.84 | 10.22±1.65a | 34.75±3.32 | 7.50±1.00a |
| Ifenprodil | ||||
| 1.5 mg/kg | 52.00±4.67 | 52.78±3.71b | 33.20±4.78 | 27.60±3.13 |
| 3 mg/kg | 60.11±7.32 | 45.67±5.86b | 36.90±3.20 | 28.90±3.55a |
| 6 mg/kg | 57.30±4.86 | 37.40±4.43b | 33.33±2.99 | 24.44±2.73a |
| 12 mg/kg | 60.20±4.98 | 35.20±5.89b | 34.20±2.84 | 15.30±5.16a |
A significant difference between baseline and test day for a given age and dose
A main effect of day, regardless of dose (p <0.05)
Social activity following MK-801 challenge
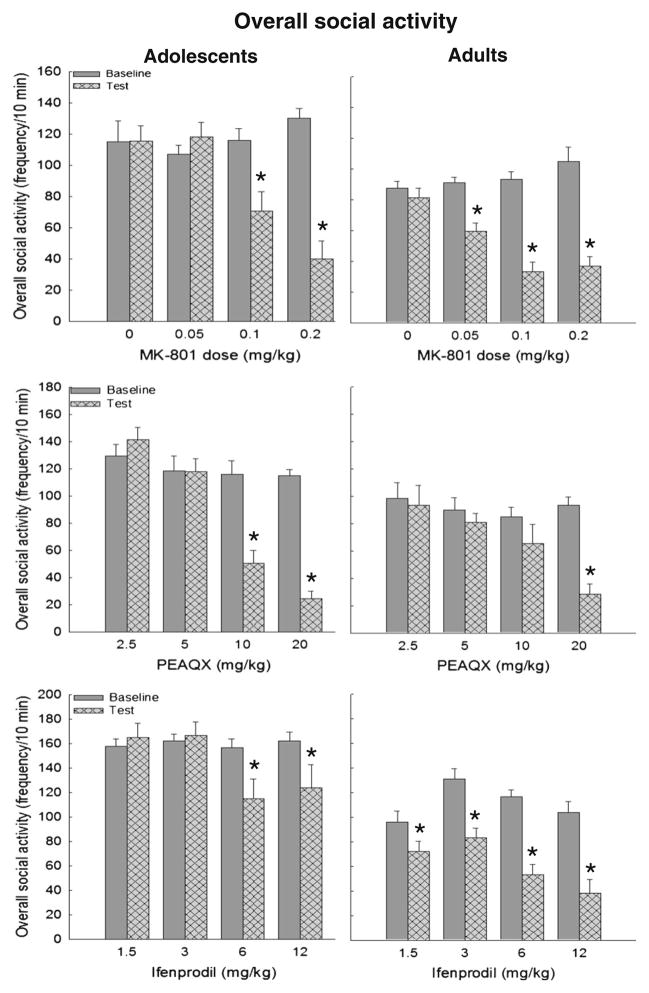
In adolescents, the ANOVA of overall social activity following challenge with MK-801 revealed a main effect of dose [F(3,34)=2.93, p <0.05] and day [F(1,34)= 42.47, p <0.0001] and their interaction, [F(3,34)=23.85, p <0.0001]. MK-801 at doses of 0.1 and 0.2 mg/kg significantly reduced the social activity of adolescents while saline and 0.05 mg/kg had no effect (Fig. 1, top left). In adults, analysis of overall social activity revealed main effects of dose [F(3,36)=3.66, p <0.05] and day [F(1,36)=122.33, p <0.0001], and a dose×day interaction [F(3,36)=14.11, p <0.0001], with all doses of MK-801 (i.e., 0.05 mg/kg and higher) significantly reducing overall social activity (Fig. 1, top right).
Fig. 1.
Overall social activity in adolescent (left panels) and adult (right ▶ panels) rats challenged acutely with vehicle on baseline (solid bars) and MK-801 (top), PEAQX (middle), or ifenprodil (bottom) on test day (hatched bars). Significant differences between baseline and test days are represented by asterisk
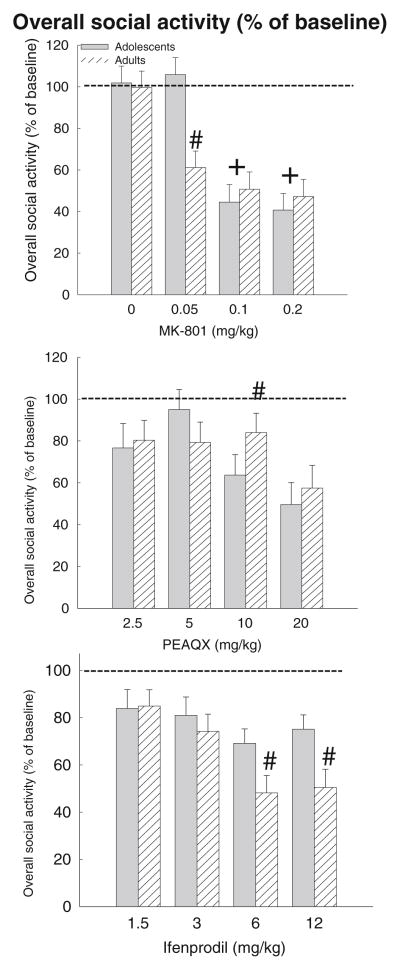
The ANCOVA for percentage of baseline overall social activity in MK-801-challenged animals revealed a significant main effect of locomotor activity [F(1,66)=7.09, p <0.01], dose [F(3,66)=36.01, p <0.00001] and an interaction between age and dose [F(3,66)=4.34, p <0.01]. Post hoc tests revealed that percentage of baseline social activity of animals challenged with 0.1 and 0.2 mg/kg MK-801 was significantly lower than vehicle-treated animals at both ages. Additionally, percentage of baseline social activity of adults was significantly lower than that of adolescents after 0.05 mg/kg MK-801, suggesting that adults were more impaired than adolescents at this dose (Fig. 2, left).
Fig. 2.
Percent (%) of baseline overall social activity in adolescent and adult rats on test day after challenge with MK-801 (top), PEAQX (middle), or ifenprodil (bottom) Significant differences between adolescents and adults are represented by number sign whereas significant differences from vehicle-treated control group is indicated with a plus sign
Social preference/avoidance
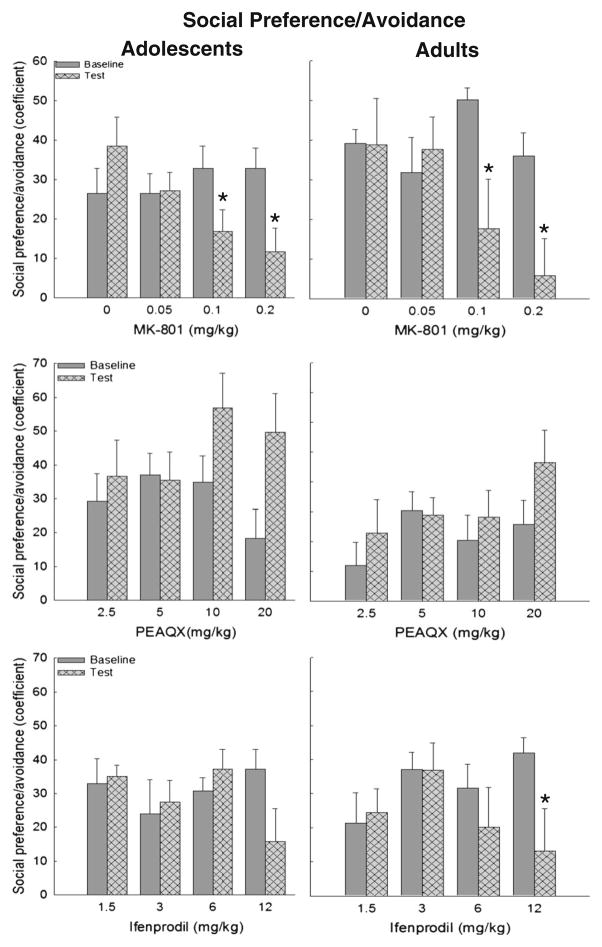
The ANOVA of the social preference/avoidance coefficient in adolescents revealed a dose×day interaction [F(3,34)=4.13, p <0.01], with 0.1 and 0.2 mg/kg significantly reducing social motivation. In adults, a main effect of day [F(1,36)=7.68, p < 0.01] and an interaction of dose and day [F(3,36)=3.74, p < 0.05] emerged, also revealing significant decreases in social motivation was significantly after 0.1 and 0.2 mg/kg MK-801 (Fig. 3, top panels).
Fig. 3.
Social preference/avoidance coefficients in adolescent (left ▶ panels) and adult (right panels) rats after MK-801 (top), PEAQX (middle), or ifenprodil (bottom). Significant differences between baseline and test day are represented by asterisk
Experiment 2: effects of PEAQX on social interactions
Baseline levels of overall social activity
The ANOVA of overall social activity revealed a main effect of age [F(1,73)=20.76, p <0.00001]. As expected, adolescents demonstrated significantly higher levels of social interactions relative to adults (119.89±4.48 and 91.69±4.27, respectively).
Total number of crosses following challenge with PEAQX
The ANOVA of locomotor activity in adolescents revealed a main effect of dose [F(3,33)=8.83, p <0.001], day [F(1,33)= 184.43, p <0.00001] and a dose×day interaction [F(3,33)= 23.83, p <0.00001], with locomotor activity significantly reduced following 5, 10, and 20 mg/kg in adolescents. In adults, effects of dose [F(3,33)=6.88, p <0.01], day [F(1,33)=47.54, p <0.00001] and their interaction [F(3,33)=11.62, p <0.0001] emerged, with locomotor activity significantly reduced at 10 and 20 mg/kg (Table 1).
Social activity following PEAQX challenge
In adolescents, the ANOVA of overall social activity revealed significant main effects of dose [F(3,32)=20.65, p <0.00001], day [F(1,32)=34.25, p <0.00001], and a dose×day interaction [F(3,32)=15.71, p <0.00001]. Adolescents demonstrated significant social inhibition following doses of 10 and 20 mg/kg (Fig. 1, middle left). In adults, the ANOVA of overall social activity revealed main effects of dose [F(3,35)=2.87, p <0.05], day [F(1,35)=23.04, p <0.0001], and an interaction between dose and day [F(3,35)=7.15, p <0.0001]. In contrast to adolescents, adults only showed significant social inhibition after 20 mg/kg (Fig. 1, middle right).
The ANCOVA for percentage of baseline overall social activity yielded main effects of locomotor activity [F(1,62)= 10.21, p <0.01], dose [F(3,62)=20.70, p <0.0001] and an age×dose interaction [F(3,62)=2.71, p <0.05]. Post hoc tests revealed that percentage of baseline social activity was significantly lower in adolescents than adults after 10 mg/kg, suggesting that adolescents were more sensitive to PEAQX-induced social inhibition (Fig. 2, middle).
Social preference/avoidance
In adolescents, the ANOVA of the social preference/avoidance coefficient revealed a main effect of dose [F(3,32)=3.23, p <0.05], day [F(1,32)=11.93, p <0.01] and a dose×day interaction [F(3,32)=5.86, p <0.01]. Adolescent rats demonstrated significantly higher levels of social motivation on test than baseline day following 10 and 20 mg/kg. However, in the ANCOVA of these data where total locomotion was included as a covariate, no main effects or interactions reached significance, suggesting that this drug effect was not reliable and was likely associated with alterations in general locomotion. In adults, no significant main effects of interaction emerged (Fig. 3, middle).
Experiment 3: effects of ifenprodil on social interactions
Baseline levels of overall social activity
The ANOVA of overall social activity at baseline revealed a main effect of age [F (1,73) = 73.42, p < 0.000001]. Adolescents displayed significantly higher levels of social activity than adults did (159.84±3.26 and 112.32±4.51, respectively).
Total number of crosses following challenge with ifenprodil
In adolescents, the ANOVA of locomotor activity yielded a main effect of day [F(1,35)=19.69, p <0.0001], with locomotor activity being significantly lower on test day. In adults, a main effect of day [F(1,35)=43.09, p <0.000001] and a day× dose interaction [F(3,35)=3.55, p <0.05] emerged, with locomotor activity being significantly reduced after 3 mg/kg and higher (Table 1).
Social activity following ifenprodil challenge
In adolescents, the ANOVA of overall social activity yielded a main effect of day [F(1,34)=5.44, p <0.05] and a dose×day interaction [F(3,34)=3.30, p <0.05]. Significant reductions in overall social activity were observed following the 6- and 12-mg/kg doses (Fig. 1, bottom left). The ANOVA of overall social activity in adults yielded main effects of dose [F (3,33) =4.23, p < 0.05], day [F (1,33)= 142.73, p < 0.00001], and a dose×day interaction [F(3,33)=5.18, p < 0.01]. Adults demonstrated a significant reduction in overall social interactions at all doses of ifenprodil tested (Fig. 1, bottom right).
The ANCOVA of percentage of baseline overall social activity revealed a main effect of locomotor activity [F(1,56)=34.83, p <0.00001], age [F(1,56)=4.20, p <0.05], dose [F(3,56)=5.62, p <0.01], and an age×dose interaction [F (3,56)=4.10, p <0.05]. Post hoc tests revealed that adolescents had significantly higher percentage of baseline levels of social activity after 6 and 12 mg/kg than did adults, indicating that adults were more socially impaired than adolescents. (Fig. 2, right panel).
Social preference/avoidance
In adolescents, no significant effects or interactions emerged for the social preference/avoidance coefficient. In adults, a main effect of day [F(1,33)=4.34, p <0.05] as well as an interaction of day with dose [F(3,34)=3.65, p <0.05] revealed that adults challenged with the highest dose of ifenprodil (12 mg/kg) demonstrated significantly lower levels of social motivation (Fig. 3, bottom panels).
Discussion
The results from the current experiments demonstrate that pharmacological blockade of the NMDA receptor with the non-competitive antagonist, MK-801, and subtype-selective NR2A and NR2B antagonists, PEAQX and ifenprodil, respectively, resulted in age-related differences in sensitivity to social inhibition. Adolescent rats required a higher dose of MK-801 and ifenprodil to reduce social interactions than adults did, whereas an opposite age effect emerged after PEAQX. Locomotor activity (indexed via total number of crossovers) was generally suppressed in animals after administration of each compound. However, ANCOVAs conducted using locomotor activity as a covariate revealed similar age differences in sensitivity as observed in the ANOVAs of overall social activity.
Although adolescents were more resistant than adults to the social inhibitory effects of MK-801, the social preference/avoidance coefficient yielded similar findings at both ages. Given that this is the first time MK-801 has been used in the modified social interaction test, it is unclear why age differences were not evident for this measure of social motivation. These findings are reminiscent, however, of other data that administration of NMDA antagonists impairs social motivation in adolescents and adults (Audet et al. 2009; Moy et al. 2012; Rung et al. 2005). Adolescents were not affected by any dose of ifenprodil in terms of social motivation whereas adults showed a reduction at the highest dose, an age-related difference similar to that seen with the social activity data. In contrast to the age differences seen in PEAQX’s effects on social activity, the drug did not reliably alter social motivation at either age (although adolescents demonstrated a PEAQX-associated increase in social preference at the two highest doses, this effect did not hold when activity was covaried in an ANCOVA of the data). These differences in patterns of age differences in drug responsiveness for overall social activity and social motivation per se suggests that the neural substrates underlying these behaviors may differ.
To our knowledge, these are the first experiments to examine age differences in sensitivity to the non-competitive NMDA receptor antagonist, MK-801, as well as NR2A (PEAQX) and NR2B (ifenprodil) antagonists on social behavior. Socially suppressing effects of MK-801 in adolescents seen here are reminiscent of the adolescent-only data of Siviy et al. (1995) and Moy et al. (2012) in adolescent rats and mice, whereas our adult data replicate reductions in social behaviors reported in adult male rodents following drugs that block the NMDA receptor complex (e.g., MK-801, ketamine; de Moura Linck et al. 2008; Gururajan et al. 2011; Silvestre et al. 1997) and using genetically bred mice (Deutsch et al. 2011; Jacome et al. 2011; Labrie et al. 2008).
The decreased sensitivity of adolescents to the socially suppressive effects of NMDA manipulations when compared to adults may be related to maturational differences in subunit expression that occur between young and mature animals (Kornhuber et al. 1988; McDonald and Johnston 1990; Monyer et al. 1994; Wenzel et al. 1997). Indeed, adolescents were less sensitive to pharmacological blockade of the NR2B subunit with the NR2B antagonist, ifenprodil, findings consistent with other studies that have suggested a role for the NR2B subunit in socially-related behaviors in rodents (Jacobs and Tsien 2012; Knapp et al. 2004; Ma et al. 2006; Sukhotina et al. 1998). The relatively delayed ontogenetic expression of the NR2A subunit is consistent with findings of experiment 2 where adults were less affected by pharmacological blockade of the NR2A subunit, as evidenced by social inhibition after only the highest dose of PEAQX whereas adolescents showed a reduction in overall social activity after the two highest doses. Unfortunately, studies investigating effects of NR2A antagonists on social behaviors are lacking (however, see Bortolato et al. 2012; Turnock-Jones et al. 2009; Zhao et al. 2009), let alone direct age comparisons of behavioral consequences of such antagonists between adolescents and adults. This may be due partly to the lack of available NR2A-selective antagonists, with even some controversy existing regarding the subunit selectivity of PEAQX (Berberich et al. 2005; Frizelle et al. 2006; Neyton and Paoletti 2006). The results from experiment 2 provide additional evidence for the involvement of the NR2A subunit in modulating social interactions, and for age differences in sensitivity to the social inhibitory effects observed after acute challenge with an NR2A antagonist between adolescent and adult rats.
These ontogenetic differences in sensitivity to MK-801 and the NR2B antagonist, ifenprodil, are reminiscent of adolescent-characteristic insensitivities to the socially suppressing effects of ethanol in male rats (Morales et al. 2011; Varlinskaya and Spear 2006) and are in line with previous results from our laboratory demonstrating that adolescents are less sensitive to ifenprodil’s ability to block acute tolerance to the motor impairing effects of ethanol when compared to adults (Ramirez et al. 2011). Given that NMDA receptors are a major target for ethanol’s inhibitory, antagonistic effects, ontogenetically enhanced expression of NMDA receptors, especially those that are NR2B-containing (Liu et al. 2004), may contribute to the commonly reported resistance of adolescents to aversive social inhibitory and motor impairing effects of ethanol (Morales et al. 2011; Ramirez and Spear 2010; Varlinskaya and Spear 2006). In contrast, adolescent-typical insensitivities were not evident after blockade of the NR2A subunit that shows a relatively protracted ontogeny (Liu et al. 2004). Along with an adolescent-related insensitivity to intake-moderating cues that may be related in part to the NR2B subunit, adolescents also conversely appear more sensitive to the socially facilitating effects of low doses of ethanol compared to adults (Varlinskaya and Spear 2002). This ethanol-induced social facilitation could also contribute to enhanced propensities for ethanol intake in adolescence, and likewise appears to be facilitated by blockade of NR2B and not NR2A NMDA subunits (Morales et al. 2013). Together, these data support the suggestion that the NR2B subunit may play a significant role in contributing to ontogenetic differences in sensitivities to ethanol-related modulation of social behaviors, which may in turn facilitate enhanced ethanol consumption during this developmental period.
Acknowledgments
The work presented in this manuscript was funded by grant P50-AA017823 to LPS
Footnotes
Conflict of interest The authors have no conflicts of interest to disclose.
Contributor Information
Melissa Morales, Email: mmorales@wakehealth.edu, Department of Physiology & Pharmacology, Wake Forest University School of Medicine, Winston-Salem, NC 27157, USA.
Linda P. Spear, Center for Development and Behavioral Neuroscience, Department of Psychology, Binghamton University, 4400 Vestal Parkway East, Binghamton, NY 13902-6000, USA
References
- Allgaier C. Ethanol sensitivity of NMDA receptors. Neurochem Int. 2002;41:377–382. doi: 10.1016/s0197-0186(02)00046-3. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Spear LP. NMDA 2A and 2B receptor involvement in the discriminative stimulus properties of ethanol in adult male rats. Society for Neuroscience; New Orleans: 2012. [Google Scholar]
- Anderson RI, Varlinskaya EI, Spear LP. Ethanol-induced conditioned taste aversion in male Sprague–Dawley rats: impact of age and stress. Alcohol Clin Exp Res. 2010;34:2106–2115. doi: 10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audet MC, Goulet S, Dore FY. Impaired social motivation and increased aggression in rats subchronically exposed to phencyclidine. Physiol Behav. 2009;96:394–398. doi: 10.1016/j.physbeh.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Berberich S, Punnakkal P, Jensen V, Pawlak V, Seeburg PH, Hvalby O, Kohr G. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci Off J Soc Neurosci. 2005;25:6907–6910. doi: 10.1523/JNEUROSCI.1905-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt TJ. The features and effects of friendship in early adolescence. Child Dev. 1982;53:1447–1460. [Google Scholar]
- Bortolato M, Godar SC, Melis M, Soggiu A, Roncada P, Casu A, Flore G, Chen K, Frau R, Urbani A, Castelli MP, Devoto P, Shih JC. NMDARs mediate the role of monoamine oxidase A in pathological aggression. J Neurosci Off J Soc Neurosci. 2012;32:8574–8582. doi: 10.1523/JNEUROSCI.0225-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton CL, Fletcher PJ. Age and sex differences in impulsive action in rats: the role of dopamine and glutamate. Behav Brain Res. 2012;230:21–33. doi: 10.1016/j.bbr.2012.01.046. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Csikszentmihalyi M, Larson R, Prescott S. The ecology of adolescent activity and experience. J Youth Adolesc. 1977;6:281–294. doi: 10.1007/BF02138940. [DOI] [PubMed] [Google Scholar]
- de Moura Linck V, Herrmann AP, Goerck GC, Iwu MM, Okunji CO, Leal MB, Elisabetsky E. The putative antipsychotic alstonine reverses social interaction withdrawal in mice. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32:1449–1452. doi: 10.1016/j.pnpbp.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Burket JA, Jacome LF, Cannon WR, Herndon AL. D-Cycloserine improves the impaired sociability of the Balb/c mouse. Brain Res Bull. 2011;84:8–11. doi: 10.1016/j.brainresbull.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob GF, Li TK, Tabakoff B. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- File SE, Hyde JR. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Frantz K, Van Hartesveldt C. Locomotion elicited by MK801 in developing and adult rats: temporal, environmental, and gender effects. Eur J Pharmacol. 1999;369:145–157. doi: 10.1016/s0014-2999(99)00070-9. [DOI] [PubMed] [Google Scholar]
- Frizelle PA, Chen PE, Wyllie DJ. Equilibrium constants for (R)-[(S)-1-(4-bromo-phenyl)-ethylamino]-(2,3-dioxo-1,2,3,4-tetrahydroquinoxalin-5-yl)-methyl]-phosphonic acid (NVP-AAM077) acting at recombinant NR1/NR2A and NR1/NR2B N-methyl-D-aspartate receptors: implications for studies of synaptic transmission. Mol Pharmacol. 2006;70:1022–1032. doi: 10.1124/mol.106.024042. [DOI] [PubMed] [Google Scholar]
- Gururajan A, Taylor DA, Malone DT. Effect of cannabidiol in a MK-801-rodent model of aspects of schizophrenia. Behav Brain Res. 2011;222:299–308. doi: 10.1016/j.bbr.2011.03.053. [DOI] [PubMed] [Google Scholar]
- Haberny KA, Paule MG, Scallet AC, Sistare FD, Lester DS, Hanig JP, Slikker W., Jr Ontogeny of the N-methyl-D-aspartate (NMDA) receptor system and susceptibility to neurotoxicity. Toxicol Sci Off J Soc Toxicol. 2002;68:9–17. doi: 10.1093/toxsci/68.1.9. [DOI] [PubMed] [Google Scholar]
- Holstein SE, Spanos M, Hodge CW. Adolescent C57BL/6J mice show elevated alcohol intake, but reduced taste aversion, as compared to adult mice: a potential behavioral mechanism for binge drinking. Alcohol Clin Exp Res. 2011;35:1842–1851. doi: 10.1111/j.1530-0277.2011.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs SA, Tsien JZ. Genetic overexpression of NR2B subunit enhances social recognition memory for different strains and species. PloS one. 2012;7:e36387. doi: 10.1371/journal.pone.0036387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacome LF, Burket JA, Herndon AL, Deutsch SI. D-Cycloserine enhances social exploration in the Balb/c mouse. Brain Res Bull. 2011;85:141–144. doi: 10.1016/j.brainresbull.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Choleris E. Sex differences in N-methyl-D-aspartate involvement in kappa opioid and non-opioid predator-induced analgesia in mice. Brain Res. 1997;768:30–36. doi: 10.1016/s0006-8993(97)00569-6. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhuber J, Retz W, Riederer P, Heinsen H, Fritze J. Effect of antemortem and postmortem factors on [3H]glutamate binding in the human brain. Neurosci Lett. 1988;93:312–317. doi: 10.1016/0304-3940(88)90101-2. [DOI] [PubMed] [Google Scholar]
- Labrie V, Lipina T, Roder JC. Mice with reduced NMDA receptor glycine affinity model some of the negative and cognitive symptoms of schizophrenia. Psychopharmacology. 2008;200:217–230. doi: 10.1007/s00213-008-1196-6. [DOI] [PubMed] [Google Scholar]
- Liu XB, Murray KD, Jones EG. Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. J Neurosci Off J Soc Neurosci. 2004;24:8885–8895. doi: 10.1523/JNEUROSCI.2476-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez de Armentia M, Sah P. Development and subunit composition of synaptic NMDA receptors in the amygdala: NR2B synapses in the adult central amygdala. J Neurosci Off J Soc Neurosci. 2003;23:6876–6883. doi: 10.1523/JNEUROSCI.23-17-06876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YY, Guo CY, Yu P, Lee DY, Han JS, Cui CL. The role of NR2B containing NMDA receptor in place preference conditioned with morphine and natural reinforcers in rats. Exp Neurol. 2006;200:343–355. doi: 10.1016/j.expneurol.2006.02.117. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Johnston MV. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Brain Res Rev. 1990;15:41–70. doi: 10.1016/0165-0173(90)90011-c. [DOI] [PubMed] [Google Scholar]
- Mirshahi T, Woodward JJ. Ethanol sensitivity of heteromeric NMDA receptors: effects of subunit assembly, glycine and NMDAR1 Mg(2+)-insensitive mutants. Neuropharmacology. 1995;34:347–355. doi: 10.1016/0028-3908(94)00155-l. [DOI] [PubMed] [Google Scholar]
- Montemayor R. The relationship between parent-adolescent conflict and the amount of time adolescents spend alone and with parents and peers. Child Dev. 1982;53:1512–1519. [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Morales M, Varlinskaya EI, Spear LP. Age differences in the expression of acute and chronic tolerance to ethanol in male and female rats. Alcohol Clin Exp Res. 2011;35:1614–1624. doi: 10.1111/j.1530-0277.2011.01508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Varlinskaya EI, Spear LP. Low doses of the NMDA receptor antagonists, MK-801, PEAQX, and ifenprodil, induces social facilitation in adolescent male rats. Behav Brain Res. 2013;250:18–22. doi: 10.1016/j.bbr.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nonneman RJ, Shafer GO, Nikolova VD, Riddick NV, Agster KL, Baker LK, Knapp DJ. Disruption of social approach by MK-801, amphetamine, and fluoxetine in adolescent C57BL/6J mice. Neurotoxicol Teratol. 2012;36:36–46. doi: 10.1016/j.ntt.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci Off J Soc Neurosci. 2006;26:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, File SE. Multiple sites of action for anxiogenic drugs: behavioural, electrophysiological and biochemical correlations. Psychopharmacology. 1984;83:304–315. doi: 10.1007/BF00428536. [DOI] [PubMed] [Google Scholar]
- Ramirez RL, Spear LP. Ontogeny of ethanol-induced motor impairment following acute ethanol: assessment via the negative geo-taxis reflex in adolescent and adult rats. Pharmacol Biochem Behav. 2010;95:242–248. doi: 10.1016/j.pbb.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez RL, Varlinskaya EI, Spear LP. Effect of the selective NMDA NR2B antagonist, ifenprodil, on acute tolerance to ethanol-induced motor impairment in adolescent and adult rats. Alcohol Clin Exp Res. 2011;35:1149–1159. doi: 10.1111/j.1530-0277.2011.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rung JP, Carlsson A, Ryden Markinhuhta K, Carlsson ML. (+)-MK-801 induced social withdrawal in rats; a model for negative symptoms of schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2005;29:827–832. doi: 10.1016/j.pnpbp.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Anxiolytic effects of chlordiazepoxide blocked by injection of GABAA and benzodiazepine receptor antagonists in the region of the anterior basolateral amygdala of rats. Biol Psychiatry. 1995a;37:473–476. doi: 10.1016/0006-3223(94)00183-4. [DOI] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 1995b;52:701–706. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, DiFeliceantonio AG, Foscue E, Glowacz S, Haseeb N, Wang N, Zhou C, Kuhn CM. Aversive effects of ethanol in adolescent versus adult rats: potential causes and implication for future drinking. Alcohol Clin Exp Res. 2010;34:2061–2069. doi: 10.1111/j.1530-0277.2010.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. The effects of NMDA and GABAA pharmacological manipulations on ethanol sensitivity in immature and mature animals. Alcohol Clin Exp Res. 2002;26:449–456. [PubMed] [Google Scholar]
- Silvestre JS, Nadal R, Pallares M, Ferre N. Acute effects of ketamine in the holeboard, the elevated-plus maze, and the social interaction test in Wistar rats. Depress Anxiety. 1997;5:29–33. [PubMed] [Google Scholar]
- Siviy SM, Line BS, Darcy EA. Effects of MK-801 on rough-and-tumble play in juvenile rats. Physiol Behav. 1995;57:843–847. doi: 10.1016/0031-9384(94)00361-8. [DOI] [PubMed] [Google Scholar]
- Snigdha S, Neill JC. Improvement of phencyclidine-induced social behaviour deficits in rats: involvement of 5-HT1A receptors. Behav Brain Res. 2008;191:26–31. doi: 10.1016/j.bbr.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The behavioral neuroscience of adolescence. W. W. Norton & Company; New York: 2010. [Google Scholar]
- Sukhotina I, Dravolina O, Bespalov A. Place conditioning of mice with the NMDA receptor antagonists, eliprodil and dizocilpine. Eur J Pharmacol. 1998;362:103–110. doi: 10.1016/s0014-2999(98)00737-7. [DOI] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Achterberg EJ, Vanderschuren LJ. Nucleus accumbens mu-opioid receptors mediate social reward. J Neurosci Off J Soc Neurosci. 2011;31:6362–6370. doi: 10.1523/JNEUROSCI.5492-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnock-Jones JJ, Jennings CA, Robbins MJ, Cluderay JE, Cilia J, Reid JL, Taylor A, Jones DN, Emson PC, Southam E. Increased expression of the NR2A NMDA receptor subunit in the prefrontal cortex of rats reared in isolation. Synapse. 2009;63:836–846. doi: 10.1002/syn.20665. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Spruijt BM, Van Ree JM. Mu- and kappa-opioid receptor-mediated opioid effects on social play in juvenile rats. Eur J Pharmacol. 1995;276:257–266. doi: 10.1016/0014-2999(95)00040-r. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Doremus-Fitzwater TL, Spear LP. Repeated restraint stress alters sensitivity to the social consequences of ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2010;96:228–235. doi: 10.1016/j.pbb.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Dev Psychobiol. 2006;48:146–161. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague–Dawley rats: impact of social deprivation and test context familiarity. Behav Brain Res. 2008;188:398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Ethanol-induced social facilitation in adolescent rats: role of endogenous activity at mu opioid receptors. Alcohol Clin Exp Res. 2009;33:991–1000. doi: 10.1111/j.1530-0277.2009.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Increases in anxiety-like behavior induced by acute stress are reversed by ethanol in adolescent but not adult rats. Pharmacol Biochem Behav. 2012;100:440–450. doi: 10.1016/j.pbb.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, Spear NE. Social behavior and social motivation in adolescent rats: role of housing conditions and partner’s activity. Physiol Behav. 1999;67:475–482. doi: 10.1016/s0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Fritschy JM, Mohler H, Benke D. NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins. J Neurochem. 1997;68:469–478. doi: 10.1046/j.1471-4159.1997.68020469.x. [DOI] [PubMed] [Google Scholar]
- Willey AR, Varlinskaya EI, Spear LP. Social interactions and 50 kHz ultrasonic vocalizations in adolescent and adult rats. Behav Brain Res. 2009;202:122–129. doi: 10.1016/j.bbr.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Sun L, Jia H, Meng Q, Wu S, Li N, He S. Isolation rearing induces social and emotional function abnormalities and alters glutamate and neurodevelopment-related gene expression in rats. Prog Neuro-Psychopharmacol Biol Psychiatry. 2009;33:1173–1177. doi: 10.1016/j.pnpbp.2009.06.016. [DOI] [PubMed] [Google Scholar]