Abstract
The mammalian stress response is an integrated physiological and psychological reaction to real or perceived adversity. Glucocorticoids (GCs) are an important component of this response, acting to redistribute energy resources to both optimize survival in the face of challenge and restore homeostasis after the immediate threat has subsided. Release of GCs is mediated by the hypothalamo–pituitary–adrenocortical (HPA) axis, driven by a neural signal originating in the paraventricular nucleus (PVN). Stress levels of GCs bind to glucocorticoid receptors (GRs) in multiple body compartments, including brain, and consequently have wide-reaching actions. For this reason, GCs serve a vital function in feedback inhibition of their own secretion. Fast, non-genomic feedback inhibition of the HPA axis is mediated at least in part by GC signaling in the PVN, acting by a cannabinoid-dependent mechanism to rapidly reduce both neural activity and GC release. Delayed feedback termination of the HPA axis response is mediated by forebrain GRs, presumably by genomic mechanisms. GCs also act in the brainstem to attenuate neuropeptidergic excitatory input to the PVN via acceleration of mRNA degradation, providing a mechanism to attenuate future responses to stressors. Thus, rather than having a single defined feedback switch, GCs work through multiple neurocircuits and signaling mechanisms to coordinate HPA axis activity to suit the overall needs of multiple body systems.
Keywords: Hypothalamo–pituitary–adrenocortical axis, Corticotropin-releasing hormone, Corticosterone, Glucocorticoid receptor, Hypothalamus, Amygdala, Prefrontal cortex
Stress and the Hypothalamo–Pituitary–Adrenocortical Axis
The organismal response to stress (defined as a real or perceived threat to homeostasis or well-being) promotes survival via adjustments to ongoing physiological processes and behavior. The activation of multiple interacting processes, including the behavioral, autonomic, endocrine, and immune systems, acts to produce an integrated stress response. This article focuses on the neuroregulatory processes governing activity of the primary endocrine stress response, initiated by the hypothalamo–pituitary–adrenocortical (HPA) axis. Stimulation of the HPA axis occurs in reaction to or in anticipation of stress. Physiological threats (systemic stressors) initiate largely reflexive responses that can be triggered without conscious perception. However, the ability to anticipate threat requires the organism to interpret the significance of multi-modal sensory information with respect to previous experience. Thus, stimuli that predict adversity (psychogenic stressors) can generate an HPA response in the absence of an existing physiologic insult. The relevance of the anticipatory glucocorticoid (GC) response hinges on the predicted need for adaptive hormonal secretion in order to redistribute resources (e.g., energy) to meet the challenge. GC hormones (corticosterone in rodents, cortisol in humans), which are the ultimate product of HPA axis activation, act on multiple bodily systems to maintain homeostasis. Appropriate activation of the HPA axis by acute stress is critical, as impaired reactivity hinders physiological resilience and cognitive processes (e.g., learning and memory). However, many of the effects of GCs that are beneficial for short-term survival can be counterproductive or even deleterious if prolonged. Therefore, the activation and inhibition of GC release is a temporally regulated process involving rapid neuronal activation and efficient inhibition.
The hypothalamic paraventricular nucleus (PVN) is responsible for initiating HPA axis stress responses. Activation of the HPA axis is mediated by neurosecretory neurons localized in the medial parvocellular portion of the PVN (Fig. 1). These neurons project to blood vessels in the median eminence, where they release adrenocorticotropin (ACTH) secretagogues, the most potent of which are corticotropin-releasing hormone (CRH) and arginine vasopressin (Vale et al. 1981; Swanson et al. 1983). Adrenocorticotropin secretion from the anterior pituitary then leads to the synthesis and release of GCs from the adrenal cortex (Dallman and Jones 1973; Dallman et al. 1987). Paraventricular CRH neurons are activated by neural inputs from a number of sources. Stressors signaling systemic challenge are communicated by neurons providing direct excitation of the PVN via sensory input from sources detecting neural or humoral homeostatic imbalance (e.g., the nucleus of the solitary tract (NTS), circumventricular organs) (Ulrich-Lai and Herman 2009). In contrast, anticipatory HPA axis responses are mediated by multisynaptic limbic forebrain circuits. These brain structures have no direct inputs to the PVN and require synaptic relays in subcortical structures (Herman et al. 2003). In many cases, subcortical relay sites also receive homeostatic information, providing a means for integrating descending limbic input with the ongoing physiological state. Given the need to temporally constrain secretion, activity of the HPA axis is controlled by negative feedback, a process wherein end-products of the stress response (GCs) inhibit their own release.
Fig. 1.
The HPA axis regulates the endocrine stress response with activation mediated by CRH-containing neurons in the hypothalamic PVN. The release of CRH onto cells of the anterior pituitary induces the secretion of ACTH into systemic circulation. At the adrenal cortex, ACTH stimulates synthesis and release of GCs (cortisol in humans and corticosterone in rodents). GCs then activate MRs and GRs providing a feedback signal to regulate HPA axis activity
GC-mediated feedback involves both genomic and nongenomic mechanisms (de Kloet et al. 2005; Groeneweg et al. 2011). Fast feedback inhibition occurs within minutes, resulting in rapid termination of PVN activation and ACTH release following stress. Delayed feedback occurs over a longer time frame, and is mediated in part by genomic actions of the nuclear corticosteroid receptors (glucocorticoid receptor (GR) and mineralocorticoid receptor (MR)). Both GR and MR act as ligand-activated transcription factors, modifying the expression of a large number of genes. The MR has a very high affinity for endogenous GCs and regulates circadian secretory rhythms and ultradian pulsatility (de Kloet et al. 1998; de Kloet and Sarabdjitsingh 2008). Although the permissive actions of MR maintain cellular responses to GCs, only stress levels of GCs extensively bind GR, which is necessary for the inhibition of HPA stress responses (Reul and de Kloet 1985; de Kloet and Reul 1987; Boyle et al. 2005; de Kloet et al. 2005). The GR is densely expressed in the PVN as well as numerous brain regions implicated in HPA axis regulation, all of which may contribute to feedback regulation (Reul and de Kloet 1985; de Kloet and Reul 1987; Jankord and Herman 2008).
Local Fast Feedback Regulation at the PVN
The rapid effects of GCs were observed as early as the 1960’s. However, the mechanisms underlying nongenomic feedback were not understood until recently (Dallman and Yates 1969; Dallman 2005). The PVN is well positioned to receive direct input from blood–brain barrier permeable factors and appears to be a primary site for GC negative feedback of the HPA axis. GCs rapidly inhibit PVN CRH neurons by way of a membrane-associated GR (mGR) that suppresses excitatory synaptic inputs to the PVN (Di et al. 2003, 2005; Tasker and Herman 2011). Rapid GC feedback is mediated by GC-induced suppression of excitation (GSE), occurring as a result of postsynaptic G-protein activation and release of retrograde messengers that provide presynaptic inhibition of glutamate release (Di et al. 2003). Interestingly, GR antagonists do not completely block GSE, suggesting that GSE may be mediated by a novel membrane receptor or modification of the nuclear GR. There is electron microscopy and neurochemical evidence for GR localization to neuronal membranes, in particular postsynaptic membranes, supporting an alternative role for the so-called nuclear receptor (Johnson et al. 2005; Komatsuzaki et al. 2005; Wang and Wang 2009; Prager et al. 2010). This is further supported by recent data demonstrating that GSE is not present in animals bearing GR deletion in PVN neurons (Haam et al. 2010). Moreover, GCs conjugated to bovine serum albumin, which prevents the steroids from crossing the cell membrane, suppress restraint-induced HPA axis activation in the PVN in vivo (Evanson et al. 2010).
Nongenomic regulation of PVN neurons is mediated by intracellular signaling networks promoting the synthesis of endocannabinoids (eCBs) in the PVN (Di et al. 2009) (Fig. 2). Endocannabinoids are synthesized from lipid precursors in the membrane and bind the type 1 cannabinoid receptor (CB1) (Breivogel and Childers 1998; Malcher-Lopes et al. 2008). Arachidonoyl ethanolamide (also known as anandamide) and 2-arachidonoylglycerol, eCBs rapidly produced in the PVN following GC exposure, act as dendritic retrograde messengers to mediate GSE (Di et al. 2003; Malcher-Lopes et al. 2006; Evanson et al. 2010). Cannabinoid receptors are predominately localized in presynaptic terminals, and CB1 antagonism or knockout leads to elevated CRH expression in the PVN and increased plasma ACTH and CORT (Patel et al. 2004; Cota et al. 2007; Ginsberg et al. 2010; Hill and McEwen 2010). Furthermore, co-application of GCs and a CB1 inverse agonist block the GC-induced suppression of HPA axis responses to acute stress (Evanson et al. 2010). Collectively these findings suggest GCs act nongenomically to provide rapid feedback inhibition of the PVN via GC actions at mGR and subsequent dendritic synthesis and release of eCBs.
Fig. 2.
GCs can rapidly inhibit CRH release from PVN neurons by acting on membrane-associated receptors. Receptor activation leads to retrograde eCB signaling at CB1 receptors which suppresses excitation of presynaptic glutamatergic neurons
Monosynaptic Feedback Inputs to the PVN
The medial parvocellular PVN receives synaptic innervation from a relatively circumscribed set of central nervous system structures. In general, PVN afferent neurons are localized to regions known to receive inputs from somatic nociceptors, visceral afferents, and/or humoral sensory pathways (Kiss et al. 1996; Li et al. 1996). Thus, the majority of excitatory PVN-projecting neurons are positioned to evoke rapid, reflexive activation of the HPA axis. Importantly, these PVN-projecting neurons can be influenced by inputs from the forebrain, a process that may integrate anticipatory activation with physiological demand. The PVN receives a large input from brainstem noradrenergic and adrenergic neurons of the NTS and C1–C3 (Cunningham and Sawchenko 1988; Cunningham et al. 1990). Lesions of these neurons attenuate reflexive HPA axis responses to stressors, while adrenergic receptor stimulation activates CRH and ACTH secretion, indicating that norepinephrine and epinephrine are necessary and sufficient for HPA axis activation (Plotsky 1987; Plotsky et al. 1989). PVN-projecting neurons in the NTS also contain peptide neuromodulators (e.g., glucagon-like peptide-1 (GLP-1), neuropeptide Y) that are HPA axis excitatory (Sawchenko et al. 1985; Harfstrand 1987; Merchenthaler et al. 1999; Tauchi et al. 2008). In addition, serotonin participates in HPA axis activation by way of projections from the midbrain and pontine raphe nuclei (Sawchenko et al. 1983; Lowry 2002). Lesion studies indicate serotonin provides excitation of the HPA axis (Jorgensen et al. 1998). However, direct serotonin input to the PVN is somewhat limited as the majority of serotoninergic fibers terminate in the peri-PVN (Sawchenko et al. 1983). The raphe nuclei also heavily innervate limbic structures including the hippocampus, prefrontal cortex, and amygdala (Lowry 2002), suggesting that serotonin has both direct and indirect effects on HPA axis regulation.
The PVN receives heavy input from numerous regions of the hypothalamus, a large portion of which is inhibitory (Cullinan et al. 1993; Roland and Sawchenko 1993). GABAergic inputs to the PVN originate in hypothalamic nuclei involved in homeostatic regulation, including the dorsomedial hypothalamus (DMH), preoptic area (POA), arcuate nucleus, and lateral hypothalamus (Roland and Sawchenko 1993). The PVN also receives input from hypothalamic neuropeptide (e.g., CRH, alpha-melanoctye stimulation hormone) and glutamate expressing neurons (Ziegler and Herman 2000; Zhang and Felder 2004; Rinaman 2007; Ulrich-Lai et al. 2011). These inputs are predominately excitatory and serve to activate the HPA axis in response to homeostatic imbalance (Ziegler and Herman 2000; Bartanusz et al. 2004). The peri-PVN region contains large numbers of GABAergic neurons, some of which project into the PVN proper (Boudaba et al. 1996). This region also receives rich input from hypothalamic cell groups, serotonin neurons, and limbic structures, suggesting that this area may play a role in limiting information coming into the PVN (Cole and Sawchenko 2002; Herman et al. 2002). Thus, hypothalamic inputs both excite and inhibit the PVN, consistent with a role in the maintenance of systemic homeostasis.
Direct forebrain inputs to the parvocellular PVN are largely confined to discrete subnuclei of the bed nucleus of the stria terminalis (BST) (Sawchenko and Swanson 1983). In general, posterior regions of the BST inhibit HPA axis responses to stress whereas anterior BST neurons provide excitatory input to the PVN, a heterogeneity that may be related to differential limbic innervation of these regions (Herman et al. 1994; Dong et al. 2001b; Dong and Swanson 2004b, 2006; Choi et al. 2007). However, regional heterogeneity of BST effects on the HPA axis may be limited to acute stress as both the anterior and posterior BST provide inhibition of GC responses following chronic stress (Choi et al. 2008a, b).
Recent evidence suggests that monosynaptic PVN inputs may be targets of GC feedback. In the NTS, expression of mRNA encoding the stress-excitatory neuropeptide GLP-1 (preproglucagon (PPG)) is effectively suppressed by acute and chronic stress in a GC-dependent manner (Zhang et al. 2009). Loss of PPG mRNA is correlated with a reduction in GLP-1 fiber density in the PVN, indicative of reduced capacity for NTS neurons to release peptide and thereby stimulate ACTH release (Tauchi et al. 2008; Zhang et al. 2009). Stress/GC-induced reductions in PPG mRNA occur within 30 min and are not accompanied by reduced PPG gene transcription, indicating that GC effects are likely mediated by destabilization of existing RNA pools (Zhang et al. 2009). GCs are known to have effects on RNA stability in vitro, and may utilize this mechanism to limit excitation of the PVN following stress (Stellato 2004). Importantly, GRs are also localized in numerous hypothalamic PVN-projecting neurons, including the arcuate nucleus, POA, and DMH (Fuxe et al. 1987). It remains to be determined whether GCs can alter this largely GABAergic input to PVN neurons.
Forebrain Feedback (and Feedforward?) Regulation of HPA Axis
Limbic brain structures including the hippocampus, prefrontal cortex, amygdala, septum, and midline thalamus are critical for emotional responses and memory, making them prime candidates for modulating GC secretion with respect to prior experience. However, these regions have little or no direct interactions with medial parvocellular PVN neurons, requiring intermediary neurons to relay their influence on CRH release (Sawchenko and Swanson 1983; Ulrich-Lai and Herman 2009). Multiple limbic brain regions express GR and contribute to feedback integration (McEwen et al. 1968; Reul and de Kloet 1985; Boyle et al. 2005). While hippocampal and medial prefrontal cortical GRs are required for inhibition of the HPA axis (Diorio et al. 1993; Boyle et al. 2005) (Fig. 3), amygdaloid GRs can stimulate HPA responses (Beaulieu et al. 1986, 1987; Shepard et al. 2003; Myers and Greenwood-Van Meerveld 2011). Regulation of the HPA axis by forebrain limbic sites appears to be mediated by synapses onto neurons of the BST, hypothalamic nuclei, and brainstem nuclei that innervate the PVN directly.
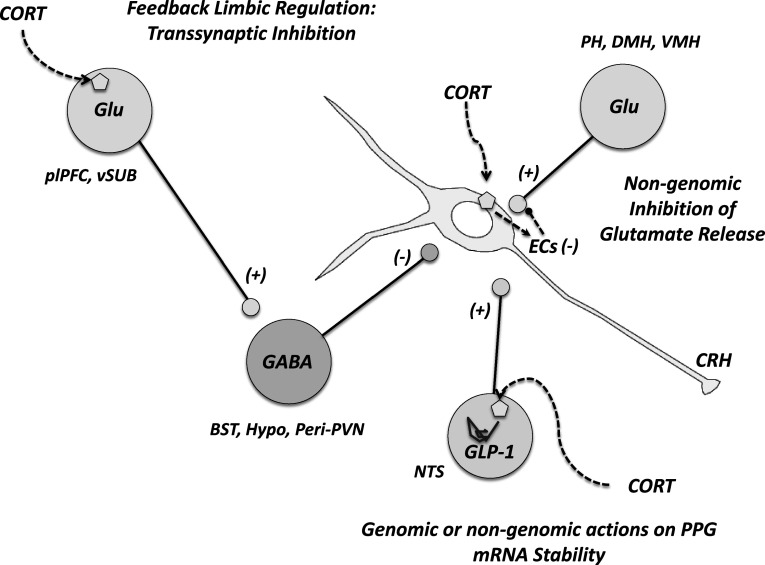
Fig. 3.
GC negative feedback can generally be divided into three interacting domains. First, GCs provide rapid, nongenomic inhibition of excitatory inputs to the PVN. In addition, GCs affect RNA stability in brain structures with direct, excitatory innervation of the PVN. Forebrain genomic GC signaling is also a key component of feedback regulation. Importantly, these structures have little or no direct interactions with the PVN and require intermediary synapses in PVN-projecting cell groups. Specifically, GCs act in the plPFC and the ventral subiculum to inhibit the PVN via GABAergic synaptic relays. BST: bed nucleus of stria terminalis, DMH dorsomedial hypothalamus, EC endocannabinoid, GLP-1 glucagon-like peptide-1, Glu glutamate, NTS nucleus of the solitary tract, PH posterior hypothalamus, plPFC prelimbic prefrontal cortex, VMH ventromedial hypothalamus, vSub ventral subiculum, + denotes excitation and − inhibition
The hippocampus is involved in terminating anticipatory HPA axis responses, consistent with its role in memory and emotion processing (Herman et al. 1989; Cullinan et al. 1993; Mueller et al. 2004; Radley and Sawchenko 2011). A role for the hippocampus in GC negative feedback is supported by the dense expression of GRs in the hippocampus (Reul and de Kloet 1985), as well as functional studies demonstrating diminished feedback efficacy following hippocampal lesions or local GR inactivation (van Haarst et al. 1997; Herman and Mueller 2006; Mueller et al. 2006). Forebrain GR knockout mice, which sustain GR deletion in the hippocampus (as well as prefrontal cortex and basolateral amygdala), also show delayed inhibition of the HPA axis response to psychogenic (but not systemic) stressors, further suggesting a role for the hippocampal GR in feedback (Furay et al. 2008). The output of the hippocampus is largely glutamatergic, and therefore excitatory. Hence, inhibition is thought to be mediated by activation of PVN-projecting GABAergic neurons in the BST, POA, DMH, and peri-PVN region (Cullinan et al. 1993; Choi et al. 2007; Radley and Sawchenko 2011).
The medial prefrontal cortex (mPFC) also modulates HPA axis activation, albeit in a more complex fashion. Numerous studies indicate that dorsal components of the mPFC (prelimbic (pl) PFC) inhibit HPA axis responses (Diorio et al. 1993; Figueiredo et al. 2003; Boyle et al. 2005; Radley et al. 2006, 2009). Indeed, anatomical studies suggest that plPFC activates inhibitory relays in the BST (Radley et al. 2009). Acute activation of the plPFC reduces GC secretion after psychogenic stress (Jones et al. 2011) and restraint-activated neurons of the plPFC express GR (Ostrander et al. 2003), suggesting that GC-sensitive neurons are engaged by stressors. Moreover, GC implants in the dorsal mPFC decrease GC release following acute restraint (Diorio et al. 1993), indicating that the inhibitory effect of the plPFC is at least in part GR-dependent.
Lesion studies indicate that the more ventral components of the mPFC (infralimbic (il) PFC) may be involved in stress excitation (Sullivan and Gratton 1998, 1999; Radley et al. 2006). Neurons of the ilPFC also appear to process GC information as they co-express c-Fos and GR after acute restraint (Ostrander et al. 2003). Notably, subcortical ilPFC projections differ substantially from those of the plPFC, targeting known stress-excitatory regions such as the central nucleus of the amygdala (CeA) and NTS (Hurley et al. 1991; Vertes 2004). Taken together, these studies suggest that the ilPFC may be involved in conveying GC feedforward rather than feedback information to subcortical HPA axis effector pathways.
Similar to ilPFC, the amygdala provides excitation of HPA axis responses (Roozendaal et al. 1991; Van de Kar et al. 1991; Roozendaal et al. 1992; Feldman et al. 1994; Feldman and Weidenfeld 1998; Shepard et al. 2003), although there is considerable functional differentiation among individual amygdalar regions (Dunn and Whitener 1986; Swanson and Petrovich 1998; Sah et al. 2003; Ulrich-Lai and Herman 2009). The CeA appears to be involved in activation of the HPA axis in response to systemic but not psychogenic stressors (Dayas et al. 1999; Xu et al. 1999; Dayas et al. 2001). Conversely, the medial amygdala may be selectively involved in generating anticipatory responses (Dayas et al. 1999, 2001; Ma and Morilak 2005; Solomon et al. 2010). Although the basolateral amygdala (BLA) is activated by both psychogenic and systemic stressors (Cullinan et al. 1995; Jones et al. 2011), relatively few studies have examined the role of this area in GC secretion. However, lesions of the BLA dampen HPA axis responses to psychogenic stress (Bhatnagar et al. 2004) while intra-BLA administration of CRH increases GC secretion (Daniels et al. 2004), indicating this area may also provide feedforward regulation of the HPA axis. The output of the basolateral amygdala is predominantly glutamatergic, suggesting that modulation of GC responses is mediated by excitatory PVN-projecting neurons as well as extensive interactions with other regions of the amygdala (Krettek and Price 1977; Pare et al. 1995; Dong et al. 2001a).
Amygdalar excitation of the HPA axis may be mediated by either transsynaptic disinhibition or excitation. Most projection neurons of the central and medial amygdala are primarily GABAergic, and both regions project heavily to the BST and POA, which contain populations of GABAergic PVN-projecting neurons (Prewitt and Herman 1998; Sah et al. 2003). Therefore, excitatory effects on HPA axis stress responses may be mediated by inhibition of GABAergic neurons in the hypothalamus or BST, effectively resulting in activation by disinhibition. There is also a specific projection from the CeA to the anterolateral BST, a stress-excitatory region that innervates the PVN (Dong et al. 2001a; Dong and Swanson 2004a; Choi et al. 2007). GCs localized to the CeA increase CRH mRNA in the CeA, anterolateral BST, and PVN (Shepard et al. 2000, 2003, 2006). Thus, in addition to GABAergic outflow, the CeA may provide peptidergic innervation of PVN-projecting, CRH-containing neurons in the BST that are in position to mediate trans-synaptic excitation from the CeA as well as excitatory outflow from regions such as the BLA.
Stress-regulatory amygdalar subnuclei also express GRs. Unlike the plPFC and hippocampus, amygdalar GRs appear to have excitatory effects on stress responses (Fig. 4). For example, systemic GCs increase CRH mRNA expression in the CeA (Schulkin et al. 1998) and stress-induced CeA CRH release is blocked by pretreatment with a GR antagonist (Cook 2002). Thus, GCs may have feedforward effects in the amygdala that can be linked to enhanced stress excitability. Dallman et al. (2003) propose that recruitment of amygdalar CRH may be a deleterious consequence of elevated GC release during chronic stress or stress-related diseases. Furthermore, the GR appears vital for normal function of amygdala circuits, as mice with specific disruption of the CeA GR have deficiencies in fear conditioning (Kolber et al. 2008).
Fig. 4.
Depending on physiological demand and anticipatory signals from the forebrain, GCs may provide feedforward excitation of the HPA axis. GCs can upregulate CRH signaling in the amygdala and BST, potentially prolonging GC secretion. GCs may also act on glutamatergic neurons in the ilPFC and BLA to excite PVN CRH neurons via synaptic relays in the hypothalamus. BLA basolateral amygdala, CeA central amygdala, ilPFC infralimbic prefrontal cortex, MeA medial amygdala, NE norepinephrine, + denotes excitation and − inhibition
The lateral septum inhibits HPA axis stress responses, and the anatomy of the septal region suggests interactions with hypothalamic and brainstem regions projecting to the PVN (Staiger and Nurnberger 1991; Singewald et al. 2011). As the majority of lateral septal neurons express GABA as their transmitter, the HPA inhibitory effects of this region are likely mediated by inhibition of excitatory projections to the PVN (Stevens et al. 1987). Expression of the MR is particularly pronounced in the lateral septum (Grillo et al. 1990), suggesting that this site may be highly sensitive to modulatory effects of low physiological GCs, perhaps as a means to set the tone of this stress-regulatory region.
Several reports implicate midline thalamic nuclei in HPA axis regulation, likely communicated via hypothalamic nuclei (Jaferi et al. 2003). Of the known stress-responsive thalamic nuclei, the paraventricular thalamus appears to play a major role in integrating HPA axis responses, mediating both habituation to familiar stressors and sensitization to novel stressors (Bhatnagar et al. 2002; Jaferi and Bhatnagar 2006). Once again, the paraventricular thalamus is rich in GRs (Fuxe et al. 1987), suggesting that GCs are capable of tuning the overall influence of this region on stress responses.
Consequences of Impaired Negative (or Enhanced Positive) Feedback
In the short-term, GC release is essential for mobilizing energy stores and suppressing non-essential processes to promote survival of the organism. However, chronic activation of the HPA axis can be directly detrimental (e.g., catabolic effects in muscle and bone). In addition, abnormally elevated or suppressed GCs can increase susceptibility to multiple pathological conditions including neuropsychiatric disorders and metabolic dysregulation. Major depressive disorder (MDD) and post-traumatic stress disorder (PTSD) are two of the most prevalent psychiatric conditions attributed to dysregulation of GC feedback. MDD is commonly associated with hypercortisolemia, whereas PTSD is frequently linked to GC hyposecretion (Yehuda et al. 2004; Radley et al. 2011; Yehuda and Seckl 2011). Both affective states correlate with altered activation or volume of the prefrontal cortex, amygdala, and hippocampus, suggesting that the disease processes involve the anticipatory stress response (Drevets 1999; Mayberg et al. 2005; Yehuda and LeDoux 2007; Krishnan and Nestler 2010; Myers-Schulz and Koenigs 2011). In addition, sensitization of reward pathways due to aberrant HPA axis activity has been proposed to drive vulnerability to addictive disorders, as GCs promote perseverance of addictive behavior (de Jong and de Kloet 2004; Uhart and Wand 2009; Frank et al. 2011).
Although GCs acutely enhance cognitive performance and improve memory consolidation, high circulating levels of GCs impair memory retrieval (Roozendaal 2002). Further, GCs can negatively affect processes governing dendritic plasticity, neurogenesis, and neuronal viability (Sapolsky et al. 1986; Pham et al. 2003; Radley et al. 2008). Stress is also a prominent risk factor for neurological conditions including epilepsy, Parkinson’s disease, Alzheimer’s disease, and chronic pain, suggesting that GCs play a role in the onset and progression of these disorders (Smith et al. 2002; Myers et al. 2007; Sotiropoulos et al. 2008; Kanner 2009; McEwen and Kalia 2010). Dysregulation of the HPA axis is also related to metabolic and cardiovascular disorders. Accumulating evidence suggests that heightened GCs contribute to visceral obesity, especially in the propensity to consume rewarding foods (Adam and Epel 2007). Cushing’s syndrome (characterized by hypercortisolemia) further illustrates the physiological consequences of prolonged GC exposure. The vast majority of these patients exhibit obesity, hypertension, impaired glucose tolerance, diabetes, and dyslipidemia (Sharma and Nieman 2011). Thus, appropriate initiation and cessation of HPA axis responses is essential for maintaining homeostatic balance and promoting adaptation in the face of adversity.
Mechanisms underlying HPA axis dysfunction in disease prominently involve alterations in GC feedback mechanisms. For example, a high proportion of depressed individuals fail to suppress HPA axis secretory activity following exogenous GC (dexamethasone) treatment, indicating a loss of feedback sensitivity (Carroll et al. 1980). Moreover, treatment with a GR antagonist can ameliorate symptoms in patients with psychotic depression (Belanoff et al. 2001), suggesting that the GR may be a viable therapeutic target for MDD. In contrast, HPA axis activity is often decreased in individuals with PTSD (Radley et al. 2011). In this case, GC negative feedback is enhanced, resulting in pathologically low HPA axis responsiveness to stress (Yehuda et al. 2004). Despite GC hyposecretion, PTSD patients may actually have prolonged GC exposure following stress due to reduced activity of GC-metabolizing enzymes (Yehuda et al. 2009; Yehuda and Seckl 2011). Notably, reduced HPA axis activity is thought to be a trait, rather than state variable, placing individuals that hyposecrete GCs at risk for development of disease.
Conclusion
Overall, the data suggest that GC feedback regulation of the HPA axis employs all realms of GC signaling: fast membrane-mediated inhibition of PVN excitation; trans-synaptic delayed feedback, involving genomic signaling; and even actions on RNA stability, effectively taking excitatory stress afferents ‘offline’. In some brain regions, such as the amygdala and perhaps ilPFC, GCs may even have positive feedback effects, promoting the actions of CRH. Furthermore, feedback is a distributed process, involving local signaling in hypothalamus, regulation of limbic inhibitory (and perhaps excitatory) outflow, and control of ascending brainstem projections to the PVN. In addition, it is important to consider that GC feedback is not the exclusive province of the brain. There are known inhibitory actions of GCs (albeit at high physiologic levels) on ACTH release by the pituitary (Miller et al. 1992). Numerous other organ systems with the capacity to signal into the CNS (e.g., adipose tissue) express GR (Bronnegard et al. 1990). Thus, GC feedback is best thought of as an integrative process involving central and peripheral compartments.
Control of the GC stress response is of substantial health significance, as dysregulation of GC secretion can lead to affective disease states and impaired metabolic and cardiovascular function. However, only a subset of stressed individuals develops these disorders, suggesting that genetic, social, and/or experiential factors determine individual vulnerability to HPA axis dysfunction. Additional research is needed to elucidate the factors that are responsible for vulnerability in some individuals and resistance in others.
Acknowledgments
The authors would like to acknowledge support from NIH grants DK059803 (BM), NS007453 (JMM), MH049698, MH069725, MH069860, and MH090574 (JPH). We are also grateful for the artistic contributions of Anne Christiansen and Nathan Evanson.
Abbreviations
- ACTH
Adrenocorticotropin
- BLA
Basolateral amygdala
- BST
Bed nucleus of the stria terminalis
- CB1
Type 1 cannabinoid receptor
- CeA
Central nucleus of the amygdala
- CRH
Corticotropin-releasing hormone
- DMH
Dorsomedial hypothalamus
- eCB
Endocannabinoid
- GC
Glucocorticoid
- GLP-1
Glucagon-like peptide-1
- GR
Glucocorticoid receptor
- GSE
Glucocorticoid-induced suppression of excitation
- HPA
Hypothalamo–pituitary–adrenocortical
- ilPFC
Infralimbic prefrontal cortex
- MDD
Major depressive disorder
- mGR
Membrane-associated GR
- mPFC
Medial prefrontal cortex
- MR
Mineralocorticoid receptor
- NTS
Nucleus of the solitary tract
- plPFC
Prelimbic prefrontal cortex
- POA
Preoptic area
- PPG
Preproglucagon
- PTSD
Post-traumatic stress disorder
- PVN
Paraventricular nucleus
Footnotes
Special Issue on Stress (Kvetnansky and Saavedra, Editors).
References
- Adam TC, Epel ES (2007) Stress, eating and the reward system. Physiol Behav 91:449–458 [DOI] [PubMed] [Google Scholar]
- Bartanusz V, Muller D, Gaillard RC, Streit P, Vutskits L, Kiss JZ (2004) Local gamma-aminobutyric acid and glutamate circuit control of hypophyseotrophic corticotropin-releasing factor neuron activity in the paraventricular nucleus of the hypothalamus. Eur J Neurosci 19:777–782 [DOI] [PubMed] [Google Scholar]
- Beaulieu S, Di Paolo T, Barden N (1986) Control of ACTH secretion by the central nucleus of the amygdala: implication of the serotoninergic system and its relevance to the glucocorticoid delayed negative feedback mechanism. Neuroendocrinology 44:247–254 [DOI] [PubMed] [Google Scholar]
- Beaulieu S, Di Paolo T, Cote J, Barden N (1987) Participation of the central amygdaloid nucleus in the response of adrenocorticotropin secretion to immobilization stress: opposing roles of the noradrenergic and dopaminergic systems. Neuroendocrinology 45:37–46 [DOI] [PubMed] [Google Scholar]
- Belanoff JK, Flores BH, Kalezhan M, Sund B, Schatzberg AF (2001) Rapid reversal of psychotic depression using mifepristone. J Clin Psychopharmacol 21:516–521 [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Huber R, Nowak N, Trotter P (2002) Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J Neuroendocrinol 14:403–410 [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C, Denski K (2004) Regulation of chronic stress-induced changes in hypothalamic-pituitary-adrenal activity by the basolateral amygdala. Ann N Y Acad Sci 1032:315–319 [DOI] [PubMed] [Google Scholar]
- Boudaba C, Szabo K, Tasker JG (1996) Physiological mapping of local inhibitory inputs to the hypothalamic paraventricular nucleus. J Neurosci 16:7151–7160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y, Muglia LJ (2005) Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci USA 102:473–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR (1998) The functional neuroanatomy of brain cannabinoid receptors. Neurobiol Dis 5:417–431 [DOI] [PubMed] [Google Scholar]
- Bronnegard M, Arner P, Hellstrom L, Akner G, Gustafsson JA (1990) Glucocorticoid receptor messenger ribonucleic acid in different regions of human adipose tissue. Endocrinology 127:1689–1696 [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Schroeder K, Mukhopadhyay S, Greden JF, Feinberg M, Ritchie J, Tarika J (1980) Plasma dexamethasone concentrations and cortisol suppression response in patients with endogenous depression. J Clin Endocrinol Metab 51:433–437 [DOI] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP (2007) Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci 27:2025–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Evanson NK, Furay AR, Ulrich-Lai YM, Ostrander MM, Herman JP (2008a) The anteroventral bed nucleus of the stria terminalis differentially regulates hypothalamic-pituitary-adrenocortical axis responses to acute and chronic stress. Endocrinology 149:818–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ulrich-Lai YM, Nguyen MM, Ostrander MM, Herman JP (2008b) The role of the posterior medial bed nucleus of the stria terminalis in modulating hypothalamic-pituitary-adrenocortical axis responsiveness to acute and chronic stress. Psychoneuroendocrinology 33:659–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RL, Sawchenko PE (2002) Neurotransmitter regulation of cellular activation and neuropeptide gene expression in the paraventricular nucleus of the hypothalamus. J Neurosci 22:959–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CJ (2002) Glucocorticoid feedback increases the sensitivity of the limbic system to stress. Physiol Behav 75:455–464 [DOI] [PubMed] [Google Scholar]
- Cota D, Steiner MA, Marsicano G, Cervino C, Herman JP, Grubler Y, Stalla J, Pasquali R, Lutz B, Stalla GK, Pagotto U (2007) Requirement of cannabinoid receptor type 1 for the basal modulation of hypothalamic-pituitary-adrenal axis function. Endocrinology 148:1574–1581 [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Watson SJ (1993) Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol 332:1–20 [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ (1995) Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 64:477–505 [DOI] [PubMed] [Google Scholar]
- Cunningham ET Jr, Sawchenko PE (1988) Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol 274:60–76 [DOI] [PubMed] [Google Scholar]
- Cunningham ET Jr, Bohn MC, Sawchenko PE (1990) Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol 292:651–667 [DOI] [PubMed] [Google Scholar]
- Dallman MF (2005) Fast glucocorticoid actions on brain: back to the future. Front Neuroendocrinol 26:103–108 [DOI] [PubMed] [Google Scholar]
- Dallman MF, Jones MT (1973) Corticosteroid feedback control of ACTH secretion: effect of stress-induced corticosterone secretion on subsequent stress responses in the rat. Endocrinology 92:1367–1375 [DOI] [PubMed] [Google Scholar]
- Dallman MF, Yates FE (1969) Dynamic asymmetries in the corticosteroid feedback path and distribution-metabolism-binding elements of the adrenocortical system. Ann N Y Acad Sci 156:696–721 [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Cascio CS, Darlington DN, Jacobson L, Levin N (1987) Regulation of ACTH secretion: variations on a theme of B. Recent Prog Horm Res 43:113–173 [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S (2003) Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci USA 100:11696–11701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels WM, Richter L, Stein DJ (2004) The effects of repeated intra-amygdala CRF injections on rat behavior and HPA axis function after stress. Metab Brain Dis 19:15–23 [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA (1999) Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur J Neurosci 11:2312–2322 [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA (2001) Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci 14:1143–1152 [DOI] [PubMed] [Google Scholar]
- de Jong IE, de Kloet ER (2004) Glucocorticoids and vulnerability to psychostimulant drugs: toward substrate and mechanism. Ann N Y Acad Sci 1018:192–198 [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Reul JM (1987) Feedback action and tonic influence of corticosteroids on brain function: a concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology 12:83–105 [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Sarabdjitsingh RA (2008) Everything has rhythm: focus on glucocorticoid pulsatility. Endocrinology 149:3241–3243 [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Vreugdenhil E, Oitzl MS, Joels M (1998) Brain corticosteroid receptor balance in health and disease. Endocr Rev 19:269–301 [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F (2005) Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6:463–475 [DOI] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Halmos KC, Tasker JG (2003) Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci 23:4850–4857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Marcheselli VL, Bazan NG, Tasker JG (2005) Rapid glucocorticoid-mediated endocannabinoid release and opposing regulation of glutamate and gamma-aminobutyric acid inputs to hypothalamic magnocellular neurons. Endocrinology 146:4292–4301 [DOI] [PubMed] [Google Scholar]
- Di S, Maxson MM, Franco A, Tasker JG (2009) Glucocorticoids regulate glutamate and GABA synapse-specific retrograde transmission via divergent nongenomic signaling pathways. J Neurosci 29:393–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ (1993) The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci 13:3839–3847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Swanson LW (2004a) Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol 468:277–298 [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW (2004b) Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol 471:396–433 [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW (2006) Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol 494:142–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW (2001a) Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev 38:192–246 [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW (2001b) Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol 436:430–455 [DOI] [PubMed] [Google Scholar]
- Drevets WC (1999) Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci 877:614–637 [DOI] [PubMed] [Google Scholar]
- Dunn JD, Whitener J (1986) Plasma corticosterone responses to electrical stimulation of the amygdaloid complex: cytoarchitectural specificity. Neuroendocrinology 42:211–217 [DOI] [PubMed] [Google Scholar]
- Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP (2010) Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology 151:4811–4819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman S, Weidenfeld J (1998) The excitatory effects of the amygdala on hypothalamo-pituitary-adrenocortical responses are mediated by hypothalamic norepinephrine, serotonin, and CRF-41. Brain Res Bull 45:389–393 [DOI] [PubMed] [Google Scholar]
- Feldman S, Conforti N, Itzik A, Weidenfeld J (1994) Differential effect of amygdaloid lesions on CRF-41, ACTH and corticosterone responses following neural stimuli. Brain Res 658:21–26 [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP (2003) The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci 18:2357–2364 [DOI] [PubMed] [Google Scholar]
- Frank MG, Watkins LR, Maier SF (2011) Stress- and glucocorticoid-induced priming of neuroinflammatory responses: potential mechanisms of stress-induced vulnerability to drugs of abuse. Brain Behav Immun 25(Suppl 1):S21–S28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furay AR, Bruestle AE, Herman JP (2008) The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology 149:5482–5490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Cintra A, Agnati LF, Harfstrand A, Wikstrom AC, Okret S, Zoli M, Miller LS, Greene JL, Gustafsson JA (1987) Studies on the cellular localization and distribution of glucocorticoid receptor and estrogen receptor immunoreactivity in the central nervous system of the rat and their relationship to the monoaminergic and peptidergic neurons of the brain. J Steroid Biochem 27:159–170 [DOI] [PubMed] [Google Scholar]
- Ginsberg AB, Pecoraro NC, Warne JP, Horneman HF, Dallman MF (2010) Rapid alteration of stress-induced hypothalamic-pituitary-adrenal hormone secretion in the rat: a comparison of glucocorticoids and cannabinoids. Stress 13:248–257 [DOI] [PubMed] [Google Scholar]
- Grillo C, Vallee S, McEwen BS, De Nicola AF (1990) Properties and distribution of binding sites for the mineralocorticoid receptor antagonist [3H]ZK 91587 in brain. J Steroid Biochem 35:11–15 [DOI] [PubMed] [Google Scholar]
- Groeneweg FL, Karst H, de Kloet ER, Joels M (2011) Mineralocorticoid and glucocorticoid receptors at the neuronal membrane, regulators of nongenomic corticosteroid signalling. Mol Cell Endocrinol. 10.1016/j.mce.2011.06.020 [DOI] [PubMed]
- Harfstrand A (1987) Brain neuropeptide Y mechanisms. Basic aspects and involvement in cardiovascular and neuroendocrine regulation. Acta Physiol Scand Suppl 565:1–83 [PubMed] [Google Scholar]
- Haam J, Halmos KC, Muglia LJ, Tasker JG (2010) Rapid synaptic modulation of hypothalamic neurons by glucocorticoids requires the glucocorticoid receptor. In: Society for Neuroscience. Society for Neuroscience, San Diego, CA, Program No. 389, 19 p
- Herman JP, Mueller NK (2006) Role of the ventral subiculum in stress integration. Behav Brain Res 174:215–224 [DOI] [PubMed] [Google Scholar]
- Herman JP, Schafer MK, Young EA, Thompson R, Douglass J, Akil H, Watson SJ (1989) Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J Neurosci 9:3072–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Watson SJ (1994) Involvement of the bed nucleus of the stria terminalis in tonic regulation of paraventricular hypothalamic CRH and AVP mRNA expression. J Neuroendocrinol 6:433–442 [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Ziegler DR, Tasker JG (2002) Role of the paraventricular nucleus microenvironment in stress integration. Eur J Neurosci 16:381–385 [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE (2003) Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 24:151–180 [DOI] [PubMed] [Google Scholar]
- Hill MN, McEwen BS (2010) Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuropsychopharmacol Biol Psychiatry 34:791–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB (1991) Efferent projections of the infralimbic cortex of the rat. J Comp Neurol 308:249–276 [DOI] [PubMed] [Google Scholar]
- Jaferi A, Bhatnagar S (2006) Corticosterone can act at the posterior paraventricular thalamus to inhibit hypothalamic-pituitary-adrenal activity in animals that habituate to repeated stress. Endocrinology 147:4917–4930 [DOI] [PubMed] [Google Scholar]
- Jaferi A, Nowak N, Bhatnagar S (2003) Negative feedback functions in chronically stressed rats: role of the posterior paraventricular thalamus. Physiol Behav 78:365–373 [DOI] [PubMed] [Google Scholar]
- Jankord R, Herman JP (2008) Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci 1148:64–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LR, Farb C, Morrison JH, McEwen BS, LeDoux JE (2005) Localization of glucocorticoid receptors at postsynaptic membranes in the lateral amygdala. Neuroscience 136:289–299 [DOI] [PubMed] [Google Scholar]
- Jones KR, Myers B, Herman JP (2011) Stimulation of the prelimbic cortex differentially modulates neuroendocrine responses to psychogenic and systemic stressors. Physiol Behav 104:266–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen H, Knigge U, Kjaer A, Vadsholt T, Warberg J (1998) Serotonergic involvement in stress-induced ACTH release. Brain Res 811:10–20 [DOI] [PubMed] [Google Scholar]
- Kanner AM (2009) Depression and epilepsy: do glucocorticoids and glutamate explain their relationship? Curr Neurol Neurosci Rep 9:307–312 [DOI] [PubMed] [Google Scholar]
- Kiss A, Palkovits M, Aguilera G (1996) Neural regulation of corticotropin releasing hormone (CRH) and CRH receptor mRNA in the hypothalamic paraventricular nucleus in the rat. J Neuroendocrinol 8:103–112 [DOI] [PubMed] [Google Scholar]
- Kolber BJ, Roberts MS, Howell MP, Wozniak DF, Sands MS, Muglia LJ (2008) Central amygdala glucocorticoid receptor action promotes fear-associated CRH activation and conditioning. Proc Natl Acad Sci USA 105:12004–12009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsuzaki Y, Murakami G, Tsurugizawa T, Mukai H, Tanabe N, Mitsuhashi K, Kawata M, Kimoto T, Ooishi Y, Kawato S (2005) Rapid spinogenesis of pyramidal neurons induced by activation of glucocorticoid receptors in adult male rat hippocampus. Biochem Biophys Res Commun 335:1002–1007 [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL (1977) Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J Comp Neurol 172:687–722 [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ (2010) Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry 167:1305–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HY, Ericsson A, Sawchenko PE (1996) Distinct mechanisms underlie activation of hypothalamic neurosecretory neurons and their medullary catecholaminergic afferents in categorically different stress paradigms. Proc Natl Acad Sci USA 93:2359–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA (2002) Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol 14:911–923 [DOI] [PubMed] [Google Scholar]
- Ma S, Morilak DA (2005) Norepinephrine release in medial amygdala facilitates activation of the hypothalamic-pituitary-adrenal axis in response to acute immobilisation stress. J Neuroendocrinol 17:22–28 [DOI] [PubMed] [Google Scholar]
- Malcher-Lopes R, Di S, Marcheselli VS, Weng FJ, Stuart CT, Bazan NG, Tasker JG (2006) Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci 26:6643–6650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcher-Lopes R, Franco A, Tasker JG (2008) Glucocorticoids shift arachidonic acid metabolism toward endocannabinoid synthesis: a non-genomic anti-inflammatory switch. Eur J Pharmacol 583:322–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH (2005) Deep brain stimulation for treatment-resistant depression. Neuron 45:651–660 [DOI] [PubMed] [Google Scholar]
- McEwen BS, Kalia M (2010) The role of corticosteroids and stress in chronic pain conditions. Metabolism 59(Suppl 1):S9–S15 [DOI] [PubMed] [Google Scholar]
- McEwen BS, Weiss JM, Schwartz LS (1968) Selective retention of corticosterone by limbic structures in rat brain. Nature 220:911–912 [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P (1999) Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 403:261–280 [DOI] [PubMed] [Google Scholar]
- Miller AH, Spencer RL, Pulera M, Kang S, McEwen BS, Stein M (1992) Adrenal steroid receptor activation in rat brain and pituitary following dexamethasone: implications for the dexamethasone suppression test. Biol Psychiatry 32:850–869 [DOI] [PubMed] [Google Scholar]
- Mueller NK, Dolgas CM, Herman JP (2004) Stressor-selective role of the ventral subiculum in regulation of neuroendocrine stress responses. Endocrinology 145:3763–3768 [DOI] [PubMed] [Google Scholar]
- Mueller NK, Dolgas CM, Herman JP (2006) Regulation of forebrain GABAergic stress circuits following lesion of the ventral subiculum. Brain Res 1116:132–142 [DOI] [PubMed] [Google Scholar]
- Myers B, Greenwood-Van Meerveld B (2011) Differential involvement of amygdala corticosteroid receptors in visceral hyperalgesia following acute or repeated stress. Am J Physiol Gastrointest Liver Physiol 302(2):G260–G266 [DOI] [PubMed] [Google Scholar]
- Myers B, Dittmeyer K, Greenwood-Van Meerveld B (2007) Involvement of amygdaloid corticosterone in altered visceral and somatic sensation. Behav Brain Res 181:163–167 [DOI] [PubMed] [Google Scholar]
- Myers-Schulz B, Koenigs M (2011) Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Mol Psychiatry. doi:10.1038/mp.2011.88 [DOI] [PMC free article] [PubMed]
- Ostrander MM, Richtand NM, Herman JP (2003) Stress and amphetamine induce Fos expression in medial prefrontal cortex neurons containing glucocorticoid receptors. Brain Res 990:209–214 [DOI] [PubMed] [Google Scholar]
- Pare D, Smith Y, Pare JF (1995) Intra-amygdaloid projections of the basolateral and basomedial nuclei in the cat: phaseolus vulgaris-leucoagglutinin anterograde tracing at the light and electron microscopic level. Neuroscience 69:567–583 [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ (2004) Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology 145:5431–5438 [DOI] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS (2003) Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci 17:879–886 [DOI] [PubMed] [Google Scholar]
- Plotsky PM (1987) Facilitation of immunoreactive corticotropin-releasing factor secretion into the hypophysial-portal circulation after activation of catecholaminergic pathways or central norepinephrine injection. Endocrinology 121:924–930 [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Cunningham ET Jr, Widmaier EP (1989) Catecholaminergic modulation of corticotropin-releasing factor and adrenocorticotropin secretion. Endocr Rev 10:437–458 [DOI] [PubMed] [Google Scholar]
- Prager EM, Brielmaier J, Bergstrom HC, McGuire J, Johnson LR (2010) Localization of mineralocorticoid receptors at mammalian synapses. PLoS One 5:e14344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prewitt CM, Herman JP (1998) Anatomical interactions between the central amygdaloid nucleus and the hypothalamic paraventricular nucleus of the rat: a dual tract-tracing analysis. J Chem Neuroanat 15:173–185 [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sawchenko PE (2011) A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. J Neurosci 31:9683–9695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Arias CM, Sawchenko PE (2006) Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci 26:12967–12976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR (2008) Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol 507:1141–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Gosselink KL, Sawchenko PE (2009) A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci 29:7330–7340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Kabbaj M, Jacobson L, Heydendael W, Yehuda R, Herman JP (2011) Stress risk factors and stress-related pathology: neuroplasticity, epigenetics and endophenotypes. Stress 14:481–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER (1985) Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117:2505–2511 [DOI] [PubMed] [Google Scholar]
- Rinaman L (2007) Visceral sensory inputs to the endocrine hypothalamus. Front Neuroendocrinol 28:50–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland BL, Sawchenko PE (1993) Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol 332:123–143 [DOI] [PubMed] [Google Scholar]
- Roozendaal B (2002) Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem 78:578–595 [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Koolhaas JM, Bohus B (1991) Attenuated cardiovascular, neuroendocrine, and behavioral responses after a single footshock in central amygdaloid lesioned male rats. Physiol Behav 50:771–775 [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Koolhaas JM, Bohus B (1992) Central amygdaloid involvement in neuroendocrine correlates of conditioned stress responses. J Neuroendocrinol 4:483–489 [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J (2003) The amygdaloid complex: anatomy and physiology. Physiol Rev 83:803–834 [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS (1986) The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev 7:284–301 [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW (1983) The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J Comp Neurol 218:121–144 [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Steinbusch HW, Verhofstad AA (1983) The distribution and cells of origin of serotonergic inputs to the paraventricular and supraoptic nuclei of the rat. Brain Res 277:355–360 [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Grzanna R, Howe PR, Bloom SR, Polak JM (1985) Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol 241:138–153 [DOI] [PubMed] [Google Scholar]
- Schulkin J, Gold PW, McEwen BS (1998) Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology 23:219–243 [DOI] [PubMed] [Google Scholar]
- Sharma ST, Nieman LK (2011) Cushing’s syndrome: all variants, detection, and treatment. Endocrinol Metab Clin North Am 40:379–391 viii-ix [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Barron KW, Myers DA (2000) Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res 861:288–295 [DOI] [PubMed] [Google Scholar]
- Shepard JD, Barron KW, Myers DA (2003) Stereotaxic localization of corticosterone to the amygdala enhances hypothalamo-pituitary-adrenal responses to behavioral stress. Brain Res 963:203–213 [DOI] [PubMed] [Google Scholar]
- Shepard JD, Schulkin J, Myers DA (2006) Chronically elevated corticosterone in the amygdala increases corticotropin releasing factor mRNA in the dorsolateral bed nucleus of stria terminalis following duress. Behav Brain Res 174:193–196 [DOI] [PubMed] [Google Scholar]
- Singewald GM, Rjabokon A, Singewald N, Ebner K (2011) The modulatory role of the lateral septum on neuroendocrine and behavioral stress responses. Neuropsychopharmacology 36:793–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AD, Castro SL, Zigmond MJ (2002) Stress-induced Parkinson’s disease: a working hypothesis. Physiol Behav 77:527–531 [DOI] [PubMed] [Google Scholar]
- Solomon MB, Jones K, Packard BA, Herman JP (2010) The medial amygdala modulates body weight but not neuroendocrine responses to chronic stress. J Neuroendocrinol 22:13–23 [DOI] [PubMed] [Google Scholar]
- Sotiropoulos I, Cerqueira JJ, Catania C, Takashima A, Sousa N, Almeida OF (2008) Stress and glucocorticoid footprints in the brain-the path from depression to Alzheimer’s disease. Neurosci Biobehav Rev 32:1161–1173 [DOI] [PubMed] [Google Scholar]
- Staiger JF, Nurnberger F (1991) The efferent connections of the lateral septal nucleus in the guinea pig: intrinsic connectivity of the septum and projections to other telencephalic areas. Cell Tissue Res 264:415–426 [DOI] [PubMed] [Google Scholar]
- Stellato C (2004) Post-transcriptional and nongenomic effects of glucocorticoids. Proc Am Thorac Soc 1:255–263 [DOI] [PubMed] [Google Scholar]
- Stevens DR, Gallagher JP, Shinnick-Gallagher P (1987) In vitro studies of the role of gamma-aminobutyric acid in inhibition in the lateral septum of the rat. Synapse 1:184–190 [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A (1998) Relationships between stress-induced increases in medial prefrontal cortical dopamine and plasma corticosterone levels in rats: role of cerebral laterality. Neuroscience 83:81–91 [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A (1999) Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J Neurosci 19:2834–2840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD (1998) What is the amygdala? Trends Neurosci 21:323–331 [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW (1983) Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36:165–186 [DOI] [PubMed] [Google Scholar]
- Tasker JG, Herman JP (2011) Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress 14:398–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauchi M, Zhang R, D’Alessio DA, Stern JE, Herman JP (2008) Distribution of glucagon-like peptide-1 immunoreactivity in the hypothalamic paraventricular and supraoptic nuclei. J Chem Neuroanat 36:144–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhart M, Wand GS (2009) Stress, alcohol and drug interaction: an update of human research. Addict Biol 14:43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP (2009) Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10:397–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Jones KR, Ziegler DR, Cullinan WE, Herman JP (2011) Forebrain origins of glutamatergic innervation to the rat paraventricular nucleus of the hypothalamus: differential inputs to the anterior versus posterior subregions. J Comp Neurol 519:1301–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J (1981) Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 213:1394–1397 [DOI] [PubMed] [Google Scholar]
- Van de Kar LD, Piechowski RA, Rittenhouse PA, Gray TS (1991) Amygdaloid lesions: differential effect on conditioned stress and immobilization-induced increases in corticosterone and renin secretion. Neuroendocrinology 54:89–95 [DOI] [PubMed] [Google Scholar]
- van Haarst AD, Oitzl MS, de Kloet ER (1997) Facilitation of feedback inhibition through blockade of glucocorticoid receptors in the hippocampus. Neurochem Res 22:1323–1328 [DOI] [PubMed] [Google Scholar]
- Vertes RP (2004) Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51:32–58 [DOI] [PubMed] [Google Scholar]
- Wang CC, Wang SJ (2009) Modulation of presynaptic glucocorticoid receptors on glutamate release from rat hippocampal nerve terminals. Synapse 63:745–751 [DOI] [PubMed] [Google Scholar]
- Xu Y, Day TA, Buller KM (1999) The central amygdala modulates hypothalamic-pituitary-adrenal axis responses to systemic interleukin-1beta administration. Neuroscience 94:175–183 [DOI] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J (2007) Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron 56:19–32 [DOI] [PubMed] [Google Scholar]
- Yehuda R, Seckl J (2011) Minireview: stress-related psychiatric disorders with low cortisol levels: a metabolic hypothesis. Endocrinology 152:4496–4503 [DOI] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Golier JA, Grossman R, Bierer LM (2004) Effects of trauma exposure on the cortisol response to dexamethasone administration in PTSD and major depressive disorder. Psychoneuroendocrinology 29:389–404 [DOI] [PubMed] [Google Scholar]
- Yehuda R, Bierer LM, Andrew R, Schmeidler J, Seckl JR (2009) Enduring effects of severe developmental adversity, including nutritional deprivation, on cortisol metabolism in aging Holocaust survivors. J Psychiatr Res 43:877–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZH, Felder RB (2004) Melanocortin receptors mediate the excitatory effects of blood-borne murine leptin on hypothalamic paraventricular neurons in rat. Am J Physiol Regul Integr Comp Physiol 286:R303–R310 [DOI] [PubMed] [Google Scholar]
- Zhang R, Packard BA, Tauchi M, D’Alessio DA, Herman JP (2009) Glucocorticoid regulation of preproglucagon transcription and RNA stability during stress. Proc Natl Acad Sci USA 106:5913–5918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DR, Herman JP (2000) Local integration of glutamate signaling in the hypothalamic paraventricular region: regulation of glucocorticoid stress responses. Endocrinology 141:4801–4804 [DOI] [PubMed] [Google Scholar]