Abstract
Induced pluripotent stem (iPS) cells are ideal sources of hepatocyte for transplantation into patients experiencing hepatic failure. Growth and transcription factors were analyzed to design a single-step protocol for the differentiation of iPS cells into hepatocytes. The expression of transcription factors was analyzed using reverse transcription-polymerase chain reaction (RT-PCR) and compared among iPS cells, as well as fetal and adult liver cells. iPS cells were cultured with growth factors and RT-PCR was performed to analyze the expression of transcription factors. iPS cells were introduced with transcription factors, cultured with growth factors and subjected to real-time quantitative PCR. Indocyanine green (ICG) was added to the medium as a hepatocyte marker. Sox17, GATA4, GATA6, FoxA2, HEX, HNF4α and C/EBPα were expressed in fetal and adult liver cells, but not in iPS cells. Sox17, GATA6 and HNF4α were expressed after exposure a combination of oncostatin M, epidermal growth factor, retinoic acid, dexamethasone and ITS (OERDITS). When iPS cells were introduced with FoxA2, GATA4, HEX and C/EBPα and cultured with OERDITS for 8 days, the cells expressed α-fetoprotein, δ-like (Dlk)-1 and γ-glutamyl transpeptidase (GTP), and ICG uptake was observed. Exposure to FoxA2, GATA4, HEX and C/EBPα and culturing with OERDITS supplementation potentially serves as a single-step inducer for the differentiation of iPS cells into hepatic progenitor-like cells within 8 days.
Keywords: growth factor, transcription factor, hepatocyte
Introduction
Fulminant hepatitis is characterized by sudden, severe liver dysfunction leading to coagulopathy and hepatic encephalopathy (1). In their study, Alam et al(2) reported the natural course of fulminant hepatitis with most of the patients developing fulminant hepatic failure within 2 weeks after the onset of jaundice, resulting in a 73.1% rate in mortality in the study population. Hepatocyte transplantation offers a potential therapeutic option for the treatment of fulminant hepatitis. Induced pluripotent stem (iPS) cells are an ideal source of autologous hepatocytes as they potentially prevent the need for immunosuppression prior to cell engraftment (3). However, the prompt differentiation of iPS cells into hepatocytes is essential.
Strategies used for the direct differentiation of iPS cells into hepatocytes have indicated the sequential supplementation of cytokines and growth factors involved in the embryonic development of the mammalian liver (3). Current strategies to generate hepatocytes from iPS cells that mimic liver embryogenesis by adding essential growth factors and simulators to serum-free culture medium have yielded more hepatocytes and hepatocyte-like cells and an increased homogeneity in the final cell population (3). Endodermal markers were previously analyzed in human embryonic stem (ES) cells, cultured with growth factors (4). Retinoic acid (RA), nerve growth factor (NGF), hepatocyte growth factor (HGF) and endodermal growth factor (EGF) are known to increase the expression of α-fetoprotein (AFP), while dexamethasone (Dex) and ITS are used to maintain the in vitro functions of hepatocytes (5). The use of activin A for the differentiation of iPS cells into endodermal cells is controversial. Activin A at a concentration of 100 ng/ml promotes the differentiation of ES cells into endoderm, whereas activin A maintains pluripotency, regulating the expression of Nanog (6,7).
Transcription factors play an important role in hepatocyte differentiation. Mice deficient in GATA-binding protein (GATA) 6 died between embryonic Days 6.5 and 7.5 and exhibited a specific defect in endodermal differentiation (8). The expression of Sry-related HMG box (Sox) 17 is restricted to the nascent primitive endodermal epithelium (9). The absence of Sox17 leads to the premature delamination and migration of the parietal endoderm. Forkhead box protein (Fox) A2 and GATA4 are the first known proteins that bind to the albumin gene enhancer in liver precursor cells in embryos (10). Disruption of the hematopoietically expressed homeobox (HEX) resulted in embryonic lethality attributable to insubstantial liver formation, making it essential for liver organogenesis (11). The CCAAT/enhancer binding protein (C/EBP) α promotes the differentiation of hepatoblasts into mature hepatocytes (12,13). Clones expressing the activating albumin promoter express C/EBPβ (14). HNF4α is essential for the hepatic specification of iPS cells (15). These data suggest that Sox17, GATA6, FoxA2, GATA4, HEX, C/EBPα, C/EBPβ and HNF4α are expected to promote the differentiation of iPS cells into hepatocytes.
Thus, the aim of this study was to analyze various growth and transcription factors involved in hepatocyte differentiation from iPS cells within 8 days.
Materials and methods
Cell culture
The iPS cell line (RIKEN Cell Bank, Tsukuba, Japan) 201B7 was cultured in ReproFF media (ReproCELL, Inc., Yokohama, Japan) as a feeder-free culture (ReproCELL, Inc., Tokyo, Japan) in dishes (Asahi Techno Glass, Funabashi, Japan) coated with Matrigel (Becton-Dickinson, Franklin Lakes, NJ) and were kept in 5% CO2 at 37°C in a humidified chamber. The cells were collected with Accutase (Innovative Cell Technologies, Inc., San Diego, CA, USA) for the experiments. 201B7 cells were cultured in Dulbecco’s modified Eagle’s medium/F12-medium (Sigma-Aldrich Corp., St. Louis, MO, USA) supplemented with 20% of knockout serum replacement (Life Technologies, Grand Island, NY, USA), 10% of minimum essential amino acids (Life Technologies), 2 mM of L-glutamine (Life Technologies), and 1 mM of 2-mercaptoethanol (iPSm(-); Sigma-Aldrich Corp.). Basic fibroblast growth factor (bFGF) (5 ng/ml; Wako Pure Chemical Industries, Ltd., Osaka, Japan), bone morphogenic protein (BMP) 4 (20 ng/ml; Wako Pure Chemical Industries, Ltd.), oncostatin M (20 ng/ml; Wako Pure Chemical Industries), EGF (20 ng/ml; Wako Pure Chemical Industries, Ltd.), NGF (100 ng/ml; R&D Systems, Inc., Minneapolis, MN), transforming growth factor (TGF)-β (1 ng/ml; R&D Systems, Inc.), RA (1 μM; Sigma-Aldrich Corp.), HGF (10 ng/ml; Sigma-Aldrich Corp.), Dex (10-7 M; Wako Pure Chemical Industries, Ltd.), and insulin, transferrin, selenium (ITS) (Wako Pure Chemical Industries, Ltd.) were added in iPSm(-).
Reverse transcriptase and real-time quantitative polymerase chain reaction
Total RNA (5 μg), isolated using Isogen (Nippon Gene, Tokyo, Japan), was used for first-strand cDNA synthesis using SuperScript III and oligo(dT), according to the manufacturer’s instructions (Life Technologies). Polymerase chain reaction (PCR) was performed using the GeneAmp® PCR System 9700 (Life Technologies) and subjected to gel electrophoresis. Real-time quantitative PCR was performed with Fast SYBR-Green Master mix (Life Technologies) and analyzed using the MiniOpticon (Bio-Rad, Hercules, CA, USA). The primer pairs used for RT-PCR were: GATA4, 5′-GAAAACGGAAGCCCAAGAACC and 5′-AGACATCGCACTGACTGAGAACG (NM_002052, 56°C, 218 bp); GATA6, 5′-TTCATCACGGCGGCTTGGATTGTC and 5′-GTGTTGTGGGGGAAGTATTTTTGC (NM_005257, 56°C, 299 bp); Sox17, 5′-CGCTTTCATGGTGTGGGCTAAGGACG and 5′-TAGTTGGGGTGGTCCTGCATGTGCTG (NM_022454, 63°C, 186 bp); FoxA2, 5′-CCACCACCAACCCCACAAAATG and 5′-TGCAACACCGTCTCCCCAAAGT (NM_021784, 60°C, 294 bp); HEX, 5′-TTCTCCAACGACCAGACCATCG and 5′-TTTTATCGCCCTCAATGTCCAC (NM_002729, 56°C, 364 bp); C/EBPα, 5′-TGGAGACGCAGCAGAAGGTG and 5′-TCGGGAAGGAGGCAGGAAAC (U34070, 69°C, 538 bp); and C/EBPβ, 5′-AGACGCAGCACAAGGTCCTG and 5′-GAGAGGGGCAGAGGGAGAGC (X52560, 60°C, 421 bp). RT-PCR was performed with 30 cycles of 1 min of denaturation, 1 min of annealing and 1 min of extension. The primer pairs for real-time quantitative PCR of AFP, ribosomal protein L19 (RPL19), δ-like (Dlk)-1, GATA6, HNF4α and γ-glutamyl transpeptidase (GTP) were 5′-ACA CAAAAAGCCCACTCCAG and 5′-GGTGCATACAGGAA GGGATG (NM_005618, 147 bp), 5′-CGAATGCCAGA GAAGGTCAC and 5′-CCATGAGAATCCGCTTGTTT (157 bp), 5′-GGATGAGTGCGTCATAGCAA and 5′-CCT CCTCTTCAGCAGCATTC (121 bp), 5′-CCACTCGTGTC TGCTTTTGTGC and 5′-CCCTTCCCTTCCATCTTCT CTCAC (139 bp), 5′-CAACGGACAGATGTGTGAGTGG and 5′-ATAACTTCCTGCTTGGTGATGGTC (NM_000457, 183 bp), and 5′-CCTCATCCTCAACATCCTCAAAGG and 5′-CACCTCAGTCACATCCACAAACTTG (J04131, 163 bp), respectively. The primer pairs for the quantitative PCR of Sox17 were the same as those for RT-PCR. Real-time quantitative PCR was performed for 40 cycles, using 5 sec for denaturation and 5 sec for annealing-extension.
Indocyanine green uptake study
Indocyanine green (ICG, 25 mg; Dai-ichi Pharmaceutical, Co., Ltd., Tokyo, Japan) was dissolved in 5 ml of water in a sterile vial and 20 ml were added to each medium at a final concentration of 1 mg/ml (16). The ICG solution was added to the cell culture and incubated at 37°C for 15 min (5). After the dish was rinsed with phosphate-buffered saline (PBS), the ICG uptake was observed through microscopy.
Results
Expression of transcription factors in iPS and liver cells
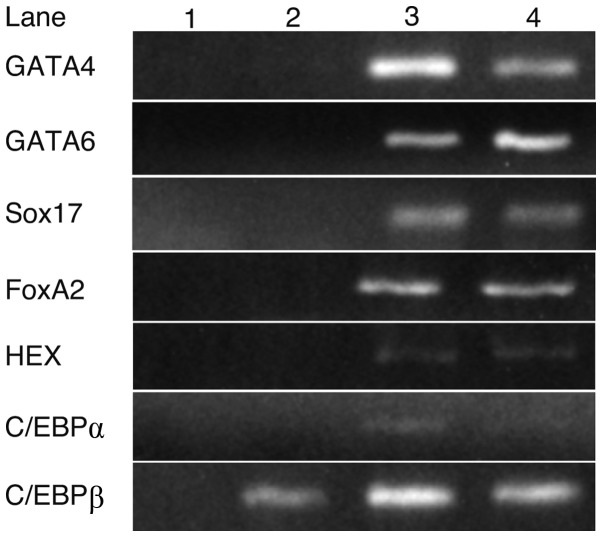
RT-PCR was performed to identify the transcription factors involved in hepatocyte differentiation (Fig. 1). GATA4, GATA6, Sox17, FoxA2, HEX and C/EBPα were expressed in fetal or adult liver cells, but not in iPS cells, whereas C/EBPβ was expressed in iPS cells as well as in fetal and adult liver cells.
Figure 1.
The expression of transcription factors in iPS and liver cells. RT-PCR analysis indicated that GATA4, GATA6, Sox17, FoxA2, HEX and C/EBPα were not expressed in iPS cells, whereas their expression was detected in fetal or adult liver cells. C/EBPβ was expressed in iPS, fetal and adult liver cells. Lanes: 1, H2O; 2, iPS cells; 3, fetal liver and 4, adult liver.
RT-PCR analysis of transcription and growth factors
Using RNA isolated from cells cultured with each growth factor for 8 days, RT-PCR was performed to detect the growth factors promoting iPS cells to express GATA4, GATA6, Sox17, FoxA2, HEX and C/EBPα (Fig. 2). C/EBPβ was not included in this experiment as it was expressed in iPS cells. GATA6 and Sox17 were strongly expressed in iPS cells cultured with EGF and oncostatin M, respectively. Sox7 and GATA6 were expressed in cells cultured with RA. C/EBPα was weakly expressed with oncostatin M. GATA4, FoxA2 and HEX were not expressed in iPS cells using any of the growth factors. These results suggest that oncostatin M, EGF and RA stimulated the expression of GATA6 and Sox17 in iPS cells.
Figure 2.
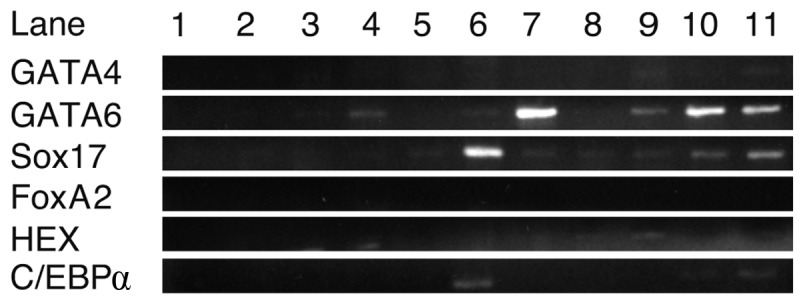
RT-PCR analysis of transcription and growth factors. Sox17 and GATA6 were strongly expressed in the presence of oncostatin M and EGF, respectively. GATA4, FoxA2, HEX and C/EBPα were not sufficiently expressed with either growth factor. Lanes: 1, H2O; 2, ReproFF; 3, no growth factors; 4, basic fibroblast growth factor; 5, BMP4; 6, oncostatin M; 7, epidermal growth factor; 8, nerve growth factor; 9, transforming growth factor β; 10, retinoic acid and 11, hepatocyte growth factor.
Expression of GATA6, Sox17 and HNF4α in the presence of growth factors
Real-time quantitative PCR conducted in cell cultures with a combination of oncostatin M, EGF and RA showed an increase in the expression of GATA6, Sox17 and HNF4α (Fig. 3). The expression of GATA6 and Sox17 showed the greatest increase during culture with a combination of oncostatin M, EGF, RA, dexamethasone and ITS. HNF4α was expressed in iPS cells cultured with oncostatin M, dexamethasone, ITS and their combinations.
Figure 3.
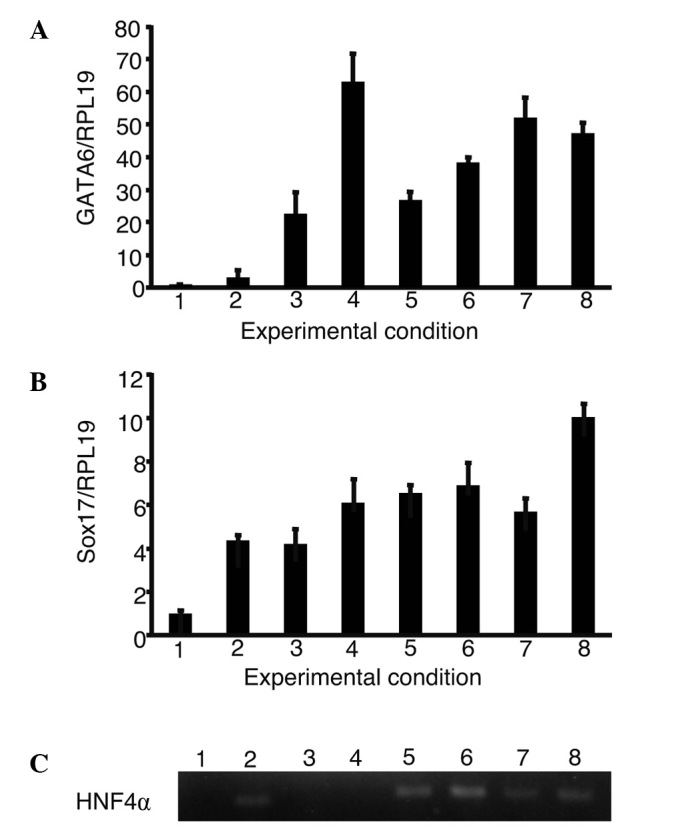
The expression of GATA6, Sox17 and HNF4α in the presence of growth factors. iPS cells were cultured using a single or a combination of growth factors. The expression of GATA6, Sox17 and HNF4α were analyzed. (A and B) The relative expression levels were normalized against iPS cells in ReproFF media. (C) The expression of HNF4α was analyzed using gel electrophoresis as it was too low to be normalized in iPS cells. (A and B) Columns and (C) lanes 1–8 show the following: 1, ReproFF; 2, oncostatin M; 3, epidermal growth factor; 4, retinoic acid; 5, dexamethasone; 6, ITS; 7, oncostatin M + epidermal growth factor + retinoic acid and 8, oncostatin M + epidermal growth factor + retinoic acid + dexamethasone + ITS. Error bar, standard error.
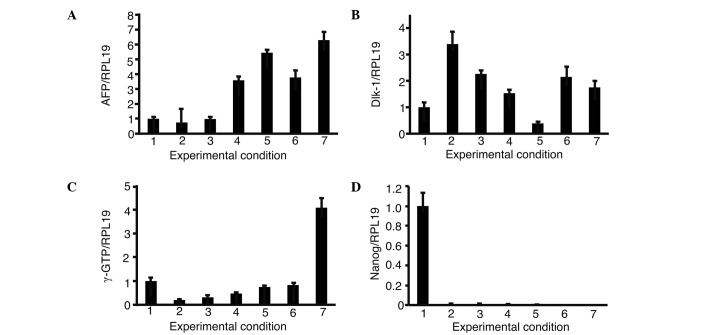
Expression of α-fetoprotein (AFP) and δ-like (Dlk)-1 in the presence of growth factors
The expression of AFP and Dlk-1, markers of hepatic progenitor cells, was then analyzed in iPS cells with single or a combination of growth factors (Fig. 4). The combination of oncostatin M, EGF, RA, dexamethasone and ITS (OERDITS) resulted in the greatest increase in the expression of AFP. Dlk-1 expression was stimulated with either oncostatin M or OERDITS. Thus, OERDITS was regarded as the most suitable combination of growth factors to promote the differentiation of iPS cells into hepatocytes.
Figure 4.
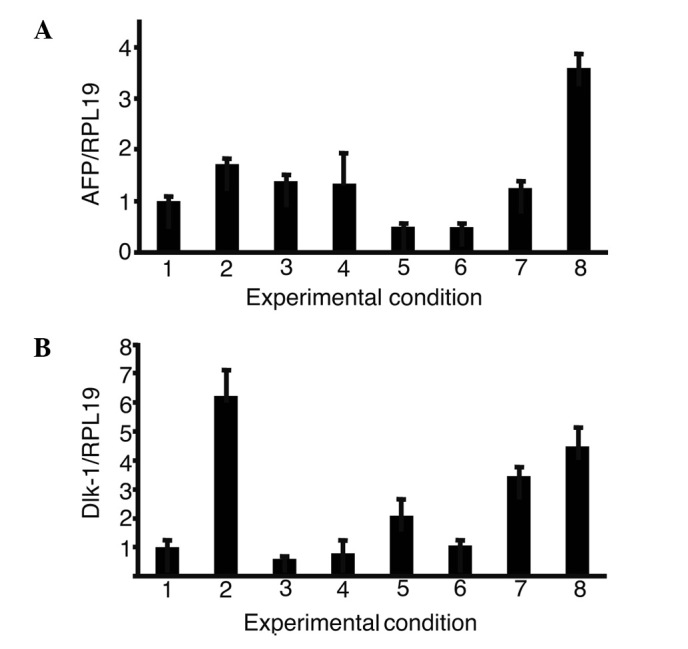
The expression of α-fetoprotein (AFP) and δ-like (Dlk)-1 in the presence of growth factors. The expression of AFP and Dlk-1 in iPS cells cultured with growth factors was analyzed. The relative expression levels were normalized against iPS cells using ReproFF media. Columns: 1, ReproFF; 2, oncostatin M; 3, epidermal growth factor; 4, retinoic acid; 5, dexamethasone; 6, ITS; 7, oncostatin M + epidermal growth factor + retinoic acid and 8, oncostatin M + epidermal growth factor + retinoic acid + dexamethasone + ITS. Error bar, standard error.
Expression of AFP, Dlk-1, γ-GTP and Nanog in the presence of growth factors
FoxA2, GATA4, HEX and C/EBPα were introduced with iPS cells and cultured with OERDITS (Fig. 5). The expression of AFP and Dlk-1 was comparable to that observed in fetal liver cells with a combination of FoxA2, GATA4, HEX and C/EBPα (FGHA). The expression of γ-GTP with FGHA was the strongest, although it was lower than that shown by fetal liver cells. All the conditions significantly suppressed the expression of Nanog.
Figure 5.
Expression of (A) AFP, (B) Dlk-1, (C) γ-GTP and (D) Nanog in the presence of growth factors. iPS cells were introduced with a combination of 3 or 4 growth factors from FoxA2, GATA4, HEX and C/EBPα and cultured in oncostatin M, EGF, RA, dexamethasone and ITS. The expression of AFP, Dlk-1, γ-GTP and Nanog was analyzed. The relative expression levels were normalized against iPS cells in ReproFF media. Columns: 1, ReproFF; 2, GHA; 3, FHA; 4, FGA; 5, FGH; 6, FGHA and 7, fetal liver. FGHA: F, FoxA2; G, GATA4; H, HEX; A, C/EBPα. Error bar, standard error.
Indocyanine green (ICG) uptake in iPS cells cultured at varying conditions
ICG was added in the iPS cell culture medium 8 days after the introduction of FGHA and cultured in OERDITS. ICG was taken-up by iPS cells (Fig. 6).
Figure 6.
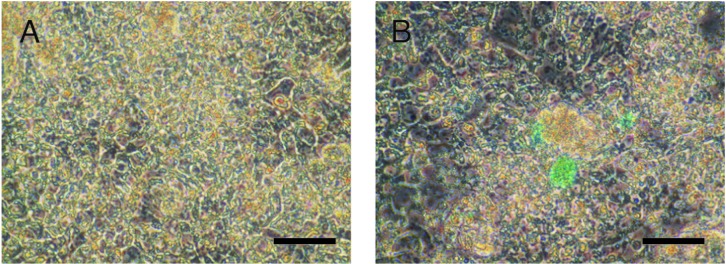
Indocyanine green (ICG) uptake in iPS cells cultured at varying conditions is shown. Indocyanine green (ICG) was added in the medium of iPS cells introduced with FoxA2, GATA4, HEX and C/EBPα and cultured with oncostatin M, EGF, RA, dexamethasone and ITS. (A) iPS cells before the addition of ICG in the medium and (B) 15 min after the addition of ICG. Original magnification, ×200; bar, 25 μm.
Discussion
Stepwise differentiation protocols are currently employed to promote the differentiation of iPS cells into hepatocytes (15,17–19). Current protocols involve the sequential application of growth factors. DeLaForest et al(15) used LY294002, an inhibitor of phosphatidyl-inositol 3 kinase. Si-Tayeb et al(17) changed the oxygen concentration to 4 and 20%. Song et al(20) added supplements (N2 and B27). Takayama et al(19) transduced Sox17, HEX and HNF4α. However, these protocols require more than 20 days for iPS cells to differentiate into hepatocytes. Shorter induction periods are necessary for the transplantation of cells to patients diagnosed with hepatic failure. Thus, we developed a new protocol that promoted the differentiation of iPS cells into hepatocytes within 8 days. This study has also investigated growth factors that increased the expression of transcription factors. The OERDITS medium was found to stimulate the expression of GATA6, Sox17 and HNF4α. Sox17 is essential for the differentiation of iPS cells into endodermal cells, whereas HNF4α promotes the maturation of hepatocytes (21). In addition, the introduction of GATA6, Sox17 and HNF4α was not necessary with the OERDITS medium. However, the OERDITS medium did not increase the expression of FoxA2, GATA4 and HEX. Therefore, the introduction of FoxA2, GATA4 and HEX was necessary for the induction of cell differentiation.
AFP expression was lowest in iPS cells introduced with FoxA2, GATA4 and HEX (Fig. 4), suggesting that C/EBPα was necessary for iPS cells to differentiate into hepatocytes. Thus, the combination of GATA4, FoxA2, HEX and C/EBPα was essential for the generation of hepatocytes from iPS cells.
The role of activin A in the endodermal differentiation of iPS cells depends on its concentration. The concentration of 100 ng/ml of activin A was shown to promote differentiation of human embryonic stem (ES) cells into endoderm cells (6). Activin A maintains pluripotency, regulating the Nanog expression (7). No endodermal markers were expressed with activin A at 20 ng/ml. Xiao et al(22) succeeded in the long-term feeder-free culture of human ES cells (H1) for >150 days and 20 passages using 5 ng/ml of activin A. However, the stepwise protocols employed high concentrations of activin A. Activin A was not analyzed in the present study as we had succeeded in the passage culture of 201B7 cells using a medium with activin A at 10 ng/ml (in preparation for submission).
Albumin was not expressed in iPS cells cultured with FGHA and OERDITS (data not shown). Future investigations should therefore be performed on the application of the extracellular matrix (23). Moreover, exposure to FoxA2, GATA4, HEX and C/EBPα and culturing with OERDITS supplementation potentially serves as a single-step inducer for the differentiation of iPS cells into hepatic progenitor-like cells.
Acknowledgments
This study was supported by the Grant-in-Aid for Encouragement of Scientists (no. 22931047) and the Research Grant-in-Aid for Scientific Research (C) (no. 23591002) from the Japan Society for the Promotion of Science (JSPS).
References
- 1.Inoue K, Watanabe T, Maruoka N, Kuroki Y, Takahashi H, Yoshiba M. Japanese-style intensive medical care improves prognosis for acute liver failure and the perioperative management of liver transplantation. Transplant Proc. 2010;42:4109–4112. doi: 10.1016/j.transproceed.2010.09.073. [DOI] [PubMed] [Google Scholar]
- 2.Alam S, Azam G, Mustafa G, et al. Natural course of fulminant hepatic failure: the scenario in Bangladesh and the differences from the west. Saudi J Gastroenterol. 2009;15:229–233. doi: 10.4103/1319-3767.56094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chistiakov DA, Chistiakov PA. Strategies to produce hepatocytes and hepatocyte-like cells from pluripotent stem cells. Hepatol Res. 2012;42:111–119. doi: 10.1111/j.1872-034X.2011.00896.x. [DOI] [PubMed] [Google Scholar]
- 4.Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2000;97:11307–11312. doi: 10.1073/pnas.97.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomizawa M, Toyama Y, Ito C, et al. Hepatoblast-like cells enriched from mouse embryonic stem cells in medium without glucose, pyruvate, arginine, and tyrosine. Cell Tissue Res. 2008;333:17–27. doi: 10.1007/s00441-008-0618-4. [DOI] [PubMed] [Google Scholar]
- 6.Yao S, Chen S, Clark J, et al. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci USA. 2006;103:6907–6912. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin M, Alev C, Wu Y, Nagai H, Sheng G. Activin/TGF-beta signaling regulates Nanog expression in the epiblast during gastrulation. Mech Dev. 2011;128:268–278. doi: 10.1016/j.mod.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Morrisey EE, Tang Z, Sigrist K, et al. GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 1998;12:3579–3590. doi: 10.1101/gad.12.22.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Artus J, Piliszek A, Hadjantonakis AK. The primitive endoderm lineage of the mouse blastocyst: sequential transcription factor activation and regulation of differentiation by Sox17. Dev Biol. 2011;350:393–404. doi: 10.1016/j.ydbio.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cirillo LA, Lin FR, Cuesta I, Friedman D, Jarnik M, Zaret KS. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol Cell. 2002;9:279–289. doi: 10.1016/s1097-2765(02)00459-8. [DOI] [PubMed] [Google Scholar]
- 11.Keng VW, Yagi H, Ikawa M, et al. Homeobox gene HEX is essential for onset of mouse embryonic liver development and differentiation of the monocyte lineage. Biochem Biophys Res Commun. 2000;276:1155–1161. doi: 10.1006/bbrc.2000.3548. [DOI] [PubMed] [Google Scholar]
- 12.Tomizawa M, Garfield S, Factor V, Xanthopoulos KG. Hepatocytes deficient in CCAAT/enhancer binding protein α (C/EBPα) exhibit both hepatocyte and biliary epithelial cell character. Biochem Biophys Res Commun. 1998;249:1–5. doi: 10.1006/bbrc.1998.8999. [DOI] [PubMed] [Google Scholar]
- 13.Yamasaki H, Sada A, Iwata T, et al. Suppression of C/EBPα expression in periportal hepatoblasts may stimulate biliary cell differentiation through increased Hnf6 and Hnf1b expression. Development. 2006;133:4233–4243. doi: 10.1242/dev.02591. [DOI] [PubMed] [Google Scholar]
- 14.Kheolamai P, Dickson AJ. Liver-enriched transcription factors are critical for the expression of hepatocyte marker genes in mES-derived hepatocyte-lineage cells. BMC Mol Biol. 2009;10:35. doi: 10.1186/1471-2199-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeLaForest A, Nagaoka M, Si-Tayeb K, et al. HNF4A is essential for specification of hepatic progenitors from human pluripotent stem cells. Development. 2011;138:4143–4153. doi: 10.1242/dev.062547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada T, Yoshikawa M, Kanda S, et al. In vitro differentiation of embryonic stem cells into hepatocyte-like cells identified by cellular uptake of indocyanine green. Stem Cells. 2002;20:146–154. doi: 10.1634/stemcells.20-2-146. [DOI] [PubMed] [Google Scholar]
- 17.Si-Tayeb K, Noto FK, Nagaoka M, et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51:297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song Z, Cai J, Liu Y, et al. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19:1233–1242. doi: 10.1038/cr.2009.107. [DOI] [PubMed] [Google Scholar]
- 19.Takayama K, Inamura M, Kawabata K, et al. Efficient generation of functional hepatocytes from human embryonic stem cells and induced pluripotent stem cells by HNF4α transduction. Mol Ther. 2012;20:127–137. doi: 10.1038/mt.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song H, Hollstein M, Xu Y. p53 gain-of-function cancer mutants induce genetic instability by inactivating ATM. Nat Cell Biol. 2007;9:573–580. doi: 10.1038/ncb1571. [DOI] [PubMed] [Google Scholar]
- 21.Takayama K, Inamura M, Kawabata K, et al. Efficient and directive generation of two distinct endoderm lineages from human ESCs and iPSCs by differentiation stage-specific SOX17 transduction. PLoS One. 2011;6:e21780. doi: 10.1371/journal.pone.0021780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao L, Yuan X, Sharkis SJ. Activin A maintains self-renewal and regulates fibroblast growth factor, Wnt, and bone morphogenic protein pathways in human embryonic stem cells. Stem Cells. 2006;24:1476–1486. doi: 10.1634/stemcells.2005-0299. [DOI] [PubMed] [Google Scholar]
- 23.Shiraki N, Yamazoe T, Qin Z, et al. Efficient differentiation of embryonic stem cells into hepatic cells in vitro using a feeder-free basement membrane substratum. PLoS One. 2011;6:e24228. doi: 10.1371/journal.pone.0024228. [DOI] [PMC free article] [PubMed] [Google Scholar]