Abstract
Objective
To compare the longitudinal changes in total bone mineral density (TBMD) across antiretroviral (ARV) regimens.
Methods
A5142 was an open-label study comparing 3 ARV regimens for the initial treatment of HIV-1. Subjects were randomized equally to efavirenz (EFV) plus 2 nucleoside reverse transcriptase inhibitors (NRTIs), lopinavir/ritonavir (LPV/r) plus 2 NRTIs, or LPV/r plus EFV without NRTI. The NRTI regimen (lamivudine [3TC] plus zidovudine [ZDV], stavudine [d4T], or tenofovir [TDF]) was selected prior to randomization. TBMD was assessed via whole-body dual-energy X-ray absorptiometry (DXA) at baseline and 48 and 96 weeks. Analysis was modified intent-to-treat (ITT) ignoring regimen changes using all evaluations.
Results
Significant mean declines in TBMD at week 48 were observed among subjects. In repeated-measures analysis of changes (including randomized regimen, NRTI used, and time), there was a significant difference in the NRTI-containing arms in mean percentage change in TBMD at week 48 according to NRTI used (P < .001). Subjects taking ZDV had similar changes to those taking d4T (P = .970), whereas those taking TDF had larger declines (P < .001). There was a nonsignificant trend toward greater mean declines among subjects taking LPV/r versus EFV (P = .080). Overall, TDF-containing regimens demonstrated the greatest losses in TBMD, while EFV regimens without TDF had lesser TBMD reductions even compared to the NRTI-sparing arm. From week 48 to 96, all treatment groups continued to lose TBMD at similar rates.
Conclusions
Among NRTI-containing arms, NRTI selection, especially use of TDF, had a greater effect on TBMD change than randomized regimen. The long-term clinical significance remains to be demonstrated.
Keywords: antiretroviral therapy, bone density, HIV
As complete viral suppression is now feasible and expected from initial antiretroviral (ARV) therapy with a number of clinically tolerable and simple regimens, the focus of clinical care and research has been redirected at assessing and minimizing the long-term effects of HIV and its therapies on end organ disease. Unfortunately, designing and implementing studies that are of sufficient size and duration to assess long-term complications is often problematic and difficult. In the interim, surrogate measures known to be correlated with organ disease progression can provide important information about potential differences between regimens.
Bone mineral density (BMD) alterations are now a well-recognized complication of ARV therapy for HIV disease, although the relative contribution of specific ARV drugs or classes, the effects of HIV disease itself, and lifestyle factors remains unclear. Prospective data have been limited, but there are a number of clinical trials of initial ARV therapies that can provide data regarding the relative contributions of therapy to changes in BMD. Several studies have demonstrated a 1% to 6% reduction of BMD in the first year of ARV therapy.1-4 Differential BMD effects according to measurement location and ARV agents and classes have been noted, but results have not been consistent.
A5142 was a phase III study of initial therapy of HIV-1 infection that prospectively compared the virologic and metabolic outcomes of 3 class-sparing regimens over a 96-week period. Bone density changes, measured by total body dual x-ray absorptiometry (DXA), were assessed as a planned secondary analysis. These analyses provide an important opportunity to gain insight into the TBMD changes associated with various classes of ARV regimens.
Methods
Overall Study Design
AIDS Clinical Trials Group (ACTG) A5142 was a phase III, randomized, multicenter, open-label, 96-week trial that compared 3 class-sparing regimens for the initial treatment of HIV-1 infection.5,6 Eligible participants were randomized equally to 3 treatment regimens: lopinavir/ritonavir (LPV/r) plus efavirenz (EFV) (nucleoside reverse transcriptase inhibitor [NRTI]–sparing arm) or 2 NRTIs plus either LPV/r (LPV/r+2NRTI arm) or EFV (EFV+2NRTI arm). The NRTI included lamivudine (3TC) for all subjects; the second NRTI was selected prior to randomization by the site investigator. At the start of the study, the choices were zidovudine (ZDV) or stavudine (d4T) extended release (investigational formulation, 100 mg once daily or 75 mg if the subjects weight was <60 kg); tenofovir (TDF) was added as an option approximately 3 months after study accrual began. Randomization was stratified based on screening plasma HIV-1 RNA (<100,000 copies/mL vs ≥100,000 copies/mL), chronic hepatitis (either B or C) infection, and choice of NRTI.
The study population consisted of HIV-1–infected, ARV-naïve, male and nonpregnant female subjects of at least 13 years of age with plasma HIV-1 RNA levels ≥2,000 copies/mL, acceptable laboratory values, and any CD4 cell count. The study opened on January 14, 2003, and closed to enrollment on May 27, 2004. Subjects were followed for metabolic and virologic endpoints at study prescribed intervals6 regardless of regimen change or discontinuation. Within-class substitutions were allowed for toxicity, and a new regimen was designed by site investigators at virologic failure based on HIV resistance testing. The study continued until the last enrolled subject completed 96 weeks of follow-up.
Bmd Measurement
Whole-body DXA was used to assess TBMD at study entry and at 48 and 96 weeks thereafter using standardized protocol. Individual subjects were assessed on the same machine at all DXA measurement visits. Validity of DXA measurements was assessed by a centralized DXA reading site (Tufts University, Boston, MA). TBMD was captured and expressed as total density in g/cm2; whole body and specific lumbar and hip T and Z scores were not recorded and could not be reconstructed from scanner data files. Bone fractures were not targeted for specific data capture, but fractures were collected as spontaneous data reports using standard ACTG adverse event recording mechanisms.
Statistical Analyses
Characteristics of subjects with versus without DXAs, and among the NRTI groups, were compared using the Kruskal-Wallis test (continuous variables) and chi-square test (categorical variables). Associations of TBMD and characteristics at study entry and associations of percentage changes in TBMD during follow-up and changes in body composition and HIV disease markers were evaluated using linear regression. Considered predictors of baseline and change in BMD included age, sex, menopausal status (pre- and perimenopausal or postmenopausal), race/ethnicity (White non-Hispanic, Black non-Hispanic, or Hispanic; the 15 subjects in other race/ethnicity categories were combined with the White non-Hispanic group), body mass index (BMI), CD4 count, log10 HIV-1 RNA, hepatitis co-infection, statin exposure, hormone replacement therapy exposure, DXA extremity fat, truncal fat and lean mass percentages, and fasting glucose and lactate levels. A multivariate repeated measures model (with unstructured covariance) was developed to describe percentage changes from baseline in TBMD at weeks 48 and 96 including main effects for time, randomized treatment arm, and NRTI used. Interaction variables involving these effects were not significant and results presented are from the model with the main effects only. This model was extended to identify other baseline predictors of change in TBMD by first including the established factors associated with TBMD, specifically sex, menopausal status, race/ethnicity, age, and BMI, as well as CD4 count and log10 HIV-1 RNA as markers of HIV disease status. Backward variable selection was then used to remove variables that were not significant at the .05 level. All other possible predictor variables were then added in turn to this model to evaluate whether any were statistically significant at the P < .05 level. For presentation purposes, the models were refitted using standard categories for each of the continuous variables that were statistically significant. Analyses of changes in TBMD are modified intent-to-treat (ITT), restricted to subjects with a baseline TBMD evaluation and using all available TBMD measurements during follow-up irrespective of changes in ARV therapy. As-treated (AT) analyses were restricted to TBMD measurements obtained while a subject was taking the same ARV therapy as initially started. Six subjects with extreme changes in TBMD values (suggesting measurement problems) between baseline and follow-up were excluded from analyses. Sensitivity analyses that included these subjects did not yield qualitatively different results.
Results
Characteristics of Subjects
Baseline TBMD data were available for 687 of the 753 subjects (91%) who were randomized in A5142. Compared to subjects with baseline DXA studies, the 66 subjects without scans were more likely to be female (35% vs 19%; P = .002) but were similar for other baseline characteristics.
Table 1 shows selected characteristics of subjects for the overall study population with TBMD data and according to NRTI taken. There was a significant difference in race/ethnicity among NRTI groups (P = .010). There were also statistically significant, but clinically small, differences in median truncal fat percentage and lactate levels across NRTI groups.
Table 1. Selected characteristics at baseline of subjects according to NRTI used.
| Characteristic | Subjects with TBMD measurements (N=687) |
NRTI used | P valuea | |||
|---|---|---|---|---|---|---|
| NRTI-sparing arm (n=232) |
ZDV+3TC (n=192) |
d4T+3TC (n=105) |
TDF+3TC (n=158) |
|||
| Gender, female | 128 (19%) | 39 (17%) | 35 (18%) | 23 (22%) | 31 (20%) | .71 |
| Race/ethnicity | .010 | |||||
| White non-Hispanic/otherb | 270 (39%) | 84 (36%) | 96 (50%) | 36 (34%) | 54 (34%) | |
| Black, non-Hispanic | 280 (41%) | 95 (41%) | 65 (34%) | 43 (41%) | 77 (49%) | |
| Hispanic | 137 (20%) | 53 (23%) | 31 (16%) | 26 (25%) | 27 (17%) | |
| Age, years | 38 (32-44) | 38 (31-44) | 38 (33-45) | 39 (34-42) | 36 (31-43) | .27 |
| Body mass index,kg/m2 | 24.8 (22.1-27.9) | 24.8 (22.1-28.4) | 24.8 (22.5-27.9) | 24.8 (22.1-28.1) | 24.6 (21.5-27.6) | .74 |
| CD4 count, cells/μL | 190 (52-308) | 186 (40-316) | 194 (76-300) | 188 (61-302) | 187 (40-308) | .86 |
| HIV-1 RNA, log10 copies/mL | 4.79 (4.43-5.24) | 4.85 (4.49-5.30) | 4.77 (4.47-5.21) | 4.77 (4.42-5.17) | 4.69 (4.27-5.19) | .14 |
| Hepatitis B or C infection | 96 (14%) | 33 (14%) | 24 (13%) | 12 (11%) | 27 (17%) | .53 |
| TBMD, g/cm2 | 1.21 (1.14-1.29) | 1.21 (1.14-1.31) | 1.22 (1.16-1.29) | 1.20 (1.14-1.26) | 1.21 (1.14-1.31) | .20 |
| Percent extremity fat percentage | 44.0 (39.8-48.3) | 44.3 (39.8-48.0) | 43.7 (39.5-48.1) | 42.7 (39.0-47.0) | 45.1 (40.8-49.7) | .10 |
| Percent truncal fat percentage | 50.0 (45.3-54.9) | 49.8 (45.6-54.6) | 50.7 (45.4-55.9) | 51.3 (46.6-55.7) | 48.6 (43.8-53.4) | .025 |
| Percent lean mass percentage | 71.9 (65.2-77.4) | 71.9 (65.3-77.5) | 71.2 (64.3-77.3) | 71.1 (65.1-77.3) | 72.4 (66.3-77.5) | .84 |
| Lactate, mmol/L | 0.46 (0.36-0.69) | 0.45 (0.36-0.67) | 0.44 (0.33-0.62) | 0.62 (0.43-0.92) | 0.45 (0.33-0.64) | <.001 |
| Glucose, mg/dL | 85 (79-92) | 84.5 (80-92) | 86.0 (80-92) | 85 (80-93) | 83 (77-91) | .17 |
Note: Values given as n (%) or median (IQR). Missing values: 1 subject had a missing baseline CD4 count, 28 subjects had missing baseline BMI measurements; 7 subjects with unknown hepatitis infection status were grouped with the subjects with neither infection. 3TC = lamivudine; d4T = stavudine; EFV = efavirenz; IQR = interquartile range; NRTI = nucleoside reverse transcriptase inhibitor; TBMD = total bone mineral density; TDF = tenofovir; ZDV = zidovudine.
P value for comparing the 4groups according to NRTI used: Kruskal-Wallis test for continuous variables, chi-square test for categorical variables.
Includes 15 subjects who classified themselves as Asian or American Indian.
Factors Associated with TBMD at Baseline
Among the 687 subjects with TBMD measurements at study entry, mean (SD) TBMD was 1.22 g/cm2 (0.11 g/cm2) and median (10th and 90th percentiles) TBMD was 1.21 g/cm2 (1.07 and 1.36 g/cm2). In univariate analyses, there were significant associations between TBMD and age, sex, menopausal status among women, race/ethnicity, BMI, and CD4 count but not HIV-1 RNA. In multivariate analysis including all of these factors except HIV-1 RNA, the same associations remained significant. There were significant adjusted TBMD associations with sex (mean 0.06 g/cm2 lower for females vs males; P < .001), menopausal status (0.07 g/cm2 lower for postmenopausal females vs pre- and perimenopausal females; P < .001), race/ethnicity (0.06 g/cm2 lower for White non-Hispanic subjects and 0.10 g/cm2 lower for Hispanic subjects vs Black non-Hispanic subjects; P < .001), and BMI (0.13, 0.11, and 0.06 g/cm2 lower among subjects with BMI <18.5, 18.5 to <25, 25 to <30 compared with ≥30 kg/m2, respectively; P < .001). Lower CD4 count was associated with higher TBMD: Compared with subjects with CD4 counts of ≥350 cells/mm3, the adjusted mean TBMD was 0.02 g/cm2 higher for subjects with counts in each of the 200 to 349 and 50 to 199 cells/mm3 categories and 0.05 g/cm2 higher for those with counts <50 cells/mm3 (P < .001). The association of TBMD with age showed a peak among subjects who were 40 to 49 years old, with mean TBMD 0.01 g/cm2 lower in each of the age categories of <30 and 30 to 39 years and 0.04 g/cm2 lower among subjects who were 50 years old or older. There were no significant TBMD associations with any of the following variables when added to this model: HIV-1 RNA, hepatitis B or C infection status, lean mass percentage, glucose or lactate levels. There was a positive association with truncal fat percentage.
Comparison of Changes in TBMD by Treatment
Of the 687 subjects with baseline TBMD measurements, 569 (83%) and 503 (73%) had measurements at weeks 48 and 96, respectively. Among the 184 without a measurement at week 96, 16 had died previously, 128 had no follow-up visit at week 96 (usually due to loss to follow-up), and 40 had a week 96 study visit but a DXA scan was not obtained. At weeks 48 and 96, 452 and 343 subjects with DXA measurements, respectively, were still on the same combination of ARV drugs that they initially started (AT population).
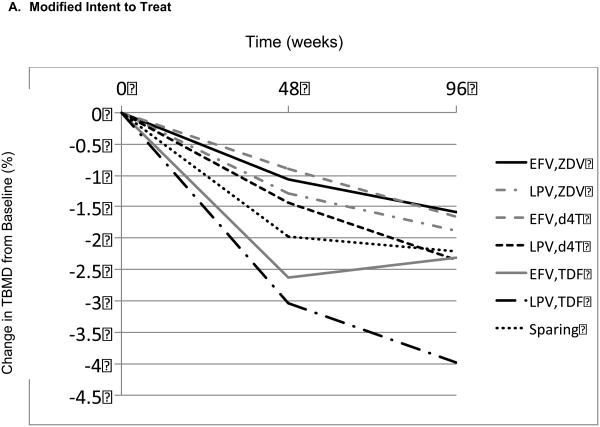
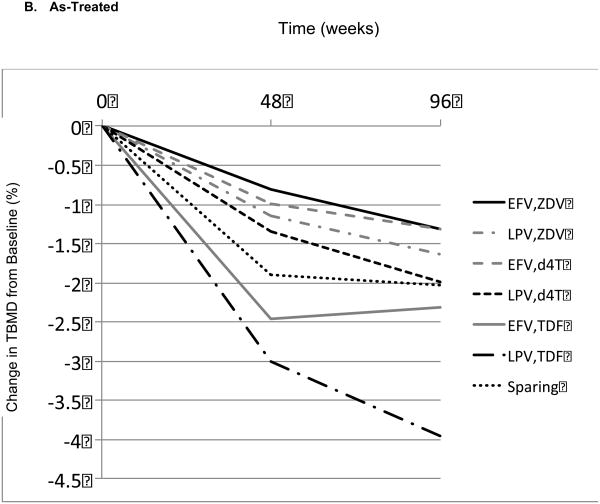
Table 2 displays the mean percentage changes in TBMD from baseline to week 96 for each of the 7 combinations of randomized regimen or selected NRTI ARV drugs. Figure 1 graphically represents the mean percentage changes from baseline for each ARV regimen, both for an ITT analysis, which used all available measurements (ignoring changes in ARV therapy; Figure 1A), and an AT analysis (Figure 1B). In both analyses, for each of the 7 regimens, there were significant mean percentage declines in TBMD from baseline to week 48 and to week 96. There were highly significant differences in mean TBMD percentage declines from baseline to week 48 between the 7 regimens (ITT, P < .001; AT, P < .001), but there was no significant difference among regimens in TBMD change from week 48 to 96 (ITT, P = .320; AT, P = .410). Thus, differences among regimens were established by week 48 and were maintained thereafter. As there was no notable difference in the pattern of changes between the ITT and AT analyses, the remainder of the results focuses on the ITT population.
Table 2. Mean percentage changes from baseline in TBMD to weeks 48 and 96 (modified intent to treat analysis) by (a) randomized treatment, (b) NRTI used, and (c) randomized regimen/NRTI used.
| (a) | ||||||
|---|---|---|---|---|---|---|
| ARV regimen | 48 weeks | 96 weeks | ||||
| n | Mean | SE | n | Mean | SE | |
| NRTI-sparing | 195 | -1.98 | 0.20 | 171 | -2.21 | 0.27 |
| EFV/2NRTI | 186 | -1.59 | 0.23 | 169 | -1.89 | 0.24 |
| LPV/2NRTI | 188 | -1.90 | 0.22 | 163 | -2.61 | 0.28 |
| (b) | ||||||
|---|---|---|---|---|---|---|
| NRTI used | 48 weeks | 96 weeks | ||||
| n | Mean | SE | n | Mean | SE | |
| NRTI-sparing | 195 | -1.98 | 0.20 | 171 | -2.21 | 0.27 |
| ZDV | 152 | -1.18 | 0.19 | 135 | -1.75 | 0.27 |
| D4T | 92 | -1.17 | 0.25 | 83 | -2.02 | 0.35 |
| TDF | 130 | -2.82 | 0.33 | 114 | -3.00 | 0.33 |
| (c) | ||||||
|---|---|---|---|---|---|---|
| ARV regimen | 48 weeks | 96 weeks | ||||
| N | Mean | SE | N | Mean | SE | |
| NRTI-sparing | 195 | -1.98 | 0.20 | 171 | -2.21 | 0.27 |
| EFV/ZDV+3TC | 73 | -1.07 | 0.29 | 62 | -1.59 | 0.38 |
| LPV/ZDV+3TC | 79 | -1.29 | 0.26 | 73 | -1.89 | 0.38 |
| EFV/d4T+3TC | 45 | -0.89 | 0.39 | 40 | -1.66 | 0.51 |
| LPV/d4T+3TC | 47 | -1.43 | 0.31 | 43 | -2.35 | 0.48 |
| EFV/TDF+3TC | 68 | -2.63 | 0.45 | 67 | -2.31 | 0.38 |
| LPV/TDF+3TC | 62 | -3.04 | 0.48 | 47 | -3.98 | 0.57 |
Note: 3TC = lamivudine; d4T = stavudine; EFV = efavirenz; LPV = lopinavir/ritonavir; NRTI = nucleoside reverse transcriptase inhibitor; SE = standard error; TBMD = total bone mineral density; TDF = tenofovir; ZDV = zidovudine.
Figure 1.
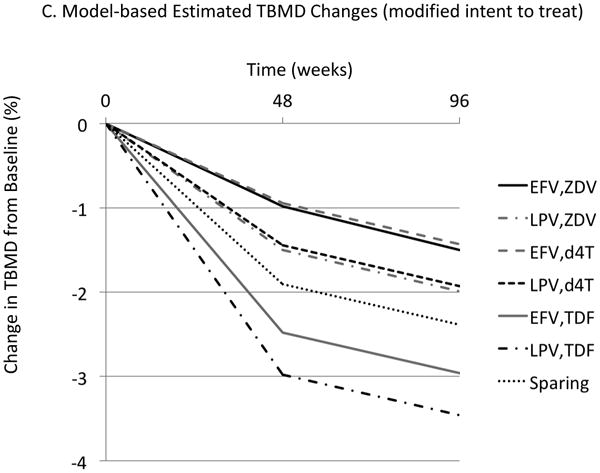
Mean total bone mineral density (TBMD) percentage change from baseline over time according to antiretroviral (ARV) regimen. (A) Modified intent-to-treat (ITT) population. (B) As-treated (AS) population. (C) Model-based estimated TBMD changes (modified ITT population). d4T = stavudine; EFV = efavirenz; LPV = lopinavir/ ritonavir; TDF = tenofovir; Sparing = efavirenz plus lopinavir/ ritonavir; ZDV = zidovudine.
Note: Changes for ZDV and d4T plots were intentionally offset for display purposes. There was no difference between ZDV and d4T found in the model.
A model for repeated measurements was used, which helped to demonstrate the differences among regimens at week 48 and to show that TBMD further declined, maintaining these differences among regimens, from week 48 to 96. The model included effects for randomized treatment and, for the NRTI-containing arms, the NRTI used. Modeled estimates of mean TBMD changes at weeks 48 and 96 (ITT analysis) are shown in Figure 1C. The estimated mean percentage ± SE TBMD decline at week 48 for the NRTI-sparing regimen from this model was 2.0 ± 0.2%. Among participants randomized to NRTI-containing regimens compared to subjects taking ZDV, those taking d4T had very similar estimated mean TBMD declines at week 48 (difference in mean change, 0.0 ± 0.4%; P = .970), whereas those taking TDF had a larger mean TBMD decline (difference in mean decline, 1.5 ± 0.3%; P < .001). After adjustment for NRTI used, there was significant variation among the 3 randomized arms (P = .013), but the interpretation of between-arm differences was complicated by the larger difference between regimens with TDF and ZDV or d4T. Subjects randomized to EFV or LPV/r (with either ZDV or d4T) had less TBMD loss than subjects assigned the NRTI-sparing regimen, whereas those who took TDF had greater TBMD loss (Figure 1C). Comparison between the NRTI-containing LPV/r and EFV arms showed a nonsignificant trend for greater mean percentage decline in TBMD for the LPV-containing arm compared with the EFV-containing arm (difference in mean decline, 0.5 ± 0.3%; P = .080). Consequently, subjects starting EFV in combination with 3TC and either ZDV or d4T had the smallest estimated mean declines at week 48 (0.9%) and those starting LPV/r in combination with 3TC and TDF had the largest (3.0%). Between week 48 and week 96, the estimated mean percentage decline was 0.5 ± 0.1%; this magnitude of decline was similar among the regimens.
Baseline Predictors of Change in TBMD
Among baseline characteristics considered in the repeated measures model, after adjusting for treatment effects, only postmenopausal status, higher HIV-1 RNA, and lower CD4 count were significantly associated with greater declines in TBMD at week 48 and only postmenopausal status was significantly associated with additional declines in TBMD from week 48 to 96. In this model, postmenopausal status was associated with a 2.5 ± 0.7% greater mean (± SE) TBMD decline by week 48 and a further 2.5 ± 0.7% greater mean TBMD decline between weeks 48 and 96. In the same model, compared with subjects with baseline HIV-1 RNA <30,000 copies/mL, subjects in the categories of 30,000 to <100,000, 100,000 to <300,000, and ≥300,000 copies/mL had mean TBMD declines that were greater by 0.5 ± 0.3%, 0.8 ± 0.3%, and 0.7 ± 0.3%, respectively. In addition, compared with subjects with baseline CD4 count ≥350 cells/mm3, subjects in the categories of 200 to 349 and 50 to 199 cells/mm3 had mean TBMD declines which were (not significantly) smaller by 0.4 ± 0.3% and 0.1 ± 0.3%, whereas subjects with CD4 counts <50 cells/mm3 had mean TBMD declines that were greater by 1.1 ± 0.4%.
Association of Changes in TBMD and Changes in Disease Status and Body Composition
Associations between changes in TBMD and changes in disease status (CD4 count and suppression of HIV-1 RNA to <50 copies/mL) and body composition (BMI, extremity and truncal fat percentages, and lean body mass percentage) at both weeks 48 and 96 were evaluated. Except for an association with change in BMI at week 48, no significant associations were found in either unadjusted analyses or with adjustment for ARV treatment, demographic factors, and baseline disease status (data not shown). The association between change in TBMD and change in BMI at week 48 was weak, corresponding to a 0.5% greater TBMD change with each 5 kg/m2 increase in BMI between baseline and week 48 (P = .014). However, this association was not significant at week 96.
Fractures
Seven subjects developed 8 bone fractures over the course of the study. The 8 fractures included 2 lower limb fractures (1 ankle, 1 hip), 5 upper limb fractures, and 1 rib fracture; details of the relationship to trauma were not available. Four subjects were randomized to the LPV/r + EFV regimen, 2 to the EFV regimen, and 1 to the LPV/r regimen; 2 subjects received TDF and 1 received d4T.
Discussion
In this analysis from a large, randomized study, significant declines in TBMD over time were observed among treatment-naïve HIV-infected subjects receiving 3 class-sparing ARV regimens. Mean decreases in TBMD of 1% to 4% in the first 2 years of treatment were observed among the ARV regimens initiated. The majority of the observed TBMD decreases occurred in the first 48 weeks of ARV. Overall, TBMD declines differed primarily according to NRTI regimen and were greatest among subjects who received TDF, while subjects exposed to ZDV and d4T had lower declines. Our original hypothesis, that a NRTI-sparing regimen would have fewer metabolic and ARV-related complications, was not supported by the data: Subjects who received the NRTI-sparing regimen had TBMD declines at week 48 that were greater than the ZDV/d4T-containing regimens but less than the TDF-containing regimens (Table 2). From 48 to 96 weeks, smaller reductions in TBMD were seen but at a similar rate across all regimens.
Our data validate prior data suggesting that BMD declines are observed with initiation of ARV therapy with all regimens2,7 particularly in the first year of therapy.8,9 Similarly, we demonstrate that tenofovir,3,10 in particular, may induce more prominent BMD changes as compared to other ARV agents. Our results are concordant with another large randomized study. In ACTG 5224s, of 269 subjects randomized equally to TDF or abacavir (ABC), given either with EFV or atazanavir/ ritonavir (ATV/r), the TDF group had greater decline in spine and hip BMD than those on ABC (percent change at week 96 was -3.0 vs -1.1, P = .040, for spine, and -3.9 vs -2.6, P = .025, for hip).4
The role of protease inhibitors (PIs) versus non-nucleoside reverse transcriptase inhibitors (NNRTIs) in protection or enhancement of the universal initial bone loss seen with ARV remains complex. In our study, among subjects randomized to NRTI-containing regimens, those receiving LPV/r tended to have greater TBMD reductions compared to those receiving EFV, but the difference was not statistically significant (P = .080). The results of ACTG 5224s were also mixed: ATV/r-treated subjects had greater loss of spine but not hip BMD compared to EFV-treated subjects. Another study, which compared LPV/r to EFV regimens given with 2 NRTIs, did not find between-regimen differences, but the study design included a simplification in the LPV/r arm to LPV/r monotherapy after 24 to 48 weeks.2
In our study, use of an NRTI-sparing regimen was associated with greater declines in TBMD compared to non-TDF NRTI regimens (ZDV or d4T). This is in contrast to in vitro research demonstrating ZDV11 and d4T12 bone toxicity and clinical evidence suggesting that dose-reducing d4T regimens may mitigate bone loss.13 Also, a small (n = 50) randomized study by van Vonderen suggested that an NRTI-sparing regimen (LPV/r + nevirapine [NVP]) was associated with significantly smaller BMD reductions than a regimen containing LPV/r+ZDV+3TC.14 After 24 months, they found a 6% reduction in the femoral neck BMD in the NRTI-containing versus only 2% reduction in the NRTI-sparing regimen. Differences between this NRTI-sparing study and ours include use of NVP versus EFV, an overall greater decline in BMD (6%) than has been seen in many studies, and baseline population differences. The mechanism of BMD toxicity among ARV agents has been postulated to involve direct effects on osteoblasts and osteoclasts,11,15,16 increased bone turnover in general,9 and perturbations of gene expression for genes involved in bone maintenance for TDF.17
These results also validate the importance of assessing traditional factors to determine the risk for bone loss. We confirm the significant effects on BMD of age18 and menopausal status in women,19,20 although younger age was not uniformly associated with greater BMD as is typically seen. Similarly, we demonstrate the important relationship between baseline body mass index and BMD, as shown in other studies,21,22 although we did not demonstrate a strong association between longitudinal changes in body mass index or body composition with BMD in our cohort. These studies among HIV-infected persons10,23,24 and our own study highlight the need to routinely assess for traditional risk factors in addition to HIV-specific variables.
In our study population, we demonstrated some unexpected findings in regard to relationships between HIV disease status and TBMD. Specifically, in our cohort, lower pretreatment CD4 cell counts were associated with higher baseline TBMD, whereas no association between pretreatment HIV-1 RNA levels and baseline TBMD was found. This is in contrast with prior research demonstrating reduced BMD with uncontrolled HIV disease manifested by low CD4 count and increased HIV-1 RNA levels.25-27 Similarly, low CD4 counts have been more recently linked with fragility fractures in HIV.28 We also demonstrated that lower pretreatment CD4 counts and higher baseline plasma HIV-1 RNA levels were associated with greater TBMD declines on therapy, similar to other studies.4 The mechanism by which BMD is reduced in uncontrolled HIV infection is likely via systemic inflammation and its effects on bone remodeling with uncoupling of bone resorption and formation processes.29-31 However, immune reconstitution with initiation of ARV therapy has also been shown to be linked to increased inflammation that, along with demonstrated evidence of direct effects of ARV therapy on bone,11,15,16 may explain the demonstrated further reduction in bone density in our cohort, as seen in other treatment studies.2,7
Taken together, these findings suggest the need for a bone health risk assessment model for the HIV-infected population that is inclusive of both traditional and HIV disease-specific (ARV regimen selection and disease characteristics) clinical variables. Current osteoporosis risk assessment models include the FRAX assessment risk tool32 developed by the National Osteoporosis Foundation and advocate for screening and determination of osteoporosis treatment eligibility among men ≥50 years and in all postmenopausal women.33 However, FRAX has not been validated in the HIV-infected population and may underestimate risk among HIV-infected persons.33 Given the prevalence of BMD reductions with any ARV therapy initiation in HIV-infected persons and recent data demonstrating increased rates of fragility fracture among HIV-infected as compared to the general US population,34 standard BMD assessment may soon be required. Recent recommendations promote universal screening among HIV-infected persons aged 50 years and older.35
Limitations to the current study include the use of whole-body densitometry to assess BMD instead of regional and site-specific densitometry, which provide clinically applicable T and Zscores that can assess fracture risk. However, data do suggest that close correlations exist between regional and whole-body BMD using DXA.36,37 Also, whole-body scans should be able to discern between-regimen differences, as the same measurements (ie, TBMD) were used longitudinally for all subjects. Nevertheless, future longitudinal ARV studies of BMD should utilize regional densitometry to allow analysis of fracture risk over time. A recent study of more than 56,000 HIV-infected persons from the Veterans Affairs' Clinical Case Registry over a 21-year period demonstrated an increased osteoporotic fracture hazard ratio among persons exposed to TDF and PIs.38 In addition, several important risk factors for reduced BMD common in HIV-infected populations were not assessed in this study, including hypogonadism,39,40 hypovitaminosis D,41 tobacco use,21,22 and duration of HIV infection.21 The lack of assessment of these risk factors may explain some of the unexpected findings in our cohort, such as increased BMD with lower CD4 counts. Also, ACTG 5142 used NRTIs that are no longer recommended (ie, ZDV and d4T). However, in spite of these limitations, several important findings are still evident: (a) TDF had an important effect on BMD (TBMD losses were greater than comparator NRTI); and (b) an NRTI-sparing regimen had less TBMD loss than TDF-containing regimens. As the vast majority of current ARV regimens contain TDF, search for successful NRTI-sparing regimens continue.42-46 For example, one ongoing study is comparing the bone effects of 2 regimens that contain darunavir/ritonavir and 3TC with TDF versus maraviroc as the third agent (NCT01400412). There are recent data to suggest that the prodrug of TDF, tenofovir alafenamide fumarate (TAF), may have less bone toxicity than TDF.47
Conclusion
Our study presents longitudinal bone density data among HIV-infected treatment-naïve men and women initiating various ARV regimens. We demonstrate significant differences in initial TBMD losses in the first year after ARV initiation, which differ by specific ARV regimen, followed by smaller TBMD losses in the second year, which are independent of treatment regimen. Our data provide further rationale for screening for BMD loss among HIV-infected persons commencing ARV therapy and potentially intervening with adjuvant therapies or specific regimen selection for those at high risk for bone loss due to demographic or HIV-related conditions.
Acknowledgments
Financial disclosures/support: The collaborating pharmaceutical companies provided lopinavir–ritonavir (Abbott), efavirenz and stavudine XR (Bristol-Myers Squibb), and tenofovir DF (Gilead). This work was supported by grants AI 068636 (AIDS Clinical Trials Group Central Grant), AI 27670, AI 068634, AI 069471, AI 27661, AI 069439, AI 25859, AI 069477, AI 069513, AI 069452, AI 27673, AI 069470, AI 069474, AI 069411, AI 069423, AI 069494, AI 069484, AI 069472, AI 38858, AI 069501, AI 32783, AI 069450, AI 32782, AI 069465, AI 069424, AI 38858, AI 069447, AI 069495, AI 069502, AI 069556, AI 069432, AI 46370, AI 069532, AI 46381, AI 46376, AI 34853, AI 069434, AI 060354, AI 36214, AI 069419, AI 069418, AI 50410, AI 45008, AI 064086, RR 00075, RR 00032, RR 00044, RR 00046, RR 02635, RR 00051, RR 00052, RR 00096, RR 00047, RR 00039, and DA 12121 from the National Institute of Allergy and Infectious Disease, National Institutes of Health.
Footnotes
Conflicts of interest: R.H. reports having received honoraria or consultant fees from Abbot, Bristol-Myers Squibb, Gilead Sciences, Vertex, and Tibotec and research support (to UCSD) from Abbott, GlaxoSmithKline, Merck, Pfizer, and ViiV. M.D.H. is a paid data monitoring committee member for Boehringer Ingelheim, Medicines Development, Pfizer, and Tibotec. S.R. and J.S.H. report no conflicts.
References
- 1.Rivas P, Gorgolas M, Garcia-Delgado R, Diaz-Curiel M, Goyenechea A, Fernandez-Guerrero ML. Evolution of bone mineral density in AIDS patients on treatment with zidovudine/lamivudine plus abacavir or lopinavir/ritonavir. HIV Med. 2008;9(2):89–95. doi: 10.1111/j.1468-1293.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- 2.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr. 2009;51(5):554–561. doi: 10.1097/QAI.0b013e3181adce44. [DOI] [PubMed] [Google Scholar]
- 3.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: A 3-year randomized trial. JAMA. 2004;292(2):191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 4.McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: AIDS Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203(12):1791–1801. doi: 10.1093/infdis/jir188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haubrich RH, Riddler SA, DiRienzo AG, et al. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS. 2009;23(9):1109–1118. doi: 10.1097/QAD.0b013e32832b4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358(20):2095–2106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duvivier C, Kolta S, Assoumou L, et al. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS (Lond) 2009;23(7):817–824. doi: 10.1097/QAD.0b013e328328f789. [DOI] [PubMed] [Google Scholar]
- 8.Cassetti I, Madruga JV, Suleiman JM, et al. The safety and efficacy of tenofovir DF in combination with lamivudine and efavirenz through 6 years in antiretroviral-naive HIV-1-infected patients. HIV Clin Trials. 2007;8(3):164–172. doi: 10.1310/hct0803-164. [DOI] [PubMed] [Google Scholar]
- 9.Yin MT, Shane E. Low bone-mineral density in patients with HIV: Pathogenesis and clinical significance. Curr Opin Endocrinol Diabetes. 2006;13(6):497–502. doi: 10.1097/MED.0b013e3280109b6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobson DL, Spiegelman D, Knox TK, Wilson IB. Evolution and predictors of change in total bone mineral density over time in HIV-infected men and women in the nutrition for healthy living study. J Acquir Immune Defic Syndr. 2008;49(3):298–308. doi: 10.1097/QAI.0b013e3181893e8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan G, Wu X, McKenna MA, Feng X, Nagy TR, McDonald JM. AZT enhances osteoclastogenesis and bone loss. AIDS Res Hum Retroviruses. 2004;20(6):608–620. doi: 10.1089/0889222041217482. [DOI] [PubMed] [Google Scholar]
- 12.Zuccotti G, Vigano A, Gabiano C, et al. Antiretroviral therapy and bone mineral measurements in HIV-infected youths. Bone. 2010;46(6):1633–1638. doi: 10.1016/j.bone.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 13.McComsey GA, Lo Re V, 3rd, O'Riordan M, et al. Effect of reducing the dose of stavudine on body composition, bone density, and markers of mitochondrial toxicity in HIV-infected subjects: A randomized, controlled study. Clin Infect Dis. 2008;46(8):1290–1296. doi: 10.1086/529384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Vonderen MG, Lips P, van Agtmael MA, et al. First line zidovudine/lamivudine/lopinavir/ritonavir leads to greater bone loss compared to nevirapine/lopinavir/ritonavir. AIDS. 2009;23(11):1367–1376. doi: 10.1097/QAD.0b013e32832c4947. [DOI] [PubMed] [Google Scholar]
- 15.Jain RG, Lenhard JM. Select HIV protease inhibitors alter bone and fat metabolism ex vivo. J Biol Chem. 2002;277(22):19247–19250. doi: 10.1074/jbc.C200069200. [DOI] [PubMed] [Google Scholar]
- 16.Fakruddin JM, Laurence J. HIV envelope gp120-mediated regulation of osteoclastogenesis via receptor activator of nuclear factor kappa B ligand (RANKL) secretion and its modulation by certain HIV protease inhibitors through interferon-gamma/RANKL cross-talk. J Biol Chem. 2003;278(48):48251–48258. doi: 10.1074/jbc.M304676200. [DOI] [PubMed] [Google Scholar]
- 17.Grigsby IF, Pham L, Mansky LM, Gopalakrishnan R, Mansky KC. Tenofovir-associated bone density loss. Ther Clin Risk Manag. 2010;6:41–47. [PMC free article] [PubMed] [Google Scholar]
- 18.Looker AC, Melton LJ, 3rd, Harris T, Borrud L, Shepherd J, McGowan J. Age, gender, and race/ethnic differences in total body and subregional bone density. Osteoporos Int. 2009;20(7):1141–1149. doi: 10.1007/s00198-008-0809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hui SL, Slemenda CW, Johnston CC, Appledorn CR. Effects of age and menopause on vertebral bone density. Bone Mineral. 1987;2(2):141–146. [PubMed] [Google Scholar]
- 20.Nuti R, Martini G. Effects of age and menopause on bone density of entire skeleton in healthy and osteoporotic women. Osteoporos Int. 1993;3(2):59–65. doi: 10.1007/BF01623374. [DOI] [PubMed] [Google Scholar]
- 21.Dolan SE, Kanter JR, Grinspoon S. Longitudinal analysis of bone density in human immunodeficiency virus-infected women. J Clin Endocrinol Metabol. 2006;91(8):2938–2945. doi: 10.1210/jc.2006-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mondy K, Yarasheski K, Powderly WG, et al. Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus-infected individuals. Clin Infect Dis. 2003;36(4):482–490. doi: 10.1086/367569. [DOI] [PubMed] [Google Scholar]
- 23.Dolan SE, Huang JS, Killilea KM, Sullivan MP, Aliabadi N, Grinspoon S. Reduced bone density in HIV-infected women. AIDS (Lond) 2004;18(3):475–483. doi: 10.1097/00002030-200402200-00014. [DOI] [PubMed] [Google Scholar]
- 24.Fausto A, Bongiovanni M, Cicconi P, et al. Potential predictive factors of osteoporosis in HIV-positive subjects. Bone. 2006;38(6):893–897. doi: 10.1016/j.bone.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Grijsen ML, Vrouenraets SM, Steingrover R, et al. High prevalence of reduced bone mineral density in primary HIV-1-infected men. AIDS (Lond) 2010;24(14):2233–2238. doi: 10.1097/QAD.0b013e32833c93fe. [DOI] [PubMed] [Google Scholar]
- 26.Bruera D, Luna N, David DO, Bergoglio LM, Zamudio J. Decreased bone mineral density in HIV-infected patients is independent of antiretroviral therapy. AIDS (Lond) 2003;17(13):1917–1923. doi: 10.1097/00002030-200309050-00010. [DOI] [PubMed] [Google Scholar]
- 27.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS (Lond) 2006;20(17):2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 28.Yong MK, Elliott JH, Woolley IJ, Hoy JF. Low CD4 count is associated with an increased risk of fragility fracture in HIV-infected patients. J Acquir Immune Defic Syndr. 2011;57(3):205–210. doi: 10.1097/QAI.0b013e31821ecf4c. [DOI] [PubMed] [Google Scholar]
- 29.Thomas J, Doherty SM. HIV infection--a risk factor for osteoporosis. J Acquir Immune Defic Syndr. 2003;33(3):281–291. doi: 10.1097/00126334-200307010-00001. [DOI] [PubMed] [Google Scholar]
- 30.Cotter EJ, Ip HS, Powderly WG, Doran PP. Mechanism of HIV protein induced modulation of mesenchymal stem cell osteogenic differentiation. BMC Musculoskel Disord. 2008;9:33. doi: 10.1186/1471-2474-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibellini D, De Crignis E, Ponti C, et al. HIV-1 triggers apoptosis in primary osteoblasts and HOBIT cells through TNFalpha activation. J Med Virol. 2008;80(9):1507–1514. doi: 10.1002/jmv.21266. [DOI] [PubMed] [Google Scholar]
- 32.National Osteoporosis Foundation. Clinician's Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2008. [Google Scholar]
- 33.Lundgren JD, Battegay M, Behrens G, et al. European AIDS Clinical Society (EACS) guidelines on the prevention and management of metabolic diseases in HIV. HIV Med. 2008;9(2):72–81. doi: 10.1111/j.1468-1293.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- 34.Young B, Dao CN, Buchacz K, Baker R, Brooks JT. Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000-2006. Clin Infect Dis. 2011;52(8):1061–1068. doi: 10.1093/cid/ciq242. [DOI] [PubMed] [Google Scholar]
- 35.McComsey GA, Tebas P, Shane E, et al. Bone disease in HIV infection: A practical review and recommendations for HIV care providers. Clin Infect Dis. 2010;51(8):937–946. doi: 10.1086/656412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franck H, Munz M. Total body and regional bone mineral densitometry (BMD) and soft tissue measurements: correlations of BMD parameter to lumbar spine and hip. Calcif Tissue Int. 2000;67(2):111–115. doi: 10.1007/s00223001124. [DOI] [PubMed] [Google Scholar]
- 37.Boyanov M. Estimation of lumbar spine bone mineral density by dual-energy X-ray absorptiometry: Standard anteroposterior scans vs sub-regional analyses of whole-body scans. Br J Radiol. 2008;81(968):637–642. doi: 10.1259/bjr/22307093. [DOI] [PubMed] [Google Scholar]
- 38.Bedimo R, Maalouf NM, Zhang S, Drechsler H, Tebas P. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS (Lond) 2012;26(7):825–831. doi: 10.1097/QAD.0b013e32835192ae. [DOI] [PubMed] [Google Scholar]
- 39.Huang JS, Wilkie SJ, Sullivan MP, Grinspoon S. Reduced bone density in androgen-deficient women with acquired immune deficiency syndrome wasting. J Clin Endocrinol Metabol. 2001;86(8):3533–3539. doi: 10.1210/jcem.86.8.7728. [DOI] [PubMed] [Google Scholar]
- 40.Teichmann J, Lange U, Discher T, Lohmeyer J, Stracke H, Bretzel RG. Bone mineral density in human immunodeficiency virus-1 infected men with hypogonadism prior to highly-active-antiretroviral-therapy (HAART) Eur J Med Res. 2009;14(2):59–64. doi: 10.1186/2047-783X-14-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bang UC, Shakar SA, Hitz MF, et al. Deficiency of 25-hydroxyvitamin D in male HIV-positive patients: A descriptive cross-sectional study. Scand J Infect Dis. 2010;42(4):306–10. doi: 10.3109/00365540903463981. [DOI] [PubMed] [Google Scholar]
- 42.Goicoechea M, Rieg G, Dube M, et al. Faster early viral decline with raltegravir (RAL) + lopinavir/ritonavir (LPV/r) vs. efavirenz (EFV)/ tenofovir disoproxil fumarate (TDF)/ emtricitabine (FTC) in antiretroviral-naive patients: CCTG 589. Paper presented at: XVIII International AIDS Conference; Vienna Austria. 2010. [Google Scholar]
- 43.Taiwo B, Zheng L, Gallien S, et al. Efficacy of a nucleoside-sparing regimen of darunavir/ritonavir plus raltegravir in treatment-naive HIV-1-infected patients (ACTG A5262) AIDS. 2011;25(17):2113–2122. doi: 10.1097/QAD.0b013e32834bbaa9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burgos J, Crespo M, Falco V, et al. Dual therapy based on a ritonavir-boosted protease inhibitor as a novel salvage strategy for HIV-1-infected patients on a failing antiretroviral regimen. J Antimicrob Chemother. 2012;67(6):1453–1458. doi: 10.1093/jac/dks057. [DOI] [PubMed] [Google Scholar]
- 45.Patterson P, Magneres C, Sued O, et al. A phase 4, single-arm, open-label, pilot study of maraviroc, raltegravir and darunavir/r in HIV-1 adults with triple class failure: TERCETO study. J Int AIDS Soc. 2012;15(6):18268. [Google Scholar]
- 46.Cenderello G, Penco G, Pontali E, Feasi M, Cassola G. Safety and efficacy of a raltegravir-based dual antiretroviral therapy in clinical practice. J Int AIDS Soc. 2012;15(6):18353. [Google Scholar]
- 47.Zolopa A, Ortiz R, Sax P, et al. Comparative study of tenofovir alafenamide vs. tenofovir disoproxil fumarate, each with elvitegravir, cobicistat, and emtricitabine, for HIV treatment. Paper presented at: 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. 2013. [Google Scholar]