Abstract
Background
Epidemiologic studies of atopic dermatitis (AD) in desert areas are still lacking.
Objective
The aim of this study was to investigate the epidemiology of AD in children in Kerman city, a desert area in Iran.
Methods
We evaluated preschool children (age, 2 to 7 years) and primary school students (age, greater than 7 up to 12 years) in Kerman. We selected 865 students to estimate the prevalence and assess other features of AD such as distribution of lesions, personal history, family history of atopy, aggravating factors, associated symptoms, and morphological variants.
Results
The prevalence of AD was 9.1% in our study population. The prevalence of AD was 9.17% and 9.09% in males and females, respectively. The prevalence of AD in the age range of 2 to 7 years was 13.53% and 8.33% among children aged greater than 7 up to 12 years. In total, 82.27% of the patients were in chronic stage of the disease, and 31.6% had a personal history of other atopic diseases. At least one first-degree family member with atopy was seen in 46.83% of the patients. The most common sites of involvement were the head and neck. The most involved areas in the limbs were extensor surfaces. The most frequent morphological variant of AD was the common type.
Conclusion
The prevalence of AD in Kerman was higher than in other Iranian cities but lower than that in developed countries. Diversity in the clinical features of AD has been observed among different studies, and the diagnostic criteria of AD should be adapted in proportion to the studied area.
Keywords: Atopic dermatitis, Epidemiology, Kerman, Preschool and primary school children, Prevalence
INTRODUCTION
The prevalence of atopic dermatitis (AD) has increased in the past 30 years1 and is associated with extremely high economic burden in European and Asian countries2. Different prevalence rates of AD have been reported and can be attributed to racial, genetic, and geographic factors3,4.
The etiology of AD is still unknown; however, hereditary, genetic, racial, and environmental factors have been identified. Environmental factors such as climate, sweating, clothing, vaccines, infections, and food allergens may cause disease exacerbation5-7. AD has three phases; infantile (0 to 2 years), childhood (2 to 12 years), and adolescent/adult (12 to 18 years). AD usually begins in infancy and childhood, and its clinical features are age-specific8. The diagnosis is based on clinical presentation, as no unique laboratory test has been specified to diagnose the disease. Different criteria have been suggested for diagnosing AD and the most important are Hanifin and Rajka and the United Kingdom Working Party's diagnostic criteria9. Moreover, the profiles of AD including aggravating factors and clinical features vary greatly in different studies3,4.
Considering the prevalence of AD and frequency of clinical features in different areas of the world, dermatologists are attempting to standardize the diagnostic criteria and classification for each region3.
Data on clinicoepidemiological features of AD in desert climates are still lacking. Kerman is a city in a desert area of Iran, and AD is the most common skin disease in the pediatric age group in this city10. Elucidating the epidemiologic features of AD in this region will be of great help to adopt the current diagnostic criteria in such desert areas. We conducted this cross-sectional study to investigate the clinical and demographic features of childhood AD in a desert area of Iran.
MATERIALS AND METHODS
Study design and data collection
In this cross-sectional descriptive study, we evaluated the clinical and demographic features of AD in preschool children (age, 2 to 7 years) and primary school students (age, greater than 7 up to 12 years) in Kerman City. Sampling was performed using multistage sampling method during the 2009 to 2010 school year. To remove the potentially confounding effect of season, equal numbers of students were selected at different times of the year. Sample size was calculated as 865 considering the type one error (α) (significant level of test)=5% and β (power of the test) =20% and an estimate of 10% for the prevalence of AD in preschool and school children (according to previous studies).
A list of all preschools and primary schools in Kerman was obtained. It consisted of 129 preschools with 5,798 children and 154 primary schools with 37,694 students. The city was divided into four geographical areas. Then, according to the number of the students and the male to female ratio in each area, nine preschools and 30 primary schools were randomly selected. Finally, 133 and 732 students were randomly selected among these nine preschools and 13 primary schools, respectively. Randomization was performed using a computer generated number table. After obtaining written informed consent from their parents or official custodians, two trained medical interns under supervision of a pediatric dermatologist examined the children physically.
This study was approved by the review board and ethics committee of Kerman University of Medical Sciences.
Diagnostic criteria and questionnaire
The United Kingdom Working Party's diagnostic criteria were used to diagnose AD11.
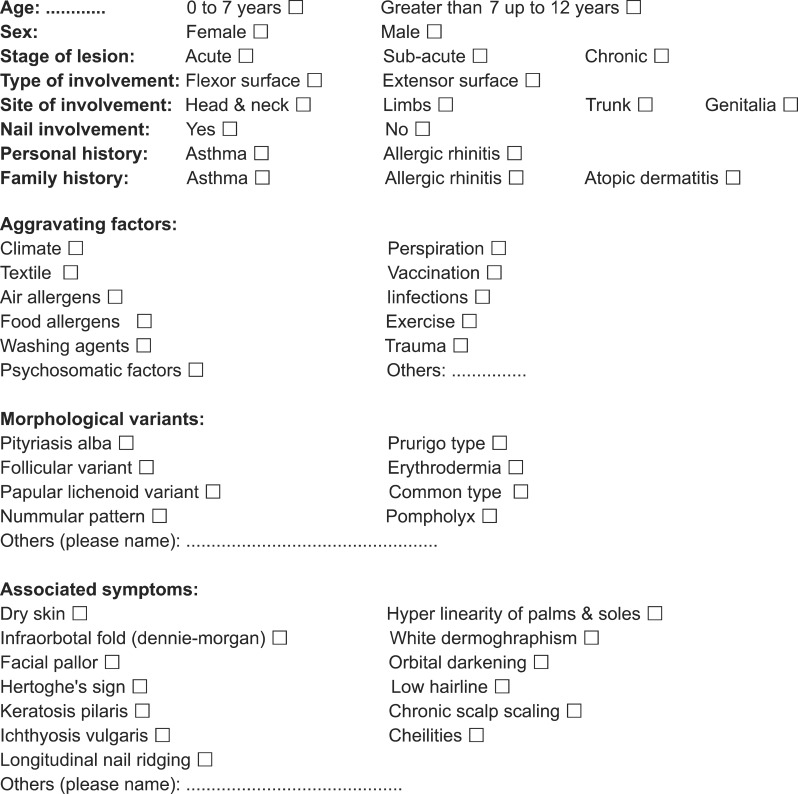
For those with a confirmed diagnosis of AD, a questionnaire (Fig. 1) was completed by two trained medical interns after a physical examination of the patients and an interview with their parents. The questionnaire consisted of questions regarding subject age, gender, history, distribution of lesions, nail involvement, family history of asthma, allergic rhinitis and AD, aggravating factors, morphological variants and accompanying symptoms. AD was classified as acute, sub-acute and chronic stages. Acute eczema was identified by intensely pruritic, erythematous and edematous papules and plaques, often with secondary excoriations. Vesicles, oozing and serous crusting were seen within affected areas. Sub-acute eczema appeared as erythematous and edematous papules and plaques with scaling and excoriation. Chronic AD is characterized by thickened, hyperkeratotic plaques with lichenification as well as prurigo nodularis12. The following morphological variants have been defined: follicular variant, papular lichenoid variant, prurigo type, nummular or discoid pattern, dyshidrotic eczema (pompholyx), common or classic eczematous reaction type (infiltrated scaling plaques with microvesiculation erosions and excoriations), pityriasis alba, and erythroderma. The patients were also evaluated for the stigma of atopy, including dry skin, hyperlinearity of palms and soles, infraorbital fold (Dennie-Morgan), white dermographism, facial pallor, orbital darkening, Hertoghe's sign, low hair line, keratosis pilaris, chronic scalp scaling and cheilitis. The association between AD, ichthyosis vulgaris, and longitudinal nail ridging has been studied8.
Fig. 1.
Data collecting sheet for demographic and clinical features of atopic dermatitis in school and preschool age children in Kerman city.
Statistical analysis
We used SPSS statistical software ver. 13 (SPSS Inc., Chicago, IL, USA) for statistical analyses. The chi-square test was used for data analysis. p-values<0.05 were considered significant.
RESULTS
Demographic findings and prevalence
In this study, 5,798 preschool and 37,964 primary school students were included. A total of 133 preschool children and 732 primary school students (totally 865 children) were selected. The prevalence of AD was 9.1% (79 patients) in our study population. The prevalence of AD according to sex and age is shown in Table 1. In the age range of 2 to 7 years there were 10 males and eight females, and in the age range of greater than 7 up to 12 years there were 31 males and 30 females with AD. The prevalence of AD in the age group of 2 to 7 years was 12.83% in females and 14.54% in males, and in the age group of greater than 7 up to 12 years it was 8.33% in females and 9.09% in males (p>0.05).
Table 1.
Demographic features of atopic dermatitis patients by age and gender
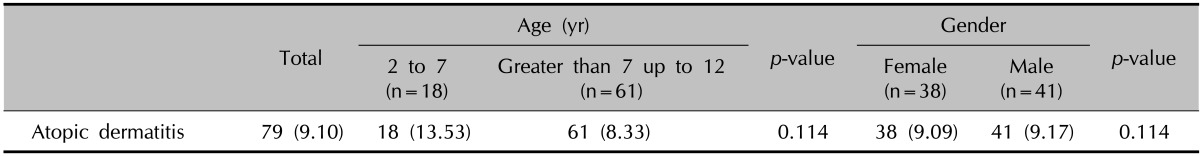
Values are presented as number (%).
Clinical features
Overall, 82.27% of patients were in the chronic, 10.12% were in the sub-acute, and 7.59% were in the acute stages. The frequencies of the sites involved are shown in Table 2. The extensor areas were involved more frequently in the age group of greater than 7 up to 12 years than those who were 2 to 7 years (p=0.021). Involvement of both flexure and extensor aspects of the limbs was seen more frequently in the age group of 2 to 7 years than in those greater than 7 up to 12 years (p=0.01).
Table 2.
Frequency of the site of involvement in atopic dermatitis patients
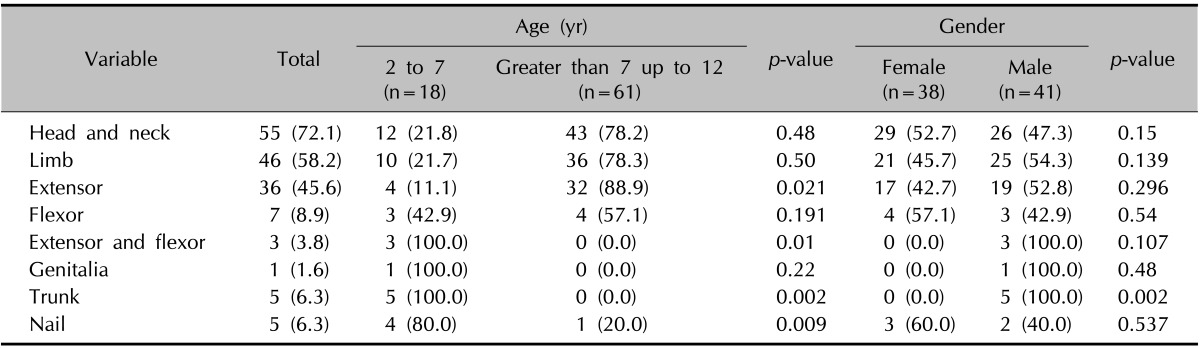
Values are presented as number (%).
The trunk was involved more frequently in the age group of 2 to 7 years than in those greater than 7 up to 12 years (p=0.002) and in males more than females (p=0.002). Nail involvement was seen more frequently in the age range of 2 to 7 years than in those greater than 7 up to 12 years (p=0.009).
Descriptive data regarding personal (asthma and allergic rhinitis) and family history of atopy (AD, asthma, and allergic rhinitis) are summarized in Table 3. Twenty-five patients (31.6%) had a personal history of at least one atopic disease. Three (3.79%) had a history of both asthma and allergic rhinitis. Thirty-seven (46.83%) patients had at least one family member with atopy. A family history of asthma was observed more frequently in the age group of 2 to 7 years than in those greater than 7 up to 12 years (p=0.001). The family history of allergic rhinitis was more frequently in the males than the females (p=0.002).
Table 3.
Frequency of atopic diseases categorized by age and gender
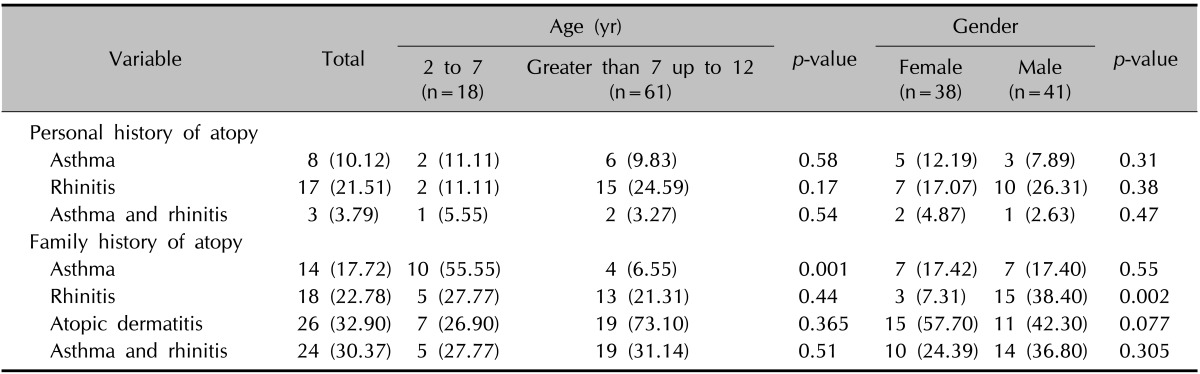
Values are presented as number (%).
Table 4 shows the frequency of aggravating factors in our study. Climate was the most frequent aggravating factor. Trauma, exercise, detergents, infections, and psychosomatic factor were the least frequent aggravating factors. Clothing and air allergens have more aggravating effects on AD in males than those in females (p=0.05 and p=0.01). Vaccination and infections had more aggravating effects on AD in the age group of 2 to 7 years than in those greater than 7 up to 12 years (p=0.01, p=0.05, respectively). The prevalence of morphological variants and associated symptoms are displayed in Table 5. Pityriasis alba, white dermographism, Hertoghe's sign and keratosis pilaris were observed more frequently in males than those in females (p=0.02, p=0.02, p=0.02, p=0.03, respectively). Dry skin was seen more frequently in females than that in males (p=0.02). Facial pallor was more frequently seen in the age group of 2 to 7 years than in those greater than 7 up to 12 years (p=0.02).
Table 4.
Distribution of aggravating factors categorized by age and gender
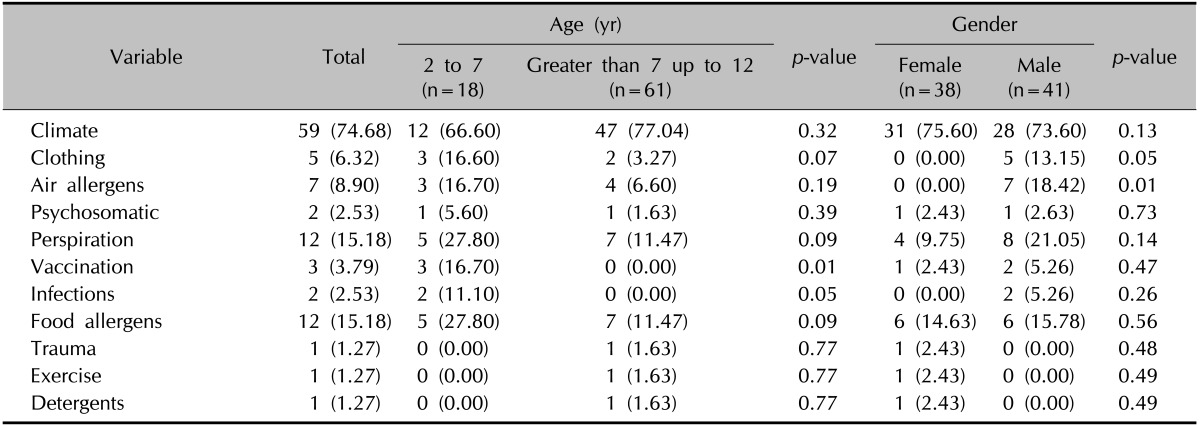
Values are presented as number (%).
Table 5.
Distribution of morphological variants and frequency of associated symptoms categorized by age and gender
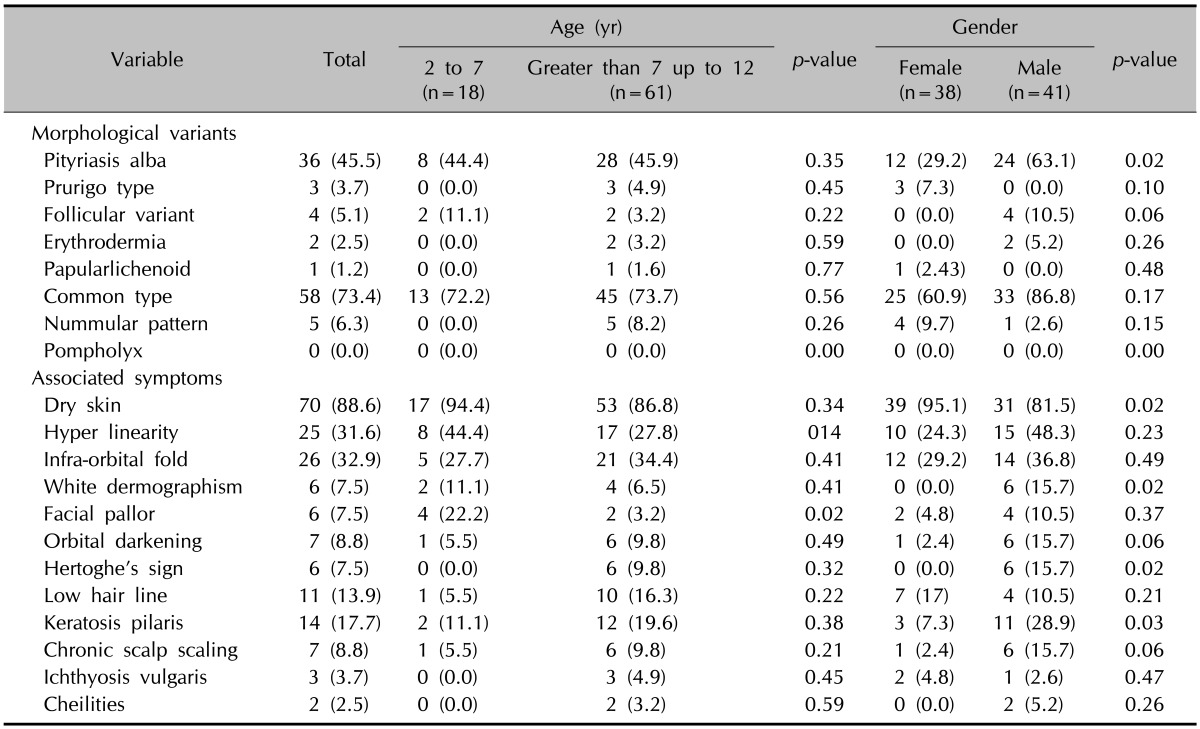
Values are presented as number (%).
DISCUSSION
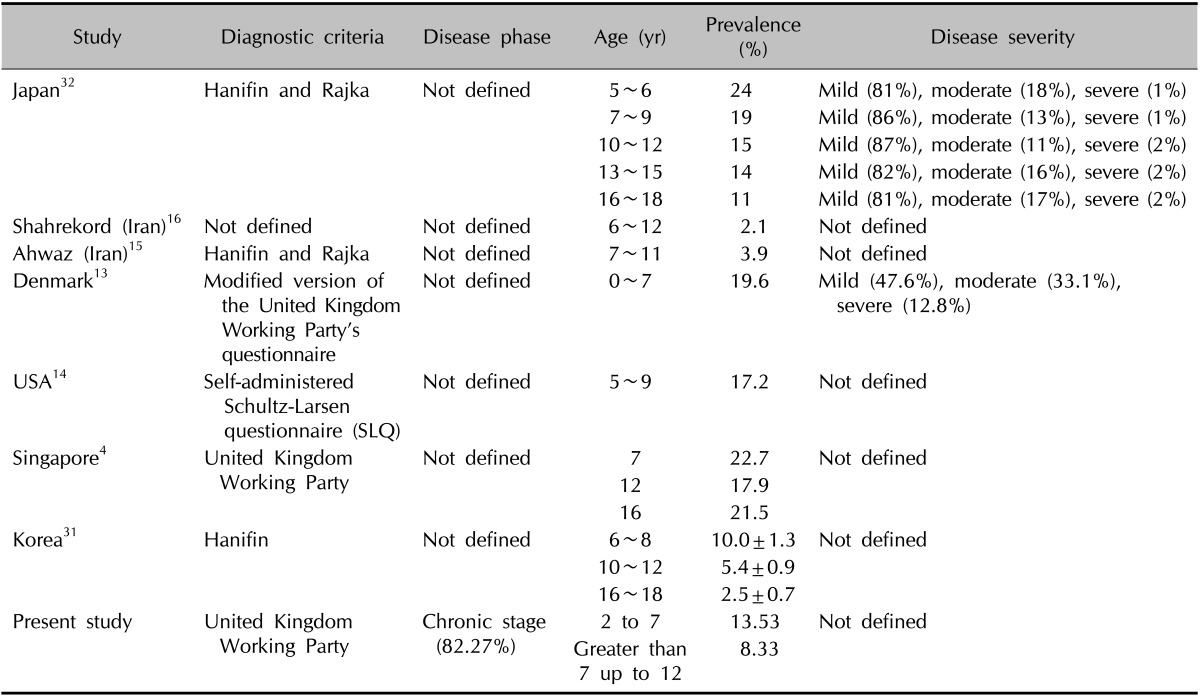
The aim of this study was to determine the prevalence and clinical features of AD in preschool and primary school children in Kerman, a desert area of Iran. We estimated the prevalence of AD to be 9.1% in children 2 to 12 year old. Available studies from Singapore, Denmark, USA, Ahwaz (a southern city in Iran) and Shahr-e-Kord (a southwest city in Iran) reported prevalence rates of AD of 20.8%, 13.9%, 17.2%, 3.9% and 2.1%, respectively4,13-16. The higher prevalence of AD in Denmark, USA and Singapore than Iran (Kerman, Shahrekord and Ahwaz) is likely explained by the higher socioeconomic conditions and smaller family size in western countries and countries with a western lifestyle1,17. These factors, in turn, resulted in a lower exposure to certain infectious agents that the developing immune system is deprived of obligatory stimulation by certain microbial antigens18. Environmental parameters such as climate and industrial factors in each region, diet regimen, genetic, and ethnic background may also play a role in the higher prevalence of AD7,8,19. This difference may also be due to the criteria utilized to diagnose AD, age range, severity, and disease phase (active or remission phase) in each study (Table 6).
Table 6.
Comparison between the present study and other studies
The following reasons explain the higher prevalence of AD in our study population than Ahwaz: (1) Kerman has a special geographic condition being located near Kavir Loot, one of the largest desert areas in Iran; it has dry, sunny and hot weather in contrast to the hot and humid weather of Ahwaz; (2) Low humidity has a demonstrated effect on exacerbating and provoking AD20. In contrast, indoor relative humidity is negatively associated with eczema symptoms21. (3) Furthermore, the ethnic origin of the population of Kerman is mainly Persians, whereas it is mainly Semitic for Ahwaz22,23. The prevalence of AD in different countries of Semitic origin varies from 5.8% in Qatar to 7.4% in Oman and 8.25% in Saudi Arabia24,25.
The prevalence of AD in Shahr-e-Kord, Tehran and Kerman (present study), people of Persian origin, was 2.1%, 17.6% and 9.1%, respectively16,26. Several studies have shown ethnicity as an etiological factor for the prevalence of AD19.
The following factors are probably involved in the higher prevalence of AD in Kerman than Shar-e-Kord (9.1% vs. 2.1%) a west city in Iran with a cold and dry weather. (1) The total hardness of the water in Kerman is higher than that in Shahr-e-Kord27,28. Children living in areas with harder tap water are more likely to develop AD29. (2) In contrast to Kerman, the majority of the people who live in Shahr-e-Kord have a rural life. These data suggest that environmental factors associated with urbanization and western lifestyle are important in the pathogenesis of AD30.
The decrease in the prevalence of AD with age was similar to reports from Korea and Japan (Table 6)31,32. This trend was not seen in a Singapore study4.
The same prevalence of AD in two genders in our study was similar to studies from Singapore, Korea, India, and Shahr-e-Kord (a city in Iran)4,31-33. However, our findings are in contrast to previous research in Nigeria that investigated the age range of 0 to 57 years and found a higher prevalence among females3. Although the prevalence of AD in the age range of greater than 7 up to 12 years was slightly more in males than females (9.09% vs. 8.33%) in our study, it was not statistically significant.
AD is divided into two main subtypes of intrinsic and extrinsic AD, and there is a female predominance in intrinsic AD34. However, whether this has any effect is unclear, as the subtypes of AD have not been determined. In our study the head and neck were the most common regions of involved skin followed by limbs. In the Nigerian study, limbs were more commonly involved than the head and neck regions. In our study, the extensor surfaces were the dominant involved sites of the limbs as well as in the studies from Nigeria and Thailand3,9. Similar to the findings from a Brazilian study, the involvement of extensor surfaces was associated with increasing age in our study. These findings were in contrast to the findings of the Nigerian study which can be attributed to the wide age range (0 to 57 years of age) and general progression of AD3,35. The distribution of AD lesions varies greatly depending on ethnic group, aggravating factors, and superinfections8. Clothing pattern and hobbies may also play a role in this regard. Trunk and genitalia involvement were more commonly observed in 2 to 7 year olds than in those greater than 7 up to 12 years, which can be ascribed to the generalized distribution of AD in younger age groups8. In our study, involvement of the head and neck regions was seen more frequently in females than that in males, which may be due to differences in clothing patterns. In Iran, females must cover their entire body surface except for the face and hands. Such a difference in the distribution of the lesions between females and males could be explained by this Islamic clothing style. Nail involvement accounted for 6.32% which was comparable with studies from India and Sweden33,36.
The majority of the patients (82.27%) in our study were in the chronic stage, supporting data from Thailand, Shahr-e-Kord, and Ahwaz9,15,16. In contrast, the sub-acute stage was the most common phase (43.3%) in the Nigerian study3. Disease phases have not been mentioned in the other studies. The common type and pityriasis alba were the most prevalent morphological variants of AD (more common in males), and had the same prevalence in different age groups. The least common morphological variants were pompholyx, papular lichenoid, and erythroderma. The most common morphological variants were papular lichenoid in Nigeria, pityriasis alba in Singapore, the common type in Thailand and India, and erythroderma in Sweden3,4,9,33,36. The difference in the prevalence of the morphological variants may be caused by genetic, geographic, and racial disparities8.
The most common accompanying sign in our study was dry skin (88.6%) which was consistent with all previous studies. The other two common accompanying signs, after dry skin, were infra-orbital folds and palmoplantar hyperlinearity. Two other accompanying signs were low hair line and orbital darkening, palmoplantar hyperlinearity and orbital darkening, infra orbital fold and orbital darkening in the studies of Nigeria, Thailand, and India, respectively3,9,33. Low hair line and orbital darkening were rare among our findings. Ethnicity and different genetic background may play major roles in these differences.
Validation of the Hanifin's and Rajka's criteria in certain regions would be under question due to the great variation in the frequencies of the clinical features that are defined as minor criteria. Further studies are required to validate the AD diagnostic criteria in our region.
In this survey, all patients were influenced by environmental factors particularly climate, sweating, and food allergies, which was similar to the studies from Singapore, Thailand, and Sweden4,9,36. Climate was the most common aggravating factor in the current study. Kerman is located in a desert area with a very low humidity. The cold and dry winter and hot and dry summer might explain the strong influence of the climate on our patients. Sunny weather in this city makes perspiration the second aggravating factor. However, the reflection of light is high in desert areas. Sun exposure can exacerbate AD due to a nonspecific intolerance to heat caused by impaired sweating in affected skin and induction of pruritus in the involved areas8.
Food allergy was another second aggravating factor. Iranian children are rapidly undergoing westernization of their lifestyles including dietary regimen. Increased consumption of foods more typical of a western diet displaces the intake of traditional foods that might be an explanation for the prominent effect of food on the exacerbation of the disease in the present study37.
Aeroallergens were the third aggravating factor. Kerman is located near one of the largest desert areas in Iran, Kavir Loot, which has nearly persistent dusty and windy conditions. These factors together with being the major resource of Iran's pistachio garden are probably responsible for the important role of aeroallergens on aggravation of AD in Kermanian children.
Similar to the results of the Thailand study, psychosomatic factors and infections were not prevalent in our population9.
Thirty-one percent of our patients had a personal history of other atopic diseases; allergic rhinitis was the most common, which was similar to the studies from Singapore and Thailand4,9. In our study, the prevalence of a family history of atopy history was 46.83% and was more common in males, which was similar to a Singapore study4. AD was the most common associated atopic disease in the family which was similar to the result of a Nigerian study3. It was in contrast to a study from Thailand that reported allergic rhinitis as the most common atopic disease in the relatives of their patients9. Similar to the findings from Shahr-e-Kord and Ahwaz15,16, a personal history of atopy increased with age, which might be attributed to the higher exposure to the environmental factors1. The main limitation of our study is that we could not use the International Society for Augmentative and Alternative Communication's questionnaire, as the validated Persian translation is not available yet. There could be recall bias in a cross-sectional study, which is another limitation of our study. Furthermore we did not assess AD severity in the patients.
The prevalence of AD in Kerman, a city with a desert climate, was higher than what was reported from two other cities of Iran. However, it was less than reported prevalence from developed countries. Variability in epidemiologic studies of AD indicates that a single diagnostic criterion may not be appropriate for diagnosing AD in different areas. Our findings may help to develop diagnostic criteria or modify the previous ones to be appropriate for such a desert areas.
ACKNOWLEDGMENT
We would like to express our sincere gratitude to Farzan Institute for Research & Technology for technical assistance.
References
- 1.Williams HC. Epidemiology of atopic dermatitis. Clin Exp Dermatol. 2000;25:522–529. doi: 10.1046/j.1365-2230.2000.00698.x. [DOI] [PubMed] [Google Scholar]
- 2.Friedman PS, Holden CA. Atopic dermatitis. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's textbook of dermatology. 7th ed. Malden: Blackwell Science; 2004. pp. 215–230. [Google Scholar]
- 3.Nnoruka EN. Current epidemiology of atopic dermatitis in south-eastern Nigeria. Int J Dermatol. 2004;43:739–744. doi: 10.1111/j.1365-4632.2004.02360.x. [DOI] [PubMed] [Google Scholar]
- 4.Tay YK, Kong KH, Khoo L, Goh CL, Giam YC. The prevalence and descriptive epidemiology of atopic dermatitis in Singapore school children. Br J Dermatol. 2002;146:101–106. doi: 10.1046/j.1365-2133.2002.04566.x. [DOI] [PubMed] [Google Scholar]
- 5.Farajzadeh S, Rahnama ZZ, Kamyabi Z, Ghavidel B. Bacterial colonization and antibiotic resistance in children with atopic dermatitis. Dermatol Online J. 2008;14:21. [PubMed] [Google Scholar]
- 6.Farajzadeh S, Behrooz V, Gadari R. The frequency of wart in children with atopic dermatitis and comparison with control group. J Med Sci Islamic Azad University Mashhad. 2008;3:182–189. [Google Scholar]
- 7.Farajzadeh S, Bazargan N, Shahesmaeili A, Shahrbabaki AG, Fekri AR. Evaluation of the frequency of food allergens by skin prick test in children with atopic dermatitis. Iranian J Dermatol. 2010;13:33–36. [Google Scholar]
- 8.Barbaral KIF, Ring J. Clinical features and diagnostic criteria of atopic dermatitis. In: Harper J, Oranje AP, Prose NS, editors. Textbook of pediatric dermatology. 2nd ed. Oxford: Blackwell Publishing; 2006. pp. 227–258. [Google Scholar]
- 9.Wisuthsarewong W, Viravan S. Diagnostic criteria for atopic dermatitis in Thai children. J Med Assoc Thai. 2004;87:1496–1500. [PubMed] [Google Scholar]
- 10.Farajzadeh S, Esfandiarpour I, Poorhamzeh B. Frequency of skin diseases in children in Kerman. Iranian J Dermatol. 2008;10:44–45. [Google Scholar]
- 11.Brenninkmeijer EE, Schram ME, Leeflang MM, Bos JD, Spuls PI. Diagnostic criteria for atopic dermatitis: a systematic review. Br J Dermatol. 2008;158:754–765. doi: 10.1111/j.1365-2133.2007.08412.x. [DOI] [PubMed] [Google Scholar]
- 12.Kang K, Polster AM, Nodorost ST, Steven SR, Cooper KD. Atopic dermatitis. In: Jorizzo JL, Bolognia JL, Schaffer JV, editors. Dermatology. 2nd ed. St. Louis: Mosby/Elsevier; 2008. pp. 181–195. [Google Scholar]
- 13.Olesen AB, Bang K, Juul S, Thestrup-Pedersen K. Stable incidence of atopic dermatitis among children in Denmark during the 1990s. Acta Derm Venereol. 2005;85:244–247. doi: 10.1080/00015550510026343. [DOI] [PubMed] [Google Scholar]
- 14.Laughter D, Istvan JA, Tofte SJ, Hanifin JM. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43:649–655. doi: 10.1067/mjd.2000.107773. [DOI] [PubMed] [Google Scholar]
- 15.Afshari F, Khadivi R, Shirzad H. Factors influencing atopic dermatitis in school children of Shahrekord. J Shahrekord Univ Med Sci. 2007;8:71–78. [Google Scholar]
- 16.Moosavi Z, Samadzadeh D. Prevalence of atopic dermatitis in 7-11 year old school children, Ahwaz. J Shahid Sadoughi University Med Sci. 2006;14:38–44. [Google Scholar]
- 17.Benn CS, Melbye M, Wohlfahrt J, Björkstén B, Aaby P. Cohort study of sibling effect, infectious diseases, and risk of atopic dermatitis during first 18 months of life. BMJ. 2004;328:1223. doi: 10.1136/bmj.38069.512245.FE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 19.Mar A, Tam M, Jolley D, Marks R. The cumulative incidence of atopic dermatitis in the first 12 months among Chinese, Vietnamese, and Caucasian infants born in Melbourne, Australia. J Am Acad Dermatol. 1999;40:597–602. doi: 10.1016/s0190-9622(99)70443-3. [DOI] [PubMed] [Google Scholar]
- 20.Reidel F. Environmental pollution and atopy. In: Ring J, Przybilla B, Ruzicka T, editors. Handbook of atopic eczema. London: Springer-Verlag; 1991. [Google Scholar]
- 21.Weiland SK, Hüsing A, Strachan DP, Rzehak P, Pearce N ISAAC Phase One Study Group. Climate and the prevalence of symptoms of asthma, allergic rhinitis, and atopic eczema in children. Occup Environ Med. 2004;61:609–615. doi: 10.1136/oem.2002.006809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Semitic [Internet] The Titi Tudorancea Learning Center; [accesed 2010 Oct 3]. Available from: http://www.tititudorancea.org/z/semitic.htm. [Google Scholar]
- 23.Iranian People & Tribes Monday [Internet] [accesed 2012 Jan 9]. Available from: http://www.iranchamber.com/people/articles/iranian_ethnic_groups.php.
- 24.Centre for Arab genomic studies. Atopic dermatitis [Internet] Dubai, United Arab Emirates: Centre for Arab Genomic Studies; [accessed 2011 Apr 4]. Available from: http://www.cags.org.ae. [Google Scholar]
- 25.Parthasaradhi A, Al Gufai AF. The pattern of skin diseases in Hail Region, Saudi Arabia. Ann Saudi Med. 1998;18:558–561. doi: 10.5144/0256-4947.1998.558. [DOI] [PubMed] [Google Scholar]
- 26.Ansarin H, Firooz A, Azimi M, Ebn-Ahmadi E, Dowlati Y. Prevalence of atopic dermatitis in children in Iran. Gulf Jl Dermatol. 1998;5:26–27. [Google Scholar]
- 27.Malakootian M, Yousefi N. The efficiency of electrocoagulation process using aluminum electrodes in removal of hardness from water. Iranian J Environ Health Sci Eng. 2009;6:131–136. [Google Scholar]
- 28.Lalehzari R, Tabatabaei SH. Groundwater quality mapping in Shahrekord Aquifer. J Environ Stud. 2010;36:55–62. [Google Scholar]
- 29.McNally NJ, Williams HC, Phillips DR, Smallman-Raynor M, Lewis S, Venn A, et al. Atopic eczema and domestic water hardness. Lancet. 1998;352:527–531. doi: 10.1016/s0140-6736(98)01402-0. [DOI] [PubMed] [Google Scholar]
- 30.McNally N, Phillips D. Geographical epidemiology of atopic dermatitis. In: Williams HC, editor. Atopic dermatitis: the epidemiology, causes, and prevention of atopic eczema. Cambridge: Cambridge University Press; 2000. pp. 113–124. [Google Scholar]
- 31.Kim CW, Park CJ, Kim JW, Koo DW, Kim KW, Kim TY. Prevalence of atopic dermatitis in Korea. Acta Derm Venereol. 2000;80:353–356. doi: 10.1080/000155500459295. [DOI] [PubMed] [Google Scholar]
- 32.Sugiura H, Umemoto N, Deguchi H, Murata Y, Tanaka K, Sawai T, et al. Prevalence of childhood and adolescent atopic dermatitis in a Japanese population: comparison with the disease frequency examined 20 years ago. Acta Derm Venereol. 1998;78:293–294. doi: 10.1080/000155598441891. [DOI] [PubMed] [Google Scholar]
- 33.De D, Kanwar AJ, Handa S. Comparative efficacy of Hanifin and Rajka's criteria and the UK working party's diagnostic criteria in diagnosis of atopic dermatitis in a hospital setting in North India. J Eur Acad Dermatol Venereol. 2006;20:853–859. doi: 10.1111/j.1468-3083.2006.01664.x. [DOI] [PubMed] [Google Scholar]
- 34.Turner JD, Schwartz RA. Atopic dermatitis. A clinical challenge. Acta Dermatovenerol Alp Panonica Adriat. 2006;15:59–68. [PubMed] [Google Scholar]
- 35.Solé D, Camelo-Nunes IC, Wandalsen GF, Mallozi MC, Naspitz CK Brazilian ISAAC Group. Prevalence of atopic eczema and related symptoms in Brazilian schoolchildren: results from the International Study of Asthma and Allergies in Childhood (ISAAC) phase 3. J Investig Allergol Clin Immunol. 2006;16:367–376. [PubMed] [Google Scholar]
- 36.Böhme M, Svensson A, Kull I, Wahlgren CF. Hanifin's and Rajka's minor criteria for atopic dermatitis: which do 2-year-olds exhibit? J Am Acad Dermatol. 2000;43:785–792. doi: 10.1067/mjd.2000.110070. [DOI] [PubMed] [Google Scholar]
- 37.Kelishadi R, Pour MH, Zadegan NS, Kahbazi M, Sadry G, Amani A, et al. Dietary fat intake and lipid profiles of Iranian adolescents: Isfahan Healthy Heart Program--Heart Health Promotion from Childhood. Prev Med. 2004;39:760–766. doi: 10.1016/j.ypmed.2004.02.047. [DOI] [PubMed] [Google Scholar]