Abstract
Background
New antitumor therapeutic strategies aim to combine different approaches that are able to induce tumor-specific effector and memory T cell responses that might control tumor growth. Dendritic cells (DCs) have the capacity to induce antigen-specific cytotoxic T lymphocytes. We have previously shown that the combined treatment of paclitaxel chemotherapy (Chemo) and injection of DCs led to complete tumor regression.
Objective
The goal of this study was to evaluate synergistic antitumor effect of a triple combination treatment comprising radiotherapy, paclitaxel Chemo and intratumoral injection of syngeneic bone marrow-derived DCs on murine fibrosarcoma, compared to other single or double combination treatments.
Methods
For the murine fibrosarcoma model, naïve C57BL/6 mice were inoculated intradermally with 2×103 MCA102 cells in the right upper flank. Mice were assigned to five groups (untreatedcontrol, RT alone, RT+Chemo, RT+DC, and RT+Chemo+DC), with eight mice in each group. In vitro cytotoxicity assays were performed to assess the immune activity. The persistence of tumor-specific immunity was determined by second tumor challenge in mice with complete tumor regression.
Results
The triple combination treatment showed a significantly enhanced therapeutic efficacy by decreasing tumor size and inducing complete tumor regression, resulting in a cure of 50% of mice. The results of in vitro cytotoxicity assays and the second tumor challenge experiment strongly indicated the induction of a tumor-specific cytotoxic T lymphocyte response and acquisition of prolonged tumor immunity.
Conclusion
These findings suggest that the triple combination treatment can be a promising strategy for the treatment of murine fibrosarcoma.
Keywords: Combined modality therapy, Dendritic cells, Fibrosarcoma, Paclitaxel, Radiotherapy
INTRODUCTION
Dendritic cells (DCs) are the most potent antigen-presenting cells that are able to induce tumor-specific immune responses leading to tumor rejection and thereby represent an attractive adjuvant for cancer immunotherapy. After DCs capture tumor cells, they present tumor antigen to T cells and generate tumor-specific cytotoxic T lymphocytes (CTLs) from naïve T cells1-3. Although tumors have immunogenic epitopes on their surface that can be recognized by the host immune system, they can achieve immune tolerance as a result of low immunogenicity of the tumor and/or lack of antigen presentation in the host immune system4. Several different strategies with the use of DCs for immunotherapy against tumors have been studied. In this study, we injected DCs into a tumor mass and these DCs engulfed the apoptotic bodies generated by the chemotherapy or radiotherapy (RT) directly in vivo.
Intratumorally injected DCs alone rarely induce curative antitumor effects as the manipulation of the tumor microenvironment is necessary to potentiate the effect of DC injection5. We have previously shown that a combination therapy of paclitaxel chemotherapy along with the intratumoral injection of DCs is a potent treatment strategy for fibrosarcoma6. Chemotherapy results in increased antigen cross-presentation, T lymphocyte expansion and T-cell infiltration of tumors7. Furthermore, suppressive regulatory T cells are depleted after the use of several chemotherapeutics, resulting in enhanced T cell reactivity8,9. Obeid et al.10 have shown that the chemotherapeutic agents induce immunogenic cell death and the exposure of tumor cells to calreticulin provides a signal that is recognized by DCs and ultimately results in phagocytosis of the tumor cells.
RT is a powerful intervention that can manipulate the tumor microenvironment. RT can kill tumor cells that release tumor antigens and also exert various immunomodulatory effects including induction of the expression of cytokines, chemokines, and release of inflammatory mediators11-13. These inflammatory mediators also increase the permeability of the local vasculature that leads to the recruitment of circulating leukocytes, including antigen-presenting cells and effector T cells into surrounding tissues14-16. Therefore, the proinflammatory microenvironment within irradiated tumors could provide DCs with maturation inducing stimuli critical for eliciting effective antigen presentation.
Fibrosarcoma is a malignant mesenchymal tumor derived from fibrous connective tissue and accounts for less than 10% of soft tissue tumors. Treatment for high grade fibrosarcoma involves a wide excision, usually combined with RT or chemotherapy. However, despite these conventional treatments, approximately 50% of patients with large, high-grade sarcoma develop distant metastasis17. Previous studies have suggested that immune-based treatments may be effective in sarcoma. For example, the MAGE-1, -3 and GAGE-1, -2 antigens are nonmutated proteins expressed in normal testis tissue, as well as in melanoma, non-small cell lung cancer, and sarcomas. Also, previous studies demonstrated that the effectiveness of DCs in treating fibrosarcoma is correlated with the sensitivity of tumor cells to action of lymphokin-activated killer cells and the level of MHC class I antigens18,19. Several previous studies investigated an antitumor strategy using DCs for treating fibrosarcoma. Kucera et al.19 evaluated the efficacy of preventive and therapeutic DC vaccination in advanced rat fibrosarcoma. Shin et al.20 tested the synergistic effect of an intratumoral (i.t.) injection of combined vincristine and DCs for the treatment of fibrosarcoma. Finkelstein et al.21 investigated the effect of combination of i.t. injection of DCs and fractionated external beam radiation on tumor-specific responses in patients with soft-tissue sarcoma.
In the current study, we investigated whether a triple combination treatment of i.t. injection of immature DCs, paclitaxel chemotherapy and RT would provide better outcomes than other single or double combination treatments.
MATERIALS AND METHODS
Animals
Female C57BL/6 mice, three to five weeks of age were purchased from Dae Han Bio Link (Eumseong, Korea). The mice were allowed to adjust to their environment for one week. DC isolation was performed at six to eight weeks of age, and tumor inoculation was performed at eight to ten weeks of age. Age-matched female C57BL/6 mice were used as a source of allogeneic DCs and for tumor inoculation. Animals were housed at the Animal Facility of the Biomedical Research Building of Inha University College of Medicine. All of the experiments were conducted according to the Inha University Laboratory Animal Research Guidelines.
Dendritic cell culture
Primary DCs were obtained from mouse bone marrow precursors. The bone marrow cells were harvested from femurs and tibias of C57BL/6 mice and were plated in complete RPMI1640 (Gibco-BRL, Rockville, MD, USA) containing recombinant murine GM-CSF (10 ng/ml; Sigma Chemical Co., St. Louis, MO, USA) and recombinant murine interleukin-4 (IL-4, 10 ng/ml; Genzyme, Farmington, MA, USA). On day 2, non-adherent granulocytes were removed, and fresh medium containing GM-CSF and IL-4 was added. On day 4, loosely adherent cells were dislodged and replaced, and immature DCs were cultured for 5 days with cytokine supplementation on alternate days. On day 6 of the culture, immature DCs and non-adherent proliferating aggregates were collected. The maturation status and the percentage of DCs were analyzed by FACScan flow cytometry (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA) with five surface markers (CD11c, CD44, CD40, CD80, and class II antigen I-Ad).
Tumor cell culture
MCA102, murine fibrosarcoma cell line of C57BL/6 mouse origin, was cultured in a complete medium containing RPMI1640 (Gibco-BRL) medium plus 10% heatinactivated fetal bovine serum (FBS; Gibco-BRL) and 100 U/ml penicillin (Gibco-BRL) in a 5% CO2 incubator at 37℃ to a subconfluent state in 75 cm2 plates. After reaching subconfluency, the cells were rinsed twice with the medium. For detachment, the cells grown to confluency were treated with 0.05% trypsin (Gibco-BRL) and EDTA. The tumor cells were washed three times and resuspended in phosphate-buffered saline (PBS) before inoculation into the mice.
In vivo tumor treatment design
Mice were assigned to five groups (untreated control, RT alone, RT+chemotherapy [Chemo], RT+DCs, and RT+ Chemo+DCs), and each group had eight mice. In this study, we omitted the other possible single or double combination treatments, such as Chemo alone, DC alone, and Chemo+DCs, because the antitumor effects of these treatments were already evaluated in our previously published study6. MCA102 cells were diluted to the appropriate concentration, and 2×103 cells were inoculated subcutaneously in the upper right flank of a C57BL/6 mouse.
On day 10, when the average tumor size reached about 4~5 mm in diameter, each group received a different combination of treatments (Fig. 1). The following treatment methods were applied in each group; 0.5 µg/ml paclitaxel was administered through an intraperitoneal injection in mice at a dose of 5 mg/kg, RT (8.5-Gy) was performed 6 hours later and both treatments were administered again on 2 days; day 12 and day 14. The total irradiation dose was 25.5-Gy. DCs were injected intratumorally four times on days 11, 13, 15 and 18 (2×106 cells/mouse for each injection in 50 µl of PBS). This injection protocol was established by Choi et al.6 with a modification described by Shin et al.20. Tumor volumes were measured using a caliper and expressed as the product of the two maximal perpendicular diameters (mm2).
Fig. 1.
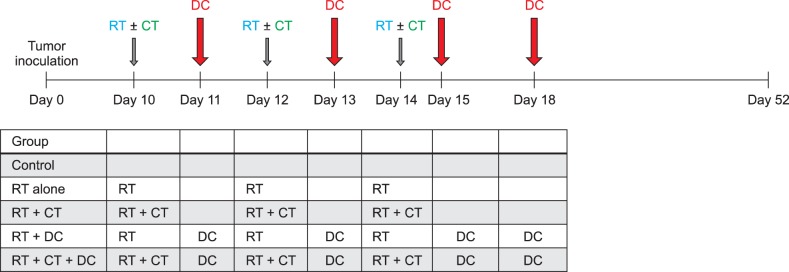
Study design. Mice were assigned to five groups (control, RT alone, RT+CT, RT+DC and RT+CT+DC) in the study. MCA102 cells were inoculated subcutaneously in the upper right flank of a C57BL/6 mouse. On day 10, when the average tumor size reached about 4~5 mm in diameter, paclitaxel was intraperitoneally administered and RT was performed 6 hours later. The same treatment was administered on two more days; day 12 and day 14. DCs were injected intratumorally four times on days 11, 13, 15 and 18. RT: radiotherapy, CT: chemotherapy, DC: dendritic cell.
Second tumor challenge experiment in the mice with complete tumor regression
A second tumor challenge experiment was performed to determine the persistence of tumor-specific immunity in mice with complete tumor regression and treated with combination therapies. Four age-matched control mice, two mice treated with RT+DCs and four mice treated with RT+Chemo+DCs were used in the second tumor challenge experiment. MCA102 cells (2×103 cells each in 50 µl PBS) were inoculated subcutaneously in the upper left flank, the side opposite to the site of the first injection.
PKH 26 labeling of target cells
MCA102 cells (2×106 cells/ml in a 2 ml total volume) were used as the target cells. These cells were transferred to polystyrene tubes and washed twice with serum-free medium before staining. Cells were then incubated for 40 minutes with freshly prepared 2 µmol/l PKH-26 (Sigma Chemical Co.) at room temperature and the staining reaction was stopped by the addition of 1 ml FBS. These cells were subsequently incubated at 37℃ for 24 hours. The splenocytes from mice with complete tumor regression after the first treatment were used as the effector cells. Labeled target cells were seeded into each well plates and incubated with the effector cells (4.8×106 cells/ml) at different effector : target ratios; 1.5 : 1, 6 : 1, and 12 : 1.
Flow cytometric analysis
After the effector cells and target cells were incubated at 37℃ in 5% CO2 for 3 hours, 10 ml of 50 mg/ml of 7-AAD (Sigma Chemical Co.) solution diluted in annexin binding buffer and 5 ml of annexin V (PharMingen, San Diego, CA, USA) was added to the cell suspension for 10 minutes on ice. Samples were analyzed by a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems) with an air-cooled argon-ion, 488 nm coherent beam laser. The instrument contained three optical filters, FL-1 (Annexin-FITC) at 530 nm, FL-2 (PKH 26) at 585 nm, and FL-3 (7-AAD) at 650 nm. Spectral overlap was electronically compensated using single color controls including target cells alone and cells treated or not treated with PKH 26, Annexin V or 7-AAD in separate tubes22. The percentage of dead cells (7-AAD positive, red or orange fluorescence) among PKH 26-stained target cells (green fluorescence) was calculated.
Statistical analysis
The measured value of the tumor size was expressed as the mean±standard error for each condition. ANOVA with nonparametric analysis of repeated measures was performed for comparisons between multiple groups23. A statistical analysis was performed using SPSS software ver. 12.0 (SPSS Inc., Chicago, IL, USA), with statistical significance set at p<0.05.
RESULTS
Isolation of bone marrow-derived dendritic cells and their phenotyping
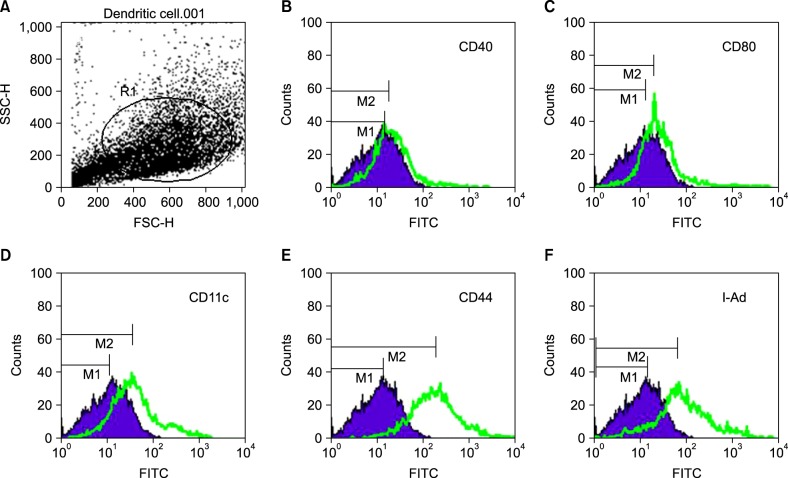
Primary DCs were obtained from syngeneic mouse bone marrow precursors in accordance with the previously described method6. The phenotypes of the DCs were verified by flow cytometry analysis (Fig. 2). Almost no expression of maturity surface markers CD40 and CD80 was detected, indicating that the DCs were in an immature state. Flow cytometry data with three other surface markers, CD11c, CD44 and I-Ad, showed the purity of the DCs to be >65%7
Fig. 2.
Phenotyping of cultured dendritic cells (DCs). The phenotypes of the primary DCs were subsequently verified by flow cytometry. (A) The circle indicates the DCs gated in the flow cytometric analysis. The results showed that the isolated DCs were positive for CD11c (D), CD44 (E), and I-Ad (F). The low expression of the maturation surface markers CD40 (B) and CD80 (C) indicates that the DCs were in an immature state. The purity of DCs was >65%.
Significant regression of tumor size in the treated groups in contrast to the untreated control group
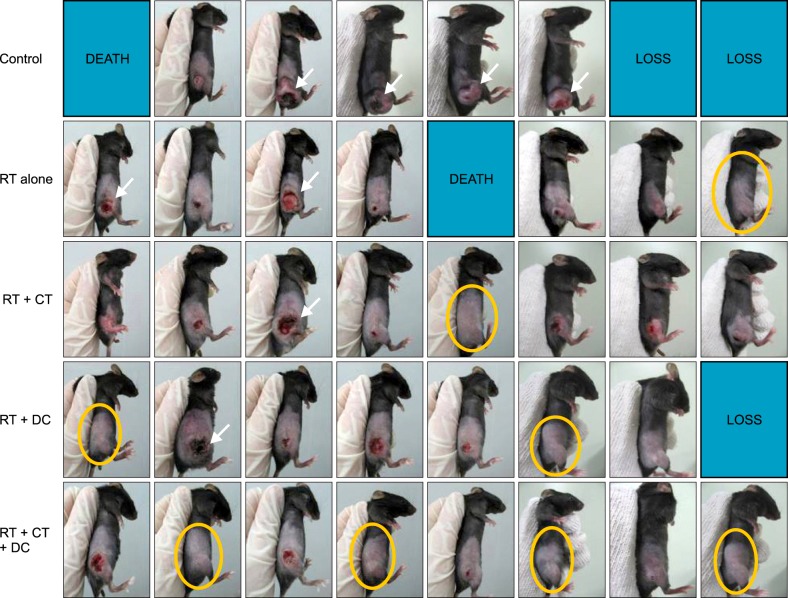
Representative photographs of the actual tumor sizes in each group on day 52 are shown in Fig. 3. Among the treated groups, the triple combination treatment group showed the highest rate of complete tumor suppression; 0% in the control group, 14.3% in the RT alone group, 12.5% in the Chemo and RT group, 28.5% in the RT and DC injection group, and 50% in the triple combination treatment group (Fig. 3). As shown in Fig. 4, the tumors grew rapidly in the untreated control mice. In contrast, the tumor growth was suppressed significantly in the treated groups (p<0.001). We observed that the triple combination therapy was more efficient than the other combination therapies in decreasing the average tumor volume. However, the statistical analysis showed no significant difference between the four treatment groups (p=0.2627). There were no side effects or any accompanying phenomenon in each group.
Fig. 3.
Photographs of animals at the end of treatment (day 52). In the control group, tumor bulk in most of the mice grew at a rapid rate with severe central necrosis (white arrows). Significant suppression of tumor growth was observed in the treated mice compared to the control group. Some mice showed complete tumor regression (yellow circles). Among the treated groups, the triple combination treatment group showed the greatest tumor suppressive effect. DEATH means tumor directly causes immediate death and LOSS means tumor does not cause death. RT: radiotherapy, CT: chemotherapy, DC: dendritic cell.
Fig. 4.
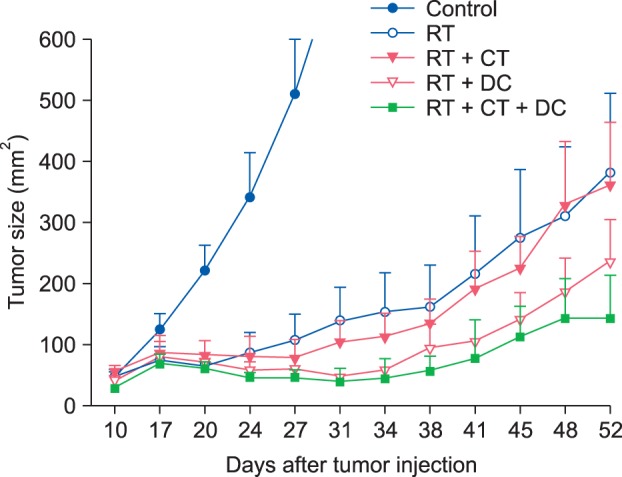
Significant suppression of tumor growth in the treated groups. Tumor growth was suppressed significantly in the treated groups in contrast to the control group. However, there was no statistically significant difference in the degree of tumor growth suppression between the treated groups. The data are expressed as the mean±standerd error. RT: radiotherapy, CT: chemotherapy, DC: dendritic cell.
Persistent antitumor memory after combined treatments of radiotherapy and dendritic cell injection with/without chemotherapy
To further evaluate the memory of antitumor immunity in vivo after the combined treatments, a second challenge with the same MCA102 tumor cells was performed in the eight mice that exhibited complete tumor regression; two age-matched control mice, two mice from the RT+DC group and four mice from the RT+Chemo+DC group. We conducted the second challenge of fibrosarcoma cell injection in the RT+DC group and the RT+Chemo+DC group because these groups showed a higher antitumor effect (Fig. 4). Also, we have already shown that the tumor-free mice treated with the combined therapy of paclitaxel chemotherapy and DC injection were able to resist a repeat challenge with the same type of tumor6. As shown in Fig. 5, a very small tumor appeared around day 10 after the injection of tumor cells, but it disappeared quickly after day 14. However, the tumors grew rapidly in the control group. These results suggested that tumor-free mice treated with RT+Chemo or RT+Chemo+DC injection were highly protected against the tumor rechallenge with the same type of tumor.
Fig. 5.
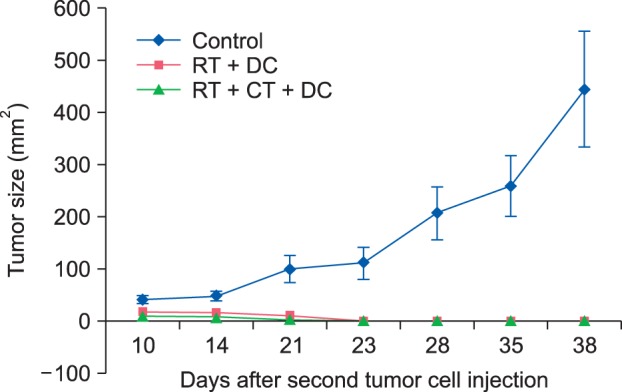
Persistent antitumor memory after combined treatment of dendritic cell (DC) and radiotherapy (RT) with/without chemotherapy (CT). A second challenge with the same MCA 102 tumor cells was performed in the mice that showed complete tumor regression in the previous experiment. A very small sized tumor appeared around day ten after the second injection, but it disappeared quickly thereafter. Age-matched normal mice, as the control group, were also injected with the same type of tumor cells and these mice showed rapid tumor growth. The data are expressed as the mean±standerd error.
Evaluation of apoptosis of MCA102 cells using an in vitro cytotoxicity assay
To examine the apoptosis of MCA102 cells after each treatment, a PKH-26 cytotoxicity assay was performed using one mouse from each group, which showed complete tumor regression. As shown in Fig. 6, the results of the flow cytometric analysis using 7-AAD and annexin-FITC showed that the CTL activity was highest in the triple combination treatment group. The splenocytes from untreated mice had a much lower CTL activity. The splenocytes from the mice treated with RT+DC also had a high level of CTL activity, but it was lesser than that in the triple combination treatment group. These results suggest that the combination of RT and DC injection can effectively induce more number of Th1 T cells and tumorspecific CTLs.
Fig. 6.
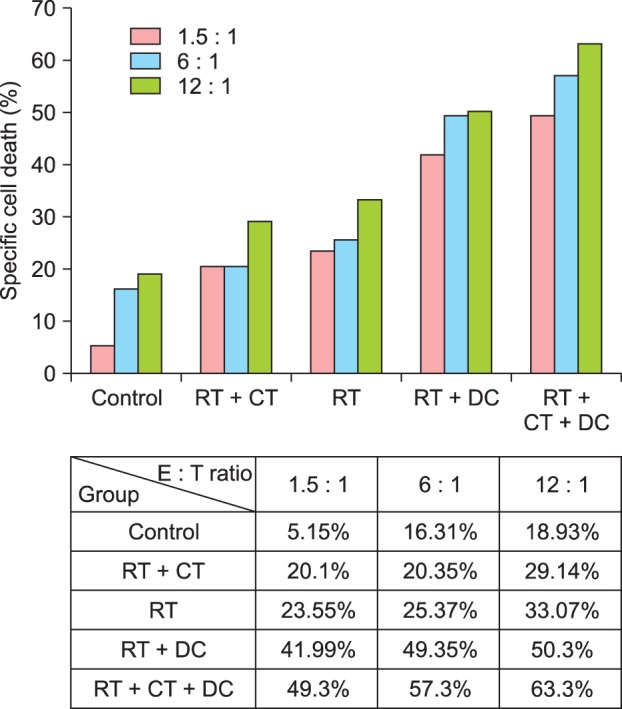
In vitro cytotoxicity assay with splenocytes from mice with complete tumor regression. The graph and table show the percentage of specific cell death at different E to T ratios in each experimental group. The PKH-26 flow cytometry evaluates MCA102 cells (T) after incubation with splenocytes after combination treatment (E). The PKH-26 assay results showed that the level of cytotoxicity was highest in the triple combination treatment group. T: target cells, E: effector cells, RT: radiotherapy, CT: chemotherapy, DC: dendritic cell.
DISCUSSION
Intratumoral injection of DCs is one of the promising methods for inducing therapeutic antitumor response. However, DCs alone rarely cause curative antitumor effects as tumor microenvironment is resistant to antitumor immune response23. Therefore, another treatment is required to potentiate the effect of DC administration23. We have previously shown that the combined treatment of i.t. injection of DCs and paclitaxel chemotherapy led to tumor regression6. In this study, we evaluated the effect of RT combined with i.t. injection of DCs as an optimal combined strategy. The data showed that the RT combined with i.t. injection of DCs caused a partial regression of the tumor. However, the combination therapy of RT and i.t. injection of DCs in the second tumor challenge experiments induced acquired long-term antitumor immunity. It is possible that RT augments the antitumor efficacy of i.t. injection of DCs by modifying tumor cells to become more immunogenic. Both chemotherapy and RT induce a pro-apoptotic environment and increase the immunogenicity resulting in increased therapeutic efficacy24. The apoptosis-inducing death receptor CD95 (Apo-1/Fas) is thought to play a key role in apoptosis and is up-regulated in response to chemotherapy and RT25. Recent studies have shown that another mechanism for the immunogenic effects of chemotherapy and radiation is the toll-like receptor-4-dependent antigen processing on DCs that is dependent upon calreticulin exposure and HMGB1 release from dying tumor cells10,26. Also, these treatments induce apoptosis and necrosis of tumor cells, thereby providing DC maturation signals27.
To the best of our knowledge, this is the first report to demonstrate that i.t. injection of DCs combined with RT and chemotherapy can show enhanced therapeutic efficacy in murine fibrosarcoma. In our study, we found that the triple combination therapy of RT, paclitaxel chemotherapy and i.t. injection of immature DCs causes a decrease in the tumor volume of fibrosarcoma among various combined strategies although there was no statistically significant difference between the experimental groups. However, the triple combination immunotherapy group demonstrated a higher rate of complete regression of tumor cells and induced anti-tumor memory immune responses.
The potential explanations regarding the efficacy of the triple combination treatment are as follows: First, paclitaxel chemotherapy and RT before DC injection would decrease the tumor burden and increase the possibility of exposure of tumor antigen to DCs. Second, the high number of DCs exposed to tumor antigens would increase the activation of T-lymphocytes in the draining lymph node and thereby induce a higher degree of tumor-specific cytotoxicity. Third, i.t. injection of immature DCs would increase the chances of tumor antigen uptake and processing in vivo, as described previously by Sauter and group27. As DCs are the most effective cells that can present an antigen to MHC class I- and class II-restricted T cells28, intratumorally injected DCs could migrate from the vicinity of the tumor to the draining lymph node and induce systemic immunity29. Intratumoral administration of DCs, therefore, can increase tumor-specific CTL and Th1-type CD4 cells and decrease tumor cells30. Furthermore, a predominant role of CD8+ T cells in tumor growth inhibition upon i.t. injection of DCs has been reported31.
This study has some limitations. Firstly, we excluded mice treated with paclitaxel chemotherapy and i.t. injection of DCs from the study. Moreover, the triple combination treatment decreased the tumor volume; however, there were no statistically significant differences on comparison with the other combined strategies. Finally, a small number of mice were subjected to the secondary challenge test, which might limit our ability to reach a conclusion about the lack of association between triple combination treatment and treatment efficacy.
In conclusion, this study provides evidence supporting the potential therapeutic use of triple combination therapy in regression of tumor size, induction of systemic immune response and acquisition of persistent tumor immunity. A further research aimed at elucidating the rationale for performing more extensive clinical studies in fibrosarcoma and other types of cancer is required.
ACKNOWLEDGMENT
This study was supported by an Inha University research grant.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 3.Celluzzi CM, Mayordomo JI, Storkus WJ, Lotze MT, Falo LD., Jr Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J Exp Med. 1996;183:283–287. doi: 10.1084/jem.183.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 5.Restifo NP, Antony PA, Finkelstein SE, Leitner WW, Surman DP, Theoret MR, et al. Assumptions of the tumor 'escape' hypothesis. Semin Cancer Biol. 2002;12:81–86. doi: 10.1006/scbi.2001.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi GS, Lee MH, Kim SK, Kim CS, Lee HS, Im MW, et al. Combined treatment of an intratumoral injection of dendritic cells and systemic chemotherapy (Paclitaxel) for murine fibrosarcoma. Yonsei Med J. 2005;46:835–842. doi: 10.3349/ymj.2005.46.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nowak AK, Robinson BW, Lake RA. Gemcitabine exerts a selective effect on the humoral immune response: implications for combination chemo-immunotherapy. Cancer Res. 2002;62:2353–2358. [PubMed] [Google Scholar]
- 8.Correale P, Cusi MG, Tsang KY, Del Vecchio MT, Marsili S, Placa ML, et al. Chemo-immunotherapy of metastatic colorectal carcinoma with gemcitabine plus FOLFOX 4 followed by subcutaneous granulocyte macrophage colony-stimulating factor and interleukin-2 induces strong immunologic and antitumor activity in metastatic colon cancer patients. J Clin Oncol. 2005;23:8950–8958. doi: 10.1200/JCO.2005.12.147. [DOI] [PubMed] [Google Scholar]
- 9.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 11.Friedman EJ. Immune modulation by ionizing radiation and its implications for cancer immunotherapy. Curr Pharm Des. 2002;8:1765–1780. doi: 10.2174/1381612023394089. [DOI] [PubMed] [Google Scholar]
- 12.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quarmby S, Kumar P, Kumar S. Radiation-induced normal tissue injury: role of adhesion molecules in leukocyteendothelial cell interactions. Int J Cancer. 1999;82:385–395. doi: 10.1002/(sici)1097-0215(19990730)82:3<385::aid-ijc12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Ganss R, Ryschich E, Klar E, Arnold B, Hämmerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002;62:1462–1470. [PubMed] [Google Scholar]
- 16.Nikitina EY, Gabrilovich DI. Combination of gammairradiation and dendritic cell administration induces a potent antitumor response in tumor-bearing mice: approach to treatment of advanced stage cancer. Int J Cancer. 2001;94:825–833. doi: 10.1002/1097-0215(20011215)94:6<825::aid-ijc1545>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Spira AI, Ettinger DS. The use of chemotherapy in soft-tissue sarcomas. Oncologist. 2002;7:348–359. doi: 10.1634/theoncologist.7-4-348. [DOI] [PubMed] [Google Scholar]
- 18.Berezhnaya NM, Vinnichuk UD, Belova OB, Baranovich VV. Antitumor action of lymphokin-activated cells of patients with soft tissue sarcomas and melanomas in dependence on expression of MHC classes I and II antigenes. Exp Oncol. 2006;28:231–234. [PubMed] [Google Scholar]
- 19.Kucera A, Pýcha K, Pajer P, Spísek R, Skába R. Dendritic cell-based immunotherapy induces transient clinical response in advanced rat fibrosarcoma - comparison with preventive anti-tumour vaccination. Folia Biol (Praha) 2009;55:119–125. [PubMed] [Google Scholar]
- 20.Shin JY, Lee SK, Kang CD, Chung JS, Lee EY, Seo SY, et al. Antitumor effect of intratumoral administration of dendritic cell combination with vincristine chemotherapy in a murine fibrosarcoma model. Histol Histopathol. 2003;18:435–447. doi: 10.14670/HH-18.435. [DOI] [PubMed] [Google Scholar]
- 21.Finkelstein SE, Iclozan C, Bui MM, Cotter MJ, Ramakrishnan R, Ahmed J, et al. Combination of external beam radiotherapy (EBRT) with intratumoral injection of dendritic cells as neo-adjuvant treatment of high-risk soft tissue sarcoma patients. Int J Radiat Oncol Biol Phys. 2012;82:924–932. doi: 10.1016/j.ijrobp.2010.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derby E, Reddy V, Kopp W, Nelson E, Baseler M, Sayers T, et al. Three-color flow cytometric assay for the study of the mechanisms of cell-mediated cytotoxicity. Immunol Lett. 2001;78:35–39. doi: 10.1016/s0165-2478(01)00226-7. [DOI] [PubMed] [Google Scholar]
- 23.Shah DA, Madden LV. Nonparametric analysis of ordinal data in designed factorial experiments. Phytopathology. 2004;94:33–43. doi: 10.1094/PHYTO.2004.94.1.33. [DOI] [PubMed] [Google Scholar]
- 24.Paulos CM, Kaiser A, Wrzesinski C, Hinrichs CS, Cassard L, Boni A, et al. Toll-like receptors in tumor immunotherapy. Clin Cancer Res. 2007;13:5280–5289. doi: 10.1158/1078-0432.CCR-07-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fulda S, Los M, Friesen C, Debatin KM. Chemosensitivity of solid tumor cells in vitro is related to activation of the CD95 system. Int J Cancer. 1998;76:105–114. doi: 10.1002/(sici)1097-0215(19980330)76:1<105::aid-ijc17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 26.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 27.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 29.Tsujitani S, Kakeji Y, Watanabe A, Kohnoe S, Maehara Y, Sugimaghi K. Infiltration of S-100 protein positive dendritic cells and peritoneal recurrence in advanced gastric cancer. Int Surg. 1992;77:238–241. [PubMed] [Google Scholar]
- 30.Machlenkin A, Goldberger O, Tirosh B, Paz A, Volovitz I, Bar-Haim E, et al. Combined dendritic cell cryotherapy of tumor induces systemic antimetastatic immunity. Clin Cancer Res. 2005;11:4955–4961. doi: 10.1158/1078-0432.CCR-04-2422. [DOI] [PubMed] [Google Scholar]
- 31.Candido KA, Shimizu K, McLaughlin JC, Kunkel R, Fuller JA, Redman BG, et al. Local administration of dendritic cells inhibits established breast tumor growth: implications for apoptosis-inducing agents. Cancer Res. 2001;61:228–236. [PubMed] [Google Scholar]