Abstract
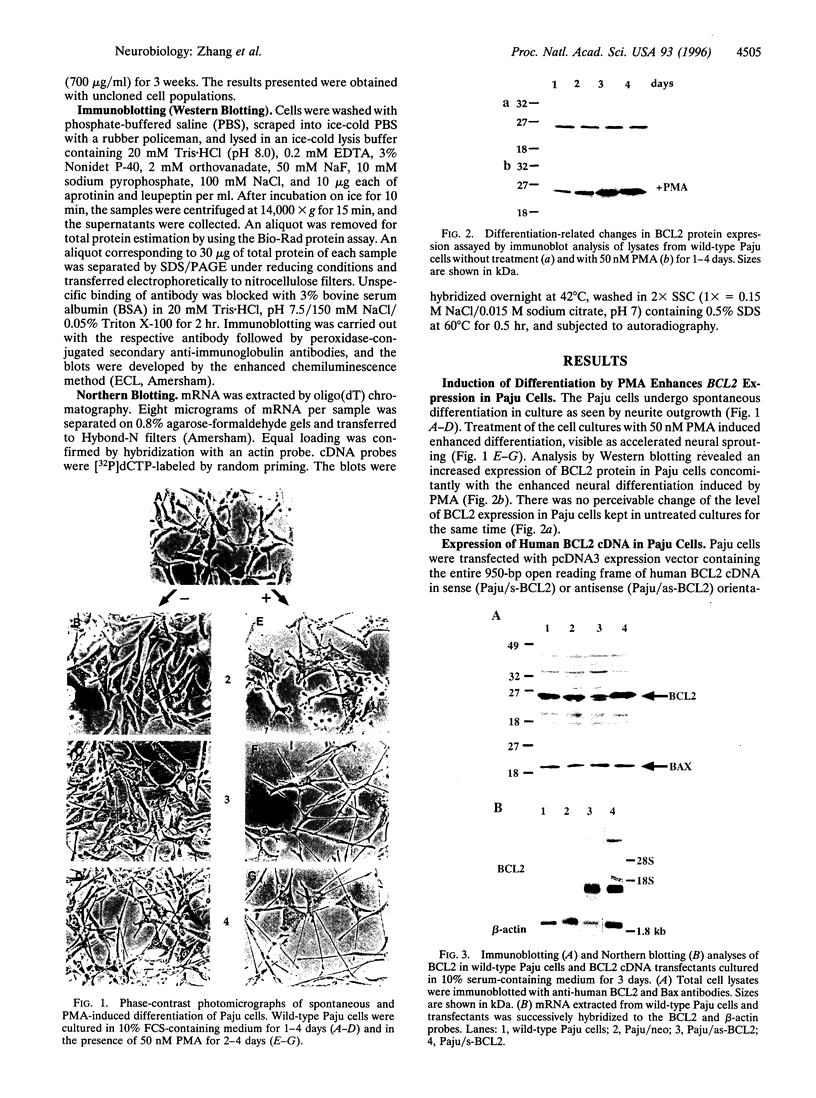
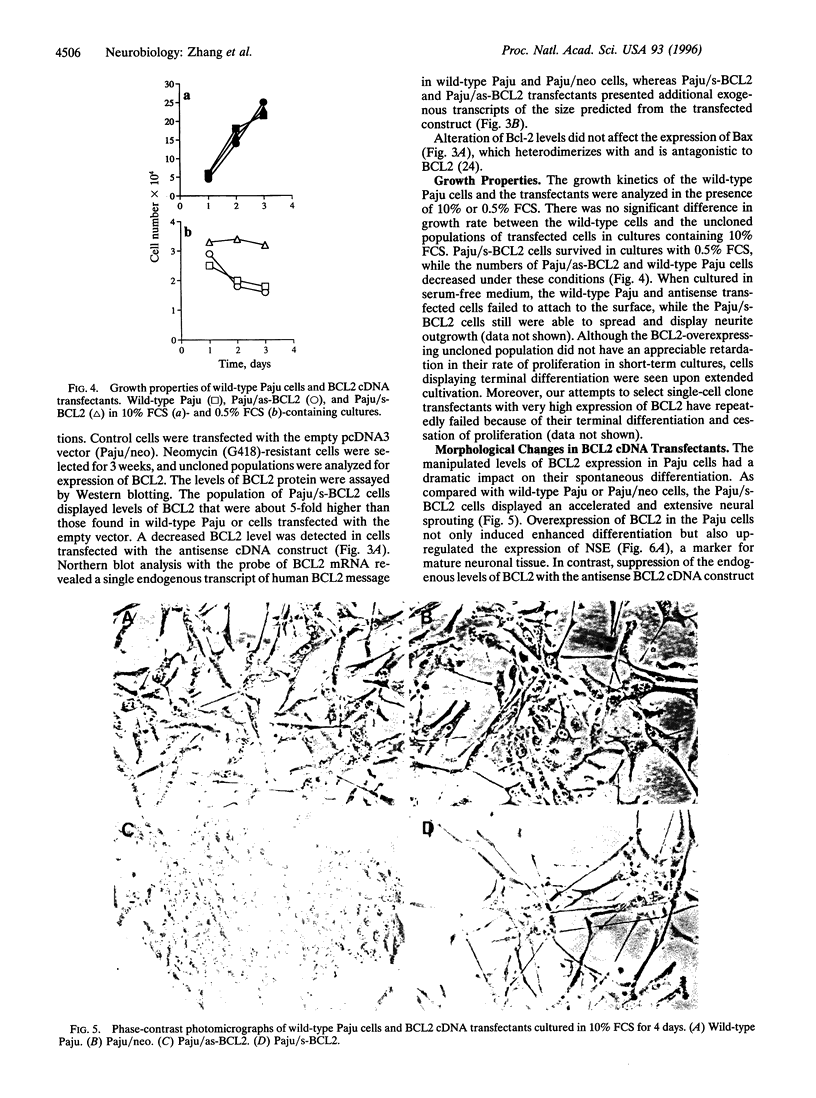
A main function attributed to the BCL2 protein is its ability to confer resistance against apoptosis. In addition to the constitutively high expression of BCL2, caused by gene rearrangement in follicular lymphomas, elevated expression of the BCL2 gene has been found in differentiating hematopoietic, neural, and epithelial tissues. To address the question of whether the expression of BCL2 is a cause or consequence of cell differentiation, we used a human neural-crest-derived tumor cell line, Paju, that undergoes spontaneous neural differentiation in vitro. The Paju cell line displays moderate expression of BCL2, the level of which increases in parallel with further neural differentiation induced by treatment with phorbol 12-myristate 13-acetate. Transfection of normal human BCL2 cDNA in sense and antisense orientations had a dramatic impact on the differentiation of the Paju cells. Overexpression of BCL2 cDNA induced extensive neurite outgrowth, even in low serum concentrations, together with an increased expression of neuron-specific enolase. Paju cells expressing the anti-sense BCL2 cDNA construct, which reduced the endogenous levels of BCL2, did not undergo spontaneous neural differentiation. These cells acquired an epithelioid morphology and up-regulated the intermediate filament protein nestin, typically present in primitive neuroectodermal cells. The manipulated levels of BCL2 did not have appreciable impact on cell survival in normal culture. Our findings demonstrate that the BCL2 gene product participates in the regulation of neural differentiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe-Dohmae S., Harada N., Yamada K., Tanaka R. Bcl-2 gene is highly expressed during neurogenesis in the central nervous system. Biochem Biophys Res Commun. 1993 Mar 31;191(3):915–921. doi: 10.1006/bbrc.1993.1304. [DOI] [PubMed] [Google Scholar]
- Baffy G., Miyashita T., Williamson J. R., Reed J. C. Apoptosis induced by withdrawal of interleukin-3 (IL-3) from an IL-3-dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production. J Biol Chem. 1993 Mar 25;268(9):6511–6519. [PubMed] [Google Scholar]
- Batistatou A., Merry D. E., Korsmeyer S. J., Greene L. A. Bcl-2 affects survival but not neuronal differentiation of PC12 cells. J Neurosci. 1993 Oct;13(10):4422–4428. doi: 10.1523/JNEUROSCI.13-10-04422.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillaud J. M., Martinez-Madrigal F., Hartmann O., Carlu C. Immunohistochemical demonstration of neurone specific enolase in bone marrow infiltrated by neuroblastoma. J Clin Pathol. 1991 Apr;44(4):309–312. doi: 10.1136/jcp.44.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Levy Z., Nourse J., Cleary M. L. The bcl-2 candidate proto-oncogene product is a 24-kilodalton integral-membrane protein highly expressed in lymphoid cell lines and lymphomas carrying the t(14;18) translocation. Mol Cell Biol. 1989 Feb;9(2):701–710. doi: 10.1128/mcb.9.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L., Sklar J. Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7439–7443. doi: 10.1073/pnas.82.21.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombel M., Symmans F., Gil S., O'Toole K. M., Chopin D., Benson M., Olsson C. A., Korsmeyer S., Buttyan R. Detection of the apoptosis-suppressing oncoprotein bc1-2 in hormone-refractory human prostate cancers. Am J Pathol. 1993 Aug;143(2):390–400. [PMC free article] [PubMed] [Google Scholar]
- Crary G. S., Singleton T. P., Neglia J. P., Swanson P. E., Strickler J. G. Detection of metastatic neuroblastoma in bone marrow biopsy specimens with an antibody to neuron-specific enolase. Mod Pathol. 1992 May;5(3):308–311. [PubMed] [Google Scholar]
- Fernandez-Sarabia M. J., Bischoff J. R. Bcl-2 associates with the ras-related protein R-ras p23. Nature. 1993 Nov 18;366(6452):274–275. doi: 10.1038/366274a0. [DOI] [PubMed] [Google Scholar]
- Flørenes V. A., Holm R., Myklebost O., Lendahl U., Fodstad O. Expression of the neuroectodermal intermediate filament nestin in human melanomas. Cancer Res. 1994 Jan 15;54(2):354–356. [PubMed] [Google Scholar]
- Gompel A., Sabourin J. C., Martin A., Yaneva H., Audouin J., Decroix Y., Poitout P. Bcl-2 expression in normal endometrium during the menstrual cycle. Am J Pathol. 1994 Jun;144(6):1195–1202. [PMC free article] [PubMed] [Google Scholar]
- Hanada M., Krajewski S., Tanaka S., Cazals-Hatem D., Spengler B. A., Ross R. A., Biedler J. L., Reed J. C. Regulation of Bcl-2 oncoprotein levels with differentiation of human neuroblastoma cells. Cancer Res. 1993 Oct 15;53(20):4978–4986. [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- LeBrun D. P., Warnke R. A., Cleary M. L. Expression of bcl-2 in fetal tissues suggests a role in morphogenesis. Am J Pathol. 1993 Mar;142(3):743–753. [PMC free article] [PubMed] [Google Scholar]
- Leek R. D., Kaklamanis L., Pezzella F., Gatter K. C., Harris A. L. bcl-2 in normal human breast and carcinoma, association with oestrogen receptor-positive, epidermal growth factor receptor-negative tumours and in situ cancer. Br J Cancer. 1994 Jan;69(1):135–139. doi: 10.1038/bjc.1994.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linette G. P., Grusby M. J., Hedrick S. M., Hansen T. H., Glimcher L. H., Korsmeyer S. J. Bcl-2 is upregulated at the CD4+ CD8+ stage during positive selection and promotes thymocyte differentiation at several control points. Immunity. 1994 Jun;1(3):197–205. doi: 10.1016/1074-7613(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Linette G. P., Korsmeyer S. J. Differentiation and cell death: lessons from the immune system. Curr Opin Cell Biol. 1994 Dec;6(6):809–815. doi: 10.1016/0955-0674(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Lu Q. L., Poulsom R., Wong L., Hanby A. M. Bcl-2 expression in adult and embryonic non-haematopoietic tissues. J Pathol. 1993 Apr;169(4):431–437. doi: 10.1002/path.1711690408. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Deane N., Platt F. M., Nunez G., Jaeger U., McKearn J. P., Korsmeyer S. J. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989 Apr 7;57(1):79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- Meikrantz W., Gisselbrecht S., Tam S. W., Schlegel R. Activation of cyclin A-dependent protein kinases during apoptosis. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3754–3758. doi: 10.1073/pnas.91.9.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merry D. E., Veis D. J., Hickey W. F., Korsmeyer S. J. bcl-2 protein expression is widespread in the developing nervous system and retained in the adult PNS. Development. 1994 Feb;120(2):301–311. doi: 10.1242/dev.120.2.301. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Liu Z. J., Kawahara A., Minami Y., Yamada K., Tsujimoto Y., Barsoumian E. L., Permutter R. M., Taniguchi T. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc, and lck cooperate in hematopoietic cell proliferation. Cell. 1995 Apr 21;81(2):223–231. doi: 10.1016/0092-8674(95)90332-1. [DOI] [PubMed] [Google Scholar]
- Nakagawa K., Yamamura K., Maeda S., Ichihashi M. bcl-2 expression in epidermal keratinocytic diseases. Cancer. 1994 Sep 15;74(6):1720–1724. doi: 10.1002/1097-0142(19940915)74:6<1720::aid-cncr2820740613>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Nakayama K., Negishi I., Kuida K., Sawa H., Loh D. Y. Targeted disruption of Bcl-2 alpha beta in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3700–3704. doi: 10.1073/pnas.91.9.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan B., Anbazhagan R., Clarkson P., Bartkova J., Gusterson B. Expression of BCL-2 in the developing human fetal and infant breast. Histopathology. 1994 Jan;24(1):73–76. doi: 10.1111/j.1365-2559.1994.tb01273.x. [DOI] [PubMed] [Google Scholar]
- Novack D. V., Korsmeyer S. J. Bcl-2 protein expression during murine development. Am J Pathol. 1994 Jul;145(1):61–73. [PMC free article] [PubMed] [Google Scholar]
- Nunez G., Seto M., Seremetis S., Ferrero D., Grignani F., Korsmeyer S. J., Dalla-Favera R. Growth- and tumor-promoting effects of deregulated BCL2 in human B-lymphoblastoid cells. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4589–4593. doi: 10.1073/pnas.86.12.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Otsuki Y., Misaki O., Sugimoto O., Ito Y., Tsujimoto Y., Akao Y. Cyclic bcl-2 gene expression in human uterine endometrium during menstrual cycle. Lancet. 1994 Jul 2;344(8914):28–29. doi: 10.1016/s0140-6736(94)91051-0. [DOI] [PubMed] [Google Scholar]
- Pilotti S., Collini P., Rilke F., Cattoretti G., Del Bo R., Pierotti M. A. Bcl-2 protein expression in carcinomas originating from the follicular epithelium of the thyroid gland. J Pathol. 1994 Apr;172(4):337–342. doi: 10.1002/path.1711720408. [DOI] [PubMed] [Google Scholar]
- Ryan J. J., Prochownik E., Gottlieb C. A., Apel I. J., Merino R., Nuñez G., Clarke M. F. c-myc and bcl-2 modulate p53 function by altering p53 subcellular trafficking during the cell cycle. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5878–5882. doi: 10.1073/pnas.91.13.5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N., Hotta K., Waguri S., Nitatori T., Tohyama K., Tsujimoto Y., Uchiyama Y. Neuronal differentiation of PC12 cells as a result of prevention of cell death by bcl-2. J Neurobiol. 1994 Oct;25(10):1227–1234. doi: 10.1002/neu.480251005. [DOI] [PubMed] [Google Scholar]
- Sejersen T., Lendahl U. Transient expression of the intermediate filament nestin during skeletal muscle development. J Cell Sci. 1993 Dec;106(Pt 4):1291–1300. doi: 10.1242/jcs.106.4.1291. [DOI] [PubMed] [Google Scholar]
- Sentman C. L., Shutter J. R., Hockenbery D., Kanagawa O., Korsmeyer S. J. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991 Nov 29;67(5):879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- Seto M., Jaeger U., Hockett R. D., Graninger W., Bennett S., Goldman P., Korsmeyer S. J. Alternative promoters and exons, somatic mutation and deregulation of the Bcl-2-Ig fusion gene in lymphoma. EMBO J. 1988 Jan;7(1):123–131. doi: 10.1002/j.1460-2075.1988.tb02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991 Nov 29;67(5):889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Croce C. M. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5214–5218. doi: 10.1073/pnas.83.14.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y. Overexpression of the human BCL-2 gene product results in growth enhancement of Epstein-Barr virus-immortalized B cells. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1958–1962. doi: 10.1073/pnas.86.6.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Oord J. J., Vandeghinste N., De Ley M., De Wolf-Peeters C. Bcl-2 expression in human melanocytes and melanocytic tumors. Am J Pathol. 1994 Aug;145(2):294–300. [PMC free article] [PubMed] [Google Scholar]








