Abstract
Introduction:
Adrenal lipomatous tumours (ALTs) are rarely encountered in clinical practice and consequently little is known about their clinical features.
Methods:
We analyze the clinical features, diagnosis and treatment of ALTs based on cases presenting at a single centre over a 31-year period. We reviewed clinical data from patients with primary adrenal tumours treated at the Ruijin Hospital, Shanghai between January 1980 and December 2010.
Results:
A total of 73 cases of primary ALTs in 22 men and 51 women (mean age 51.1±14.2 years) were reviewed. The ALTs included 65 myelolipomas (89.0%), 3 lipomas (4.1%), 2 angiomyolipomas (2.7%), 2 teratomas (2.7%), and 1 liposarcoma (1.4%). Of the total 73 patients, 24 of them had tumours in the left adrenal gland, 47 in the right gland and 2 had bilateral tumours. In total, 51 patients underwent open surgery and 22 laparoscopic surgery.
Conclusion:
Myelolipoma is predominant among the various types of lipomatous adrenal gland tumours; it accounts for about 90% of all cases. Surgery is recommended for tumours ≥3.5 cm in diameter, for all cases of symptomatic tumour, and for cases of teratoma or liposarcoma identified by preoperative imaging.
Introduction
Adrenal lipomatous tumours (ALTs) are rarely encountered in clinical practice and consequently little is known about their clinical features. Patients are usually asymptomatic on presentation and symptoms, such as abdominal pain and increased girth, occur only after enlargement of the tumour.1 Most ALTs cases are found coincidentally at radiological examination or autopsy.2 The increased availability of high resolution ultrasonography (US), computed tomography (CT) and magnetic resonance imaging (MRI) has, therefore, resulted in increased reports of ALT in recent years.3–5
The management of ALTs is uncertain in clinical practice. Numerous studies have examined the endocrine and radiologic features of these tumours in an attempt to define simple criteria for surgical intervention.4–10 Thus, unequivocal criteria for differential diagnosis are lacking and the frequency of follow-up of patients with ALTs remains a matter of debate. Pathologically, ALTs include myelolipoma, lipoma, angiomyolipoma, teratoma and liposarcoma.6,11–13 In this study, we retrospectively reviewed the features of 73 cases of ALT treated at a single centre over a 31-year period. This is by far the largest series of ALTs reported to date.
Methods
Patient recruitment
Patients with histologically confirmed primary ALTs treated at Ruijin Hospital, Shanghai between January 1980 and December 2010 were included in the study. The patients were all ≥18 years old and had adequate bone marrow, liver and renal function. We excluded patients with non-lipomatous histology, coexisting malignant tumours from the analysis. All patients provided informed consent in accordance with the institutional guidelines.
Procedures
Clinical characteristics (sex, age, presentation, biochemical analysis) and tumour characteristics (location, diameter, weight) were documented for all patients. Adrenal endocrine hormones, including plasma and urine cortisol, plasma metanephrine and normetanephrine (MN and NMN), plasma aldosterone, renin and sex hormones, were also examined.
Radiology was performed using GE Logiq-9 ultrasound machines (GE Healthcare) with a probe frequency of 3.5 MHz, a GE LightSpeed 16 CT Scanner (GE Healthcare) and a 1.5 Sigma MRI (GE Healthcare). Intravenous urography (IVU) was performed for tumours ≥8 cm in diameter to determine the best operative approach (trans-retroperitoneal, abdominal or thoracoabdominal).
All patients received intravenous-inhalation combined anesthesia. The operative procedures included open tumorectomy or tumorectomy, plus adrenalectomy and retroperitoneal laproscopic tumourectomy. In all cases the objective was to preserve normal adrenal gland tissue, unless adhesion of tumour to adrenal gland excluded this as an option.
Postoperative tumour specimens were fixed in 10% neutral formaldehyde and embedded in paraffin. Hematoxylineosin (H&E) stained sections were examined under light microscopy. After being discharged, each patient was clinically assessed at 3-month interval for the first year and once a year thereafter. All patients had both ultrasonography and CT scan check-ups and 18 patients had MRI examinations.
Statistical analysis
All data were reviewed retrospectively. Patient characteristics were expressed as means and standard deviations (±SD) for continuous variables and percentages or medians were calculated for categorical or discrete variables.
Results
The series comprised 73 patients (22 men and 51 women) with a mean age of 51.1±14.2 years. The time between diagnosis and surgery ranged from 1 to 22 months. Twenty-four patients had tumours on the left adrenal gland, 47 on the right adrenal gland and 2 had bilateral tumours. Most patients (58) were asymptomatic (Table 1, Table 2).
Table 1.
Clinical data of 65 patients with myelolipoma
| Parameter | ||
|---|---|---|
| Sex | Male | 21 |
| Female | 44 | |
| Mean age ±SD, years | 52±14 | |
| Presentation | Asymptomatic | 12 |
| Symptomatic | 53 | |
| Surgery | Open tumorectomy | 23 |
| Open tumorectomy plus adrenalectomy | 21 | |
| Laparascopic tumorectomy | 21 | |
| Location | Right | 41 |
| Left | 22 | |
| Bilateral | 2 | |
| Mean tumour diameter ±SD, cm | 6.2±3.0 | |
| Median tumour weight (range), g | 33.5 (15.5–1750.0) | |
| Median follow-up (range) years | 7.1 (1.1–31.0) | |
SD: standard deviation.
Table 2.
Clinical data of 8 patients with ALTs other than myelolipoma
| Pathology | Case | Sex | Age (years) | Presentation | Surgery | Location | Diameter (cm) | Weight (g) | Follow-up (years) |
|---|---|---|---|---|---|---|---|---|---|
| Lipoma | 1 | F | 31 | Asymptomatic | L, T | R | 4.0 | 17.5 | 6.2 |
| Lipoma | 2 | F | 60 | Asymptomatic | O, T+A | R | 10.0 | 172 | 4.6 |
| Lipoma | 3 | F | 51 | Backache on the right side | O, T+A | R | 6.0 | 112 | 3.4 |
| Angiomyolipoma | 1 | F | 47 | Asymptomatic | O, T+A | L | 6.0 | 45 | 6.2 |
| Angiomyolipoma | 2 | M | 70 | Asymptomatic | O, T+A | L | 8.0 | 121 | 5.4 |
| Teratoma | 1 | F | 21 | Backache on the right side | O, T+A | R | 6.0 | 48.5 | 6.7 |
| Teratoma | 2 | F | 35 | Asymptomatic | O, T+A | R | 8.0 | 135.5 | 4.8 |
| Liposarcoma | 1 | F | 31 | Backache on the right side | O, T+A | R | 12.3 | 1250 | 7.4 |
O: open operation; L: laparoscopic operation; T: tumourectomy; A: adrenalectomy; R: right side; L: left side.
All 73 patients had a diffuse or nebula-like hyperechoic than solid organ (e.g., kidney) on ultrasonography. CT examination indicated that in all cases the tumours were round or oval in shape. They were well-circumscribed, sharply demarcated and presented as an isolated low density mass. The CT values ranged from −60 to 5 Hounsfield units (HU), and the mass was not enhanced in contrast-enhanced tomograpy. MRI imaging showed fat tissue intensity for the diagnosis of ALTs, especially for the myelolipoma. The appearance of a myelolipoma on MRI reflects the proportion of fat and of bone marrow elements in the tumour. Fat has a high signal intensity on both T1- and T2- weighted sequences. The bone marrow elements have a low signal intensity on T1-weighted images and moderate signal intensity on T2- weighted images.
In total, 51 patients underwent open surgery (23 tumourectomy and 28 tumourectomy plus adrenalectomy) and 22 retroperitoneal laparoscopic tumourectomy. There were no surgical complications. A definitive diagnosis was made in all patients based on pathological examination. There were no cases of tumour recurrence during the follow-up (mean: 9.6 ± 7.6 years).
Myelolipoma
Sixty-five cases (89.0%) of adrenal myelolipomas (Table 1) were identified. Two bilateral myelolipomas underwent open surgery on 2 occasions 3 months apart and were successfully maintained on oral cortisone replacement therapy after the second operation.
The largest myelolipoma presented with a huge right-sided retroperitoneal hematoma accompanied by hemorrhagic shock (hemoglobin level of 40 g/L) was admitted as an emergency. A CT scan (Fig. 1, part A) in the area of the right kidney, adrenal gland and kidney margin showed hemorrhagic necrosis. The resected tumour was 20 cm in diameter and weighed 1750 g (Fig. 1, part B). All myelolipomas were composed of a mixture of mature adipose tissue and hematopoietic elements (Fig. 1, part C).
Fig. 1.
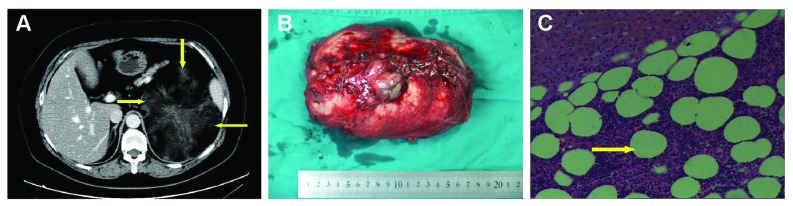
A 51-year-old woman with left adrenal tumour (myelolipoma). A. Contrast-enhanced computed tomography image shows a 20-cm low-attenuation tumour (yellow arrows) in the left adrenal gland. B. An encapsulated postoperative myelolipoma specimen weighed 1750 g. C. An adrenal myelolipoma with mature adipose cells (yellow arrow). Hematoxylin and eosin staining at 200× original magnification.
One patient had a high androgen level of 2.4 ng/mL (normal range: 0.2–0.8 ng/mL) and had suffered the consequences of growth retardation since age 10; on hospital admission, at 34 his height was only 1.28 m. One patient had an elevated plasma aldosterone level of 345.70 pg/mL (normal range: 29.4 to 161.5 pg/mL) and 1 patient had an elevated plasma cortisol level of 29.40 μg/dL (normal range: 7–22 μg/dL). The postoperative disease-free survival (DFS) of myelolipoma up to December 2010 was 10.1 ± 7.9 years (range: 1.1–31.0 years).
Lipoma
Three female patients (4.1%) presented with adrenal lipomas. The lipomas were each composed of mature adipose tissue. The DFS was 6.2, 4.6 and 3.4 years, respectively. These 3 patients are still alive, at the time of publication.
Angiomyolipoma
One male and one female patient (2.7%) were diagnosed with angiomyolipoma. The angiomyolipoma was composed of mature adipose cells, smooth muscle cells and blood vessel. Both patients are still alive, at the time of publication.
Teratoma
Two patients (2.7%) were diagnosed with teratoma. The teratoma was composed of mature tissues arising from more than 1 germinal layer and contained a large fatty component and calcified tissue or bone. Both patients are still alive, at the time of publication.
Liposarcoma
One female patient (1.4%) was diagnosed with adrenal liposarcoma (Fig. 2, part A). During surgery a large cystic tumour, 12.3 cm in diameter (Fig. 2, part B), was found in the right adrenal gland, which had caused anteriomedial displacement of the right kidney. The tumour weighed 1250 g. Microscopic examination showed that the tumour had a thick fibrous capsule and lipoblasts (Fig. 2, part C). In some areas of the tumour, a proportion of cells had morphological features compatible with round cell liposarcoma (good differentiation and low malignancy). The patient remained DFS 7.4 years postoperatively and is still alive, at the time of publication.
Fig. 2.

A 31-year-old woman with right adrenal tumour (liposarcoma). A. Contrast-enhanced computed tomography image shows a 12.3-cm low-attenuation tumour (yellow arrows) in the right adrenal gland. B. The postoperative liposarcoma specimen weighed 1250 g. C. An adrenal liposarcoma with lipoblast cells (yellow arrow). Hematoxylin and eosin staining at 200× original magnification.
Discussion
Here we present the largest series of ALTs cases to date. Disease prevalence based on our findings is likely to be accurate as it is estimated from a large adrenal tumour data bank at one of the largest adrenal disease centres in China.14–16 However, ethnic differences that may reflect the prevalence of ALT in other regions of the world fall outside the scope of this study.
Evidence from the present study and previous case reports indicate that ALTs are predominantly benign, hormonally inactive tumours.6,17,18 When the tumours reach sufficient size to suppress peripheral organs, or when intra-tumour hemorrhagic necrosis occurs, patients develop symptoms such as lower backache, abdomen pain or hypertension. These symptoms may be accompanied by hypercortisolism, hyperaldosteronism or elevated sex hormones levels. In our series, 1 patient with myelolipoma had central obesity and hypercortisolism. In another patient, myelolipoma was accompanied by hypokalemia and hyperaldosteronemia. A third patient in our study had stopped growing and developing at 10 years old possibly due to hyperandrogenism, which resulted in negative feedback and reduced growth hormone secretion.
The precise diagnosis of ALTs with imaging techniques remains challenging as imaging characteristics of ALTs vary according to the major component of the mass. Ultrasound, CT and MRI help visualize the adipose components in the ALT masses.7–10 Ultrasound is an effective screening tool for the primary detection of tumour size and adhesiveness to peripheral organs. It clearly distinguishes adipose tissue which is hyperechogenic, from hematopoietic tissues which are hypoechogenic.19 Unfortunately, ultrasound cannot be regarded as definitively diagnostic because other tumours may also be highly echogenic.19
Characteristically, ALT lesions seen on CT have a negative Hounsfield value owing to the presence of macroscopic fat.7 CT diagnosis is, therefore, based on the presence of fat tissue, which demonstrates a clear margin on low density imaging. Within the adrenal mass, fat may appear as a sporadically mottled or streak-like high density image. CT values range from −60 to 5 HU without enhancement phenomenon on enhanced scans. These features of CT provide primarily confirm the diagnosis of ALT. MRI is also useful to diagnose ALTs, because the fat has a high signal intensity on both T1- and T2- weighted sequences.
Myelolipomas were composed of a mixture of mature adipose tissue and hematopoietic elements (Fig. 1, part C). The pathogenesis of lipoma is also unknown, but many scholars agree that it has the same parenchymal features as myelolipoma.20 Angiomyolipomas are composed of vessels, smooth muscles and mature adipose tissues.21 This distinguishes them from lipomas, which are usually surrounded by a connective tissue capsule and are filled with mature adipose cells.20 Adrenal teratomas include hair, blood vessels, bone and adipose tissue and are identified as having fat or cartilage density on CT.8 Teratomas have 2 pathological subtypes: mature and immature.22,23 The phenomena of de-differentiation and increasing mitosis usually indicates malignant potential.24 The two teratomas in our study were both benign and showed no metastases or recurrences in long-term follow-up. Liposarcoma is extremely rare and is usually recognized as adrenocortical cancer because of its large volume.6 Liposarcoma often originates in retroperitoneal mesenchymal tissues, especially in the peri-renal area and is filled with lipoblast cells. Radiologically distinguishing between liposarcoma and myelolipoma or adrenocortical cancer is challenging and the final diagnosis usually requires US- or CT-guided biopsy.25,26 The case of liposarcoma in our study had previously prompted high suspicion of adrenocortical cancer (Fig. 2, part A). The large diameter (20 cm), and evidence of mucocystic degeneration indicated that it may have derived from adrenal stromal cells.
Surgery is generally recommended for ALTs tumours which are symptomatic, hormonally active or greater than 3.5 cm in diameter.6,13,18 The 2 patients in our study with bilateral myelolipoma underwent 2 tumourectomy procedures (the first 8 months after diagnosis and the second 3 months later). Simultaneous removal of both tumours was not undertaken to minimize the risk of adrenal crisis. Both patients received cortisone replacement therapy after the second operation.
Regular ultrasound or CT follow-up every 3 to 6 months is recommended for tumours less than 3.5 cm.27 Surgery should be performed without further delay if a rapid change in tumour size is detected; large ALTs carry a high risk of spontaneous rupture, hemorrhage and life-threatening shock.19,21 Indeed, it has been proposed that due to the latent risk of rupture and hemorrhage, tumours less than 3.5 cm should also be treated surgically, especially in light minimally invasive surgical techniques.28,29 The possibility of secondary infection or malignant transformation associated with tumour growth is another reason to adopt early tumour excision to achieve a better prognosis. In our study, the diameter of all the tumours was more than 3.5 cm.
Conclusion
Different types of ALTs are found in clinical practice, including myelolipoma (the most predominant form), lipoma, angiomyolipoma, teratoma and liposarcoma. Most patients present without symptoms or hormone disturbances. Tumour growth is associated with altered endocrine function, clinical symptoms and the risk of hemorrhagic rupture. Imaging examinations (ultrasound, CT and MRI) are valuable for preoperative diagnosis. Surgical intervention is warranted in ALTs ≥3.5 cm in diameter, for all symptomatic cases and all cases of preoperatively diagnosed teratoma or liposarcoma. Regular postoperative follow-up is recommended for all patients.
Footnotes
Competing interests: Dr. Zhao, Dr. Sun, Dr. Jing, Dr. Zhou, Dr. Huang, Dr. Wang, Dr. Zhu, Dr. Yuan and Dr. Shen all declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Heylen S, Hubens G, Vaneerdeweg W. Giant adrenal myelolipoma: A case report. Acta Chir Belg. 2011;111:91–3. doi: 10.1080/00015458.2011.11680714. [DOI] [PubMed] [Google Scholar]
- 2.Bayram F, Atasoy KC, Yalcin B, et al. Adrenal myelolipoma: Case report with a review of the literature. Australas Radiol. 1996;40:68–71. doi: 10.1111/j.1440-1673.1996.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 3.Gajraj H, Young AE. Adrenal incidentaloma. Br J Surg. 1993;80:422–6. doi: 10.1002/bjs.1800800405. [DOI] [PubMed] [Google Scholar]
- 4.Matsuda T, Abe H, Takase M, et al. Case of combined adrenal cortical adenoma and myelolipoma. Pathol Int. 2004;54:725–9. doi: 10.1111/j.1440-1827.2004.01686.x. [DOI] [PubMed] [Google Scholar]
- 5.Timonera ER, Paiva ME, Lopes JM, et al. Composite adenomatoid tumour and myelolipoma of adrenal gland: Report of 2 cases. Arch Pathol Lab Med. 2008;132:265–7. doi: 10.5858/2008-132-265-CATAMO. [DOI] [PubMed] [Google Scholar]
- 6.Lam KY, Lo CY. Adrenal lipomatous tumours: A 30 year clinicopathological experience at a single institution. J Clin Pathol. 2001;54:707–12. doi: 10.1136/jcp.54.9.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyoshi T, Kanematsu M, Kondo H, et al. Abdomen: angiography with 16-detector CT--comparison of image quality and radiation dose between studies with 0.625-mm and those with 1.25-mm collimation. Radiology. 2008;249:142–50. doi: 10.1148/radiol.2483071007. [DOI] [PubMed] [Google Scholar]
- 8.Lockhart ME, Smith JK, Kenney PJ. Imaging of adrenal masses. Eur J Radiol. 2002;41:95–112. doi: 10.1016/S0720-048X(01)00444-2. [DOI] [PubMed] [Google Scholar]
- 9.Korobkin M, Lombardi TJ, Aisen AM, et al. Characterization of adrenal masses with chemical shift and gadolinium-enhanced MR imaging. Radiology. 1995;197:411–8. doi: 10.1148/radiology.197.2.7480685. [DOI] [PubMed] [Google Scholar]
- 10.Dunnick NR, Korobkin M. Imaging of adrenal incidentalomas: Current status. AJR Am J Roentgenol. 2002;179:559–68. doi: 10.2214/ajr.179.3.1790559. [DOI] [PubMed] [Google Scholar]
- 11.Dieckmann KP, Hamm B, Pickartz H, et al. Adrenal myelolipoma: clinical, radiologic, and histologic features. Urology. 1987;29:1–8. doi: 10.1016/0090-4295(87)90587-5. [DOI] [PubMed] [Google Scholar]
- 12.Boudreaux D, Waisman J, Skinner DG, et al. Giant adrenal myelolipoma and testicular interstitial cell tumour in a man with congenital 21-hydroxylase deficiency. Am J Surg Pathol. 1979;3:109–23. doi: 10.1097/00000478-197904000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Akamatsu H, Koseki M, Nakaba H, et al. Giant adrenal myelolipoma: Report of a case. Surg Today. 2004;34:283–5. doi: 10.1007/s00595-003-2682-4. [DOI] [PubMed] [Google Scholar]
- 14.Wang XJ, Shen ZJ, Zhu Y, et al. Retroperitoneoscopic partial adrenalectomy for small adrenal tumours (< or =1 cm): The Ruijin clinical experience in 88 patients. BJU Int. 2010;105:849–53. doi: 10.1111/j.1464-410X.2009.08878.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhu Y, He HC, Su TW, et al. Selective alpha1-adrenoceptor antagonist (controlled release tablets) in pre-operative management of pheochromocytoma. Endocrine. 2010;38:254–9. doi: 10.1007/s12020-010-9381-x. [DOI] [PubMed] [Google Scholar]
- 16.Feng F, Zhu Y, Wang X, et al. Predictive factors for malignant pheochromocytoma: Analysis of 136 patients. J Urol. 2011;185:1583–90. doi: 10.1016/j.juro.2010.12.050. [DOI] [PubMed] [Google Scholar]
- 17.Polamaung W, Wisedopas N, Vasinanukorn P, et al. Asymptomatic bilateral giant adrenal myelolipomas: Case report and review of literature. Endocr Pract. 2007;13:667–71. doi: 10.4158/EP.13.6.667. [DOI] [PubMed] [Google Scholar]
- 18.Mukherjee S, Pericleous S, Hutchins RR, et al. Asymptomatic giant adrenal myelolipoma. Urol J. 2010;7:66–8. [PubMed] [Google Scholar]
- 19.Fontana D, Porpiglia F, Destefanis P, et al. What is the role of ultrasonography in the follow-up of adrenal incidentalomas? The Gruppo Piemontese Incidentalomi Surrenalici. Urology. 1999;54:612–6. doi: 10.1016/S0090-4295(99)00226-5. [DOI] [PubMed] [Google Scholar]
- 20.Sharma MC, Gill SS, Kashyap S, et al. Adrenal lipoma. A case report. Urol Int. 1998;60:245–7. doi: 10.1159/000030265. [DOI] [PubMed] [Google Scholar]
- 21.Fallo F, Pezzi V, Sonino N, et al. Adrenal incidentaloma in pregnancy: Clinical, molecular and immunohistochemical findings. J Endocrinol Invest. 2005;28:459–63. doi: 10.1007/BF03347228. [DOI] [PubMed] [Google Scholar]
- 22.McMillan A, Horwich A. Malignant teratoma presenting with an adrenal mass. Clin Radiol. 1987;38:327–8. doi: 10.1016/S0009-9260(87)80088-0. [DOI] [PubMed] [Google Scholar]
- 23.Sarkar K, Buckley C, Uhthoff HK. Liposarcoma simulating a Baker’s cyst: A case study. Arch Orthop Trauma Surg. 1986;105:316–9. doi: 10.1007/BF00449934. [DOI] [PubMed] [Google Scholar]
- 24.Dhawan SB, Aggarwal R, Mohan H, et al. Adrenocortical carcinoma: diagnosis by fine needle aspiration cytology. Indian J Pathol Microbiol. 2004;47:44–5. [PubMed] [Google Scholar]
- 25.Belezini E, Daskalopoulou D, Markidou S. Fine needle aspiration of adrenal myelolipoma: A case report. Cytopathology. 1992;3:31–4. doi: 10.1111/j.1365-2303.1992.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 26.Houshiar AM, Soltani M, Ercole C. Giant intra-adrenal myelolipoma associated with recurrent urinary tract infection. Int Urol Nephrol. 1997;29:130–6. doi: 10.1007/BF02551331. [DOI] [PubMed] [Google Scholar]
- 27.Del Gaudio A, Solidoro G. Myelolipoma of the adrenal gland: Report of two cases with a review of the literature. Surgery. 1986;99:293–301. [PubMed] [Google Scholar]
- 28.Yamada S, Tanimoto A, Wang KY, et al. Non-functional adrenocortical adenoma: A unique case of combination with myelolipoma and endothelial cysts. Pathol Res Pract. 2011;207:192–6. doi: 10.1016/j.prp.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Rohana AG, Ming W, Norlela S, et al. Functioning adrenal adenoma in association with congenital adrenal hyperplasia. Med J Malaysia. 2007;62:158–9. [PubMed] [Google Scholar]


