Abstract
We report on a 61-year-old woman with a history of right-sided nephrectomy for clear cell renal cell carcinoma (RCC) occurring 21 years ago; she currently presented with a bilateral ovarian tumour. Histologically, the tumour of both ovaries was clear cell carcinoma. Immunohistochemically, the tumour cells were positive for vimentin, RCC marker, epithelial membrane antigen, cytokeratin AE1/3 and CD10. Cytokeratin 7, CA125, HMWCK, estrogen and progesterone receptors were all negative. Based on the morphology and immunophenotype of the tumour, we established a diagnosis of late metastasis of RCC in the ovaries. A postoperative abdominal computed tomography scan, however, revealed a tumour mass solely in the left kidney, which had not been visible in the preoperative ultrasound. The patient underwent nephron-sparing surgery and a biopsy showed the tumour to be clear cell RCC. Metastasis of RCC to the ovaries is rare, and to the best of our knowledge, only 24 cases have been reported to date. However, due to the different treatments and prognosis, the distinction between a primary ovarian tumour and metastasis of RCC is important.
Introduction
Renal cell carcinoma (RCC) represents 2% to 3% of all cancers, with the highest incidence occurring in the Czech Republic, followed by Latvia and the United States.1 Despite the fact that this tumour is able to metastasize to different sites via hematogenous spread, there have been only 24 cases of ovarian metastases reported to date.2,3 Metastasis to the ovaries is thought to occur by retrograde venous embolization through the renal vein to the ovarian vessels.4 We describe an additional case with clinical, histological and immunohistochemical characteristics, discuss the differential diagnosis and review the literature.
Case report
A 61-year-old woman presented with a history of clear cell renal cell carcinoma (RCC) of the right kidney (the pTNM classification of the tumour was pT2aN0M0). She had been treated with nephrectomy and adjuvant chemotherapy (vinblastine 6 cycles) and had regular follow-up ultrasounds by a urologist every 6 months for more than 20 years. In November 2011, a new ovarian mass was accidentally discovered during a follow-up ultrasound and the patient was referred to the Gynecological Oncology Centre at our hospital. The detailed transvaginal ultrasound was performed by a gynecological oncologist. It revealed a bilateral ovarian tumour showing the sonomorphologic and Doppler pattern characteristic for primary or metastatic clear cell ovarian cancer with no sign of spreading to the abdomen. The tumour size was 12 × 10 × 8 cm in the right ovary and 8 × 8 × 6 cm in the left ovary. Tumour marker CA 125 was slightly elevated (48 IU/mL, standard ≤35 IU/mL). During the intraoperative pelvic and abdominal exploration, we found only a bilateral ovarian mass. Based on the result of an intraoperative ovarian tumour biopsy, which showed the adnexal mass to be clear cell carcinoma, and uncertain as to whether it was primary or metastatic, the patient underwent radical surgery. This included a total abdominal hysterectomy with a bilateral salpingo-oophorectomy, total omentectomy, appendectomy and systematic pelvic and paraortic lymphadenectomy; no residual tumour remained. The final pathology report confirmed metastatic RCC in both ovaries with negative findings in the lymph nodes (76/0), omentum and the other removed structures.
Histological results
Ovarian tumour specimen
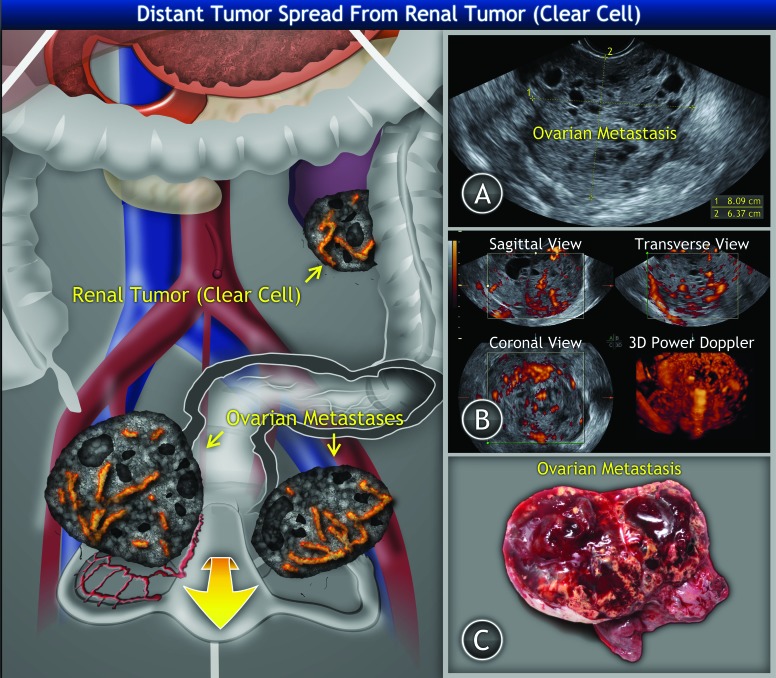
Grossly, the right ovary was replaced by a hemorrhagic solid yellowish tumour mass (size 105 × 90 × 70 mm) with few cysts, 30 mm in the greatest diameter (Fig. 1, part C). The left ovary was replaced by a tumour mass of the same structure and size 80 × 60× 50 mm.
Fig. 1.
Clear cell renal cell tumour of the left kidney with bilateral ovarian metastases: A: Ultrasound findings of right ovarian metastasis of solid structure with multiple small irregular cysts filled with hypoechogenic (blood) intracystic fluid. B: Three-dimensional power Doppler ultrasound of ovarian metastasis showing tumour structure and vessel tree. C: Gross appearance of the ovarian tumour.
Histologically, both ovaries consisted of a clear cell tumour arranged in a solid, alveolar, microcystic and macrocystic pattern and showed a prominent vascular network (Fig. 2). The tumour cell nuclei were regular without marked pleomorphism or mitoses. An intraoperative diagnosis, using the frozen section technique, revealed clear cell carcinoma, although it was uncertain as to whether it was primary ovarian or metastatic. Immunohistochemically, the neoplastic cells were positive for RCC marker, vimentin, epithelial membrane antigen, cytokeratin AE1/3 and CD10. Other markers, including cytokeratin 7, CA 125, HMWCK, estrogen and progesterone receptors, were negative. Based on the morphology and immunophenotype, we concluded that the final diagnosis was metastatic clear cell RCC.
Fig. 2.
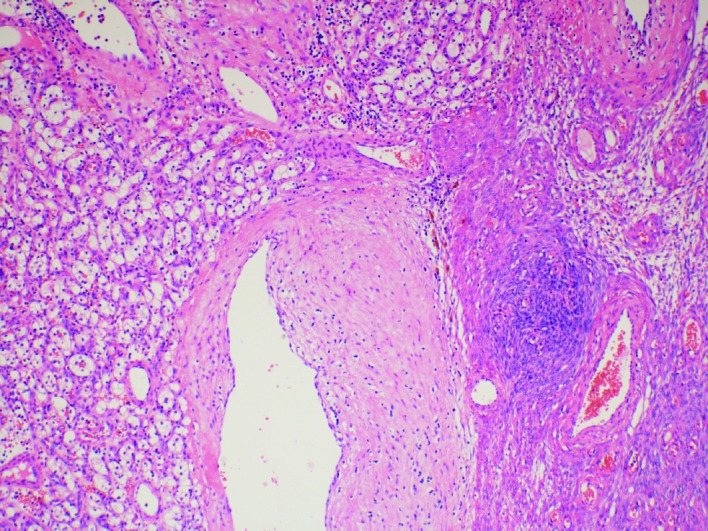
Metastasis of clear cell renal cell carcinoma: Ovary hematoxylin and eosin stain (100×).
Kidney specimen
Grossly, the resected part of the left kidney showed a well-demarcated solid yellowish tumour measuring 30 × 27 × 24 mm. Histologically, the tumour consisted of typical clear cell RCC (Fuhrman nuclear grade 2) (Fig. 3). Neither angioinvasion nor any extension into the peri-nephric tissue was found, and the pTNM classification was pT1aNXM1.
Fig. 3.
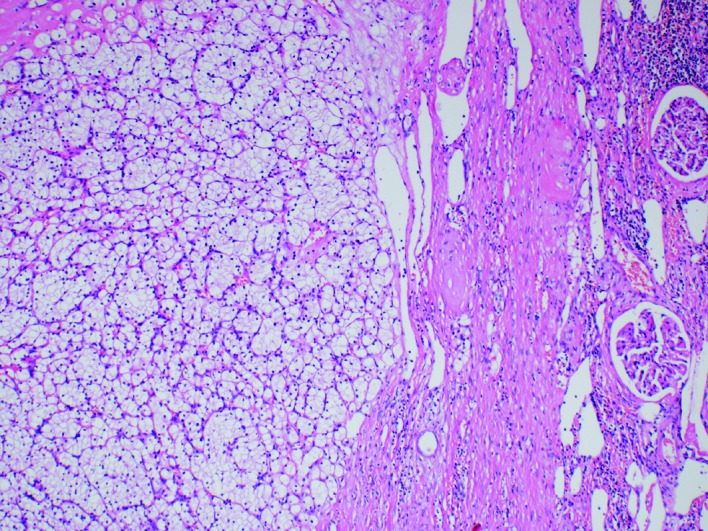
Clear cell renal cell carcinoma: Left kidney, hematoxylin and eosin stain (100×).
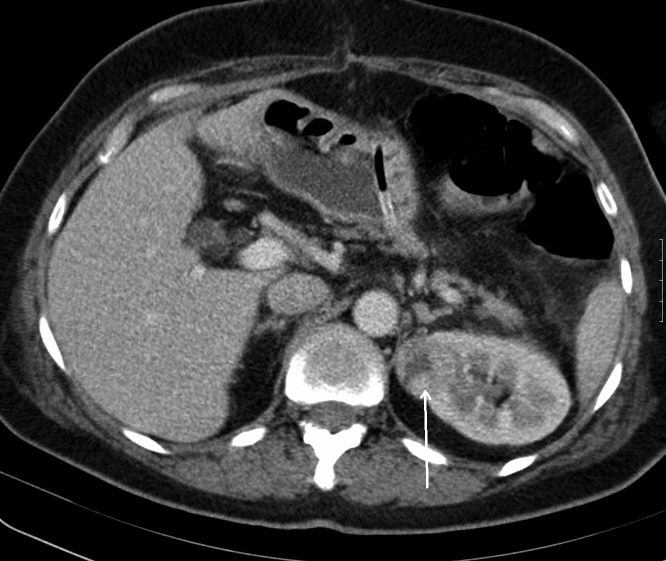
A subsequent transabdominal computed tomography (CT) scan revealed a well-encapsulated tumorous mass in the left kidney (29 × 28 × 31 mm) with heterogeneous enhancement (Fig. 4), which was not visible on the ultrasound preoperatively. A repeated CT scan after 4 months confirmed the increased size of the renal mass (32 × 30 × 40 mm); nephron-sparing surgery followed (open partial nephrectomy). The patient’s postoperative course was unremarkable, with temporarily elevated serum creatinin (maximum 405 μmol/L) and urea (maximum 12 mmo/L). Soon after surgery, her renal function was within the normal range. There was no need for dialysis or supportive care.
Fig. 4.
Computed tomography scan: Well-encapsulated tumorous mass of the left kidney with heterogenous enhancement.
She did not receive any adjuvant chemotherapy and she has had regular follow-ups with no sign of disease 1 year after the removal of ovarian tumours, as per the CT and positron emission tomography/CT scans.
Discussion
Our report concerns a rare case of bilateral secondary ovarian involvement from primary clear cell RCC. RCC is a common tumour, which is able to metastasize to different sites.5 Simon and colleagues concluded that even among those patients having clear cell RCC, who experience an initial 5-year postoperative disease-free interval, about 5% will experience a renal recurrence and 15% will develop metastases during the ensuing 10 years.7
The most frequent localizations of metastases are the lungs (50%–60%), lymph nodes (36%), bones (30%–40%), liver (30%–40%) and brain (5%).6 However, metastases of this tumour (via hematogenous spread) have been identified in virtually every organ of the human body.9
Among the reported clear cell RCC cases spreading to the female genital tract, most (about 80) metastasized to the vagina.8 Only 24 cases of RCC metastases to the ovaries have been reported to date.2,3 In our case, the metastatic tumour affected both ovaries.
Bilateral ovarian tumours were, however, detected in only 8 of the previously reported cases, and involvement of the contralateral ovary is even rarer.2,9 Ovarian metastases may develop either before, or many years after, a diagnosis of the primary renal tumour and can therefore be easily confused with primary ovarian tumours.10
Metastatic renal cell tumours typically spread hematogenously, and may be spread by a renal-ovarian axis (i.e., the direct drainage of the left ovary into the left renal vein).2 Because of this unique anatomy, incompetent gonadal veins allow a retrograde venous flow.11 This phenomenon would explain the slightly higher number of cases of left-sided clear cell RCC with metastases to the ipsilateral ovary. Synchronous primary ovarian and renal cancers are extremely rare; only 3 cases were reported in English scientific literature, and English published reports revealed only 2 cases of metastasized ovarian carcinoma presented as a renal mass.11 Most of the RCCs spreading into the ovaries were clear cell types. There is only 1 report of a papillary RCC that metastasized to the ovary.10
Clear cell carcinoma is a distinctive histological type of primary ovarian carcinoma.
A differential diagnosis of this tumour includes a steroid cell tumour (stromal luteoma, Leydig cell tumour, steroid cell tumour, not otherwise specified, dysgerminoma, and a clear cell variant of struma ovarii.12,13
Moreover, metastasis of clear cell carcinoma from other primary sites, including the kidney, should also be considered. The presence of hobnail cells and extracellular mucin are more typical of clear cell carcinoma of the ovary, while a solid and tubular growth pattern with bland cells and a prominent vascular network raises the possibility of a metastasis of clear cell RCC. A panel of immunohistochemical markers further supports this diagnosis. A useful panel for this distinction is CD10, CK7, RCC marker, mesothelin and HMWCK (clone 34 E12). Ovarian clear cell carcinoma is usually CK7+, mesothelin + and CD10-. However, CD10 can be positive in up to 20% of ovarian clear cell carcinoma, usually at the apical border.14 On the other hand, CCRCC is CK7-, mesothelin-, CD10+, and RCC marker+. HMWCK can also be helpful; they are always negative in RCC.
The most common symptoms related to clear cell renal cancer are anemia, hepatic dysfunction, gross hematuria, hypoalbuminemia, flank pain, malaise, paraneoplastic hypercalcemia and anorexia. Currently, more than 70% of all renal cancer cases are detected as an incidental finding on imaging obtained for unrelated reason.4 The tumour is usually a solid mass, mostly heterogeneous, which is associated with necrosis, hemorrhage, regressive changes or calcification. In about one-third of patients, the tumour is homogenous. RCC is hypervascular and shows strong enhancement even after intravenous contrast administration has already been in the arterial phase. The most significant prognostic factor determining the survival rate in RCC is the invasion into the renal vein or the inferior vena cava.15 In our case, the renal tumour was not identified by ultrasound, but only by a transabdominal CT scan.
Most metastatic ovarian tumours are unilateral, very often large, with the greatest dimension being 12.5 cm on average.16 If the ovarian metastasis is discovered prior to its renal source, then a subjective tumour pattern recognition on an ultrasound may lead to a misdiagnosis of primary ovarian clear cell carcinoma (also typically presented as a unilateral mass with a mean size of 13 to 15 cm,17 or as a granulosa-cell tumour.18 In an ultrasound, solid tumous containing several small, irregular locules or multilocular-solid tumours are usually depicted. The cysts are filled with a small amount of fluid or mixed echogenicity (hemorrhagic content). These sonomorphologic and Doppler patterns of ovarian mass (although found bilaterally) were identified during our patient’s transvaginal ultrasound (Fig. 1); therefore, it was suspected that a primary or metastatic clear cell ovarian cancer was present.
The ovarian masses were treated as a primary ovarian cancer with radical surgery, because the intraoperative biopsy cannot differentiate whether or not the mass is of primary or secondary ovarian origin. Similarly, the encapsulated left side tumour was treated with radical nephron-sparing surgery. Limited evidence suggests that surgical extirpation of both lesions may lead to long-term disease-free survival.11,19
Conclusion
Ovarian metastasis of CCRCC should be considered using a differential diagnosis of ovarian clear cell tumours, even if many years have passed after the diagnosis of a renal primary tumour, and even in cases without anamnestic data of RCC, since the metastasis could be discovered prior to the renal primary.
Acknowledgments
This work was supported by Charles University in Prague, project UNCE No. 204024, PRVOUK-P27/LF1/1, and BBM 1. LF UK LM2010004.
Footnotes
Competing interests: Dr. Bauerová, Dr. Dundr, Dr. Fischerová, Dr. Pešl, Dr. Zikán and Dr. Burgetová all declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008 v20, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. Lyon, France: International Agency for Research on Cancer; 2010. http://globocan.iarc.fr. Accessed February 26, 2014. [Google Scholar]
- 2.Anagnostou VK, Tiniakos DG, Chorti M, et al. Right sited renal cell carcinoma metastazing to the contra-lateral ovary: Case report and review of the literature. Pathol Oncol Res. 2009;15:123–7. doi: 10.1007/s12253-008-9039-7. [DOI] [PubMed] [Google Scholar]
- 3.Guney S, Guney N, Ozcan D, et al. Ovarian Metastasis of a primary renal cell carcinoma: Case report and review of literature. Eur J Gynaecol Oncol. 2010;31:339–41. [PubMed] [Google Scholar]
- 4.Sountoulides P, Metaxa L, Cindolo L. Atypical presentations and rare metastatic sites of renal cell carcinoma: A review of case reports. J Med Case Rep. 2011;2:429. doi: 10.1186/1752-1947-5-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eble JN, Sauter G, Epstein JI, et al. Pathology and genetics of tumours of the urinary system and male genital organs. Lyon, France: IARC Press; 2004. World Health Organization Classification of Tumours; p. 23. [Google Scholar]
- 6.Toquero L, Aboumarzouk OM, Abbasi Z. Renal cell carcinoma metastasis to the ovary: A case report. Cases J. 2009;2:7472. doi: 10.4076/1757-1626-2-7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SP, Weight CJ, Leibovich BC, et al. Outcomes and clinicopathologic variables associated with late recurrence after nephrectomy for localized renal cell carcinoma. Urology. 2011;78:1101–6. doi: 10.1016/j.urology.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Bozaci EA, Atabekoğlu C, Sertçelik A, et al. Metachronous metastases from renal cell carcinoma to uterine cervix and vagina: Case report and review of literature. Gynecol Oncol. 2005;99:232–5. doi: 10.1016/j.ygyno.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Decoene J, Ameye F, Lerut E, et al. Renal cell carcinoma with synchronous metastasis to the calcaneus and metachronous metastases to the ovary and gallbladder. Case Rep Med. 2011;2011:671645. doi: 10.1155/2011/671645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stolnicu S, Borda A, Radulescu D, et al. Metastasis from papillary renal cell carcinoma masquerading as primary ovarian clear cell tumor. Pathol Res Pract. 2007;203:819–22. doi: 10.1016/j.prp.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Gavallos G, Tawfik O, Herrell D, et al. Renal-ovarian axis: A case report and review. Urology. 2003;62:749xviii–749xxii. doi: 10.1016/s0090-4295(03)00585-5. [DOI] [PubMed] [Google Scholar]
- 12.Hammock L, Ghorab Z, Gomez-Fernandes CR. Metastatic renal cell carcinoma to the ovary: A case report and discussion of differential diagnosis. Arch Lab Med. 2003;127:123–6. doi: 10.5858/2003-127-e123-MRCCTT. [DOI] [PubMed] [Google Scholar]
- 13.Insabato L, De Rosa G, Franco R, et al. Ovarian metastasis from renal cell carcinoma: A report of three cases. Int J Surg Pathol. 2003;4:309–12. doi: 10.1177/106689690301100408. [DOI] [PubMed] [Google Scholar]
- 14.Kurman RJ, Hedrick Allenson L, et al. Blaustein’s pathology of the female genital tract. 6th ed. Springer; 2011. pp. 762–3.pp. 972–3. [DOI] [Google Scholar]
- 15.Mueller-Lisse UG, Mueller-Lisse UL, Meindl T, et al. Staging of renal cell carcinoma. Eur Radiol. 2007;17:2768–77. doi: 10.1007/s00330-006-0554-1. [DOI] [PubMed] [Google Scholar]
- 16.Lerwill MF, Young RH. Metastatic Tumors of the Ovary. In: Kurman RJ, Ellenson LH, Ronnett BM, editors. Blaustein’s Pathology of the Female Genital Tract. 6th ed. Springer Science+Business Medica; NY: 2011. [Google Scholar]
- 17.Seidman JD, Cho KR, Ronnett BM, et al. Surface Epithelial Tumors of the Ovary. In: Kurman RJ, Ellenson LH, Ronnett BM, editors. Blaustein’s Pathology of the Female Genital Tract. 6th ed. Springer Science+Business Medica; New York, NY: 2011. [Google Scholar]
- 18.Van Holsbeke C, Domali E, Holland TK, et al. Imaging of gynecological disease (3): clinical and ultrasound characteristics of granulosa cell tumors of the ovary. Ultrasound Obstet Gynecol. 2008;31:450–6. doi: 10.1002/uog.5279. [DOI] [PubMed] [Google Scholar]
- 19.Albrizio M, La Fianza A, Gorone MS. Bilateral metachronous ovarian metastases from clear cell renal carcinoma: A case report. Cases J. 2009;2:7083. doi: 10.1186/1757-1626-2-7083. [DOI] [PMC free article] [PubMed] [Google Scholar]