Abstract
Implication for health policy/practice/research/medical education:
Amyloid A (AA) amyloidosis is a systemic form of amyloidosis secondary to chronic infections and inflammatory disorders such as recurrent suppurative skin infections secondaryto subcutaneous administration of drugs (skinpopping).The diagnosis of AA amyloidosis is frequently overlooked due to the insidious nature of the disease. The renal manifestations of AA amyloidosis include proteinuria, tubular dysfunction, and progressive loss of renal function. Urinalysis and quantification of urinary protein excretion are important screening tests. Early diagnosis and treatment of AA amyloidosis can reverse end-organ damage.
Keywords: Amyloid A amyloidosis, Subcutaneous drug injection, Skin-popping, Suppurative skin infections
In this issue of the Journal, Cooper et al. reported a 37-year-old woman who developed proteinuria and severe renal insufficiency in the setting of long-standing subcutaneous heroin use and recurrent skin abscesses (1). Erythrocyte sedimentation rate (ESR) and serum concentration of C-reactive protein (CRP) were elevated. Renal biopsy revealed amyloid A (AA) amyloidosis.
Injection drug use is a global health issue (2). There are approximately 16 million people who inject drugs worldwide. Although injecting drug use has been identified in over 148 countries, the largest numbers of injection drug users were found in China, the USA, and Russia. Once intravenous access sites are exhausted, drugs are frequently injected subcutaneously, a method known as skin-popping. While reducing the chances of an overdose, skin-poppingmay prolong the duration of euphoria. AA amyloidosis is a complication of inflammatory disorders and chronic infections such as recurrent suppurative skin infections secondary to subcutaneous administration of drugs. AA amyloid is derived from serum amyloid A (SAA), which is an acute-phase protein released from the liver (3). SAA concentrations can be increased as much as 1000-fold during an acute-phase response. The SSA concentration correlated directly with amyloid burden estimated using whole-body 123I-serum amyloid P (SAP) scintigraphy (4). Renal disease is a common and serious complications of AA amyloidosis (Figure 1) (5). The SAA concentration significantly correlated with the risk of death and progression of renal disease. Renal manifestations of AA amyloidosis include polyuria due to nephrogenic diabetes insipidus, renal tubular acidosis, proteinuria, edema, and progressive renal disease. Severe proteinuria can give rise to the nephrotic syndrome.
Fig. 1.
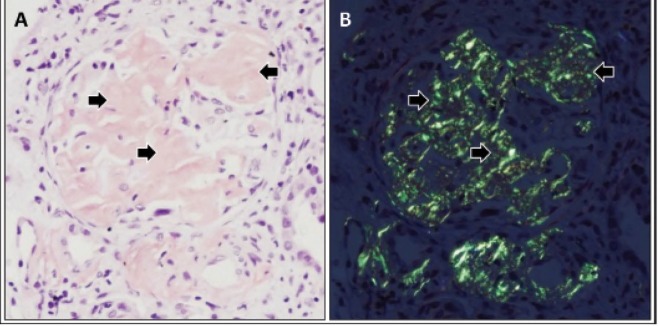
Renal amyloid A amyloidosis. A photomicrograph demonstrating accumulation of amorphous material (arrows)in a glomerulus (A). Polarizing microscopy revealing apple-green birefringence of deposits in the glomerular mesangial areas (arrows) (B). Tissue sections were stained with Congo red. Courtesy of David B. Thomas, MD.
The diagnosis of AA amyloidosis is frequently overlooked due to the insidious nature of the disease. Socioeconomic conditions, cultural background, and behavioral practices of those affected likely play a role as well. However, in some cases, it appears that both careful and casual health care providers do not recognize obvious signs and symptoms of the disease for a long period of time. Although the importance of monitoring SSA concentrations in patients at risk for AA amyloidosis has previously been emphasized, the test is not readily available. For example, SSA is not listed under the tests that can be ordered at Quest Diagnostics and LapCorp, two of the largest diagnostic laboratories in the United States. C-reactive protein (CRP) concentrations usually parallel those of SAA (3). Therefore, monitoring CRP concentrations might be a useful tool in estimating amyloid burden and assessing the risk for the development of the complications of AA amyloidosis (6). Although frequently used as a surrogate marker for systemic inflammatory responses, erythrocyte sedimentation rate (ESR) is greatly affected by the size, shape, and number of erythrocytes, as well as the concentration of other plasma proteins, particularly fibrinogen (3). Nonetheless, the measurement of ESR can be readily carried out in almost every clinical laboratory. It remains to be seen whether ESR correlates directly with amyloid burden as well as the risk of death and progression of renal disease as SAA does. With respect to renal involvement, renal function tests including urinalysis and random urine protein and creatinine concentrations are important in excluding significant renal disease.
Accumulating evidence indicates that normalization of circulating SAA concentrations arrest AA amyloid deposition and is the primary objective in the treatment of AA amyloidosis (7). Successful treatment of the underlying inflammatory disorders reduces SAA concentrations. This approach improves survival and results in regression of amyloid deposits in a significant number of patients. Colchicine prevents AA amyloidosis in high-risk patients with familial Mediterranean fever and arrest the progression of renal disease in a subset of patients (8). The use of colchicine in a patient with AA amyloidosis due to drug abuse led to favorable results (9). The institution of a treatment strategy should be accompanied by close monitoring of circulating SSA or CRP levels to ensure the effectiveness of the treatment. In a significant number of patients, normalization of circulating SAA concentrations is associated with reversal of organ damage as a result of regression of amyloid deposits. The report by Cooper et al. calls for a heightened awareness of AA amyloidosis as a potential cause of renal disease in patients with recurrent bacterial skin infections due to subcutaneous drug use.
Author’s contribution
AN is the single author of the manuscript.
Conflict of interests
The author declared no competing interests.
Funding/Support
None declared.
Please cite this paper as: Nayer A, Amyloid A amyloidosis: frequently neglected renal disease in injecting drug users. J Nephropathol. 2014; 3(1):26-28. DOI: 10.12860/jnp.2014.06
References
- 1.Cooper C, Bilbao JE, Said S, Alkhateeb H, Bizet J, Elfar A. et al. Serum amyloid A renal amyloidosis in a chronic subcutaneous (“skin popping”) heroin user. J Nephropathol. 2013;2(3):196–200. doi: 10.12860/JNP.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA. et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372(9651):1733–45. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- 3.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 4.Hawkins PN, Lavender JP, Pepys MB. Evaluation of systemic amyloidosis by scintigraphy with 123I-labeled serum amyloid P component. N Engl J Med 1990. 23;323(8):508–13. doi: 10.1056/NEJM199008233230803. [DOI] [PubMed] [Google Scholar]
- 5.Lachmann HJ, Goodman HJ, Gilbertson JA, Gallimore JR, Sabin CA, Gillmore JD. et al. Natural history and outcome in systemic AA amyloidosis. N Engl J Med. 2007;356(23):2361–71. doi: 10.1056/NEJMoa070265. [DOI] [PubMed] [Google Scholar]
- 6.Hazenberg BP, van Rijswijk MH. Clinical and therapeutic aspects of AA amyloidosis. Baillieres Clin Rheumatol. 1994;8(3):661–90. doi: 10.1016/s0950-3579(05)80121-9. [DOI] [PubMed] [Google Scholar]
- 7.Gillmore JD, Lovat LB, Persey MR, Pepys MB, Hawkins PN. Amyloid load and clinical outcome in AA amyloidosis in relation to circulating concentration of serum amyloid A protein. Lancet. 2001;358(9275):24–9. doi: 10.1016/S0140-6736(00)05252-1. [DOI] [PubMed] [Google Scholar]
- 8.Zemer D, Pras M, Sohar E, Modan M, Cabili S, Gafni J. Colchicine in the prevention and treatment of the amyloidosis of familial Mediterranean fever. N Engl J Med. 1986;314(16):1001–5. doi: 10.1056/NEJM198604173141601. [DOI] [PubMed] [Google Scholar]
- 9.Tan AU Jr, Cohen AH, Levine BS. Renal amyloidosis in a drug abuser. J Am Soc Nephrol. 1995;5(9):1653–8. doi: 10.1681/ASN.V591653. [DOI] [PubMed] [Google Scholar]


