Abstract
Context: Antiphospholipid syndrome (APS) is a systemic autoimmune disorder which commonly affects kidneys.
Evidence Acquisitions: Directory of Open Access Journals (DOAJ), Google Scholar, PubMed (NLM), LISTA (EBSCO) and Web of Science have been searched.
Results: There is sufficient epidemiological, clinical and histopathological evidence to show that antiphospholipid syndrome is a distinctive lesion caused by antiphospholipid antibodies in patients with different forms of antiphospholipid syndrome. It is now time to devise a classification for an accurate diagnosis and prognostication of the disease.
Conclusions: Now that the morphological lesions of APSN are sufficiently well characterized, it is prime time to devise a classification which is of diagnostic and prognostic utility in this disease.
Keywords: Antiphospholipid syndrome, APS-associated nephropathy, Systemic lupus erythematosus, Thrombotic microangiopathy
1. Context
Antiphospholipid syndrome (APS) is a systemic autoimmune disorder which commonly affects kidneys (1-3).
2. Evidence Acquisition
Directory of Open Access Journals (DOAJ) Google Scholar, PubMed (NLM), LISTA (EBSCO) and Web of Science were searched with key words relevant to antiphospholipid syndrome, antiphospholipid antibodies, APS-associated nephropathy and thrombotic microangiopathy.
3. Results
Twenty one research and review articles relevant to this topic directly or indirectly have been found. From the information given in these papers, the following aspects were drawn out.
3.1. Definition
The morphological features of renal involvement in APS were poorly recognized and probably underestimated till recent past (4). More recently, investigators have focused their attention on characterizing the typical morphological features of renal involvement in APS and the nephropathologists should be attentive of the lesions of APS-associated nephropathy (APSN) when they observe renal biopsies of patients with primary APS (PAPS), secondary APS (SAPS) in the setting of systemic lupus erythematosus (SLE) patients with positive antiphospholipid antibodies (aPLs), and catastrophic APS (CAPS) (5-16). Renal morphologic lesions in PAPS and SAPS are typically caused by thrombosis occurring at any location within the renal vasculature, leading to various outcomes, depending on the size, type and site of the vessel involved (4). With the frequent occurrence of thrombocytopenia and systemic hypertension in these patients often discouraging renal biopsy, the true incidence of kidney involvement in different forms of APS has not been fully known. Historically, it was thought that patients with APS have a higher risk of occurrence of biopsy-related complications (12).
3.2. Clinical features
Clinically, hypertension is one of the first major features of APSN. It is remarkably common in both PAPS and SAPS, and is considered to be a considerate sign of nephropathy in this disease (5-10). In the study conducted by Nochy et al., hypertension was present in 93% of their patients and in some cases was the only clinical sign indicative of nephropathy (7). In a small group of patients with SAPS due to SLE, Kleinknecht et al. showed that all of the patients had severe hypertension and renal insufficiency. Accordingly, the authors suggested that in patients with APS complicated by high blood pressure kidney involvement should be strongly considered and investigated (17). In addition, patients frequently present with acute and/or chronic kidney disease (CKD), and a low-grade proteinuria and/or hematuria (5-17).
3.3. Morphologic features of antiphospholipid syndrome -nephropathy
APSN denotes the renal parenchymal and vascular damage caused by intraluminal and intramural lesions in arterioles and/or interlobular arteries and in the glomeruli, in patients with aPLs (7,12-14). In APSN, the vascular lesions may be acute, termed thrombotic microangiopathy (TMA), and/or chronic, such as arteriosclerosis, fibrous intimal hyperplasia (FIH), and fibrous obliteration of arteries and arterioles, and also focal cortical atrophy (FCA). APSN has been described in patients with PAPS, CAPS, SLE-related APS and SLE/non-APS patients with aPLs (11,13,14). TMA is the best recognized and most characteristic lesion of APSN. The pathological features of TMA are characterized by fibrin thrombi in intra-renal vascular tree, especially arterioles, as well as the glomerular capillaries (Figure 1A-D). However, there is no inflammatory cell infiltration of the vessel wall or vascular immune deposits. During acute phase, swelling of endothelial cells leads to narrowing or occlusion of the capillary lumena, further aggravated by the fibrin thrombi containing fragmented blood cells. Ultimately, thrombi organize into fibro-cellular and fibrous vascular occlusions, which may be re-cannalized by endothelialized channels leading to plexiform aspect of intimal fibrosis (7,12,14). Indeed, repeated thrombosis/recanalization leads to splitting and multi-layering of the glomerular basement membrane (GBM) best seen as wrinkling and duplication, by silver stains on light microscopic (LM) examination. Other LM features comprise fibrin micro-thrombi in the capillary tufts and mesangiolysis. Chronic morphologic features, consisting of arteriosclerosis which typically is accompanied by intimal thickening of the arteries and arterioles primarily by myofibroblastic cellular proliferation and FIH, are associated with marked lumen reduction and resultant ischemia. FCA is a distinctive lesion and likely represents the morphological renal analogue of multiple cortical infarcts seen on imaging studies (7,12). It involves the area under the kidney capsule, as foci or triangles with sharp margins with the rest of the normal cortex, associated with depression of the contour of the kidney capsule. In the area of FCA, the structures of kidney tissue are transformed in a manner which is very typical of APSN. In this region, there is dense interstitial fibrosis and the tubules become atrophic and packed with eosinophilic casts, resembling thyroid tissue, and named as ‘tubular thyroidization’. The glomeruli may become voluminous or small and sclerotic. The voluminous glomeruli, almost lacking tufts, are known as “pseudocystic glomeruli”. The arterioles are occluded by fibrin thrombi or often by fibrous tissue, depending on the stage of the disease (Figure 2A-D ). Hence, the disease of small-vessel, vaso-occlusive nephropathy, designated as APSN, includes both the acute thrombotic lesions and chronic vascular lesions like FIH of interlobular arteries, re-canalizing thrombi in arteries and arterioles, fibrous obliteration of the same vessels and FCA. More recent studies have demonstrated that the spectrum of APSN is expanding and includes various lesions of glomerulonephritis (GN) (15,18). Regarding treatment and prognosis, most studies have shown a favorable response to anticoagulation therapy with preservation of renal function over short and medium-term (4). Very few long-term longitudinal studies are available to address the long-term prognosis of the condition.
Figure 1.
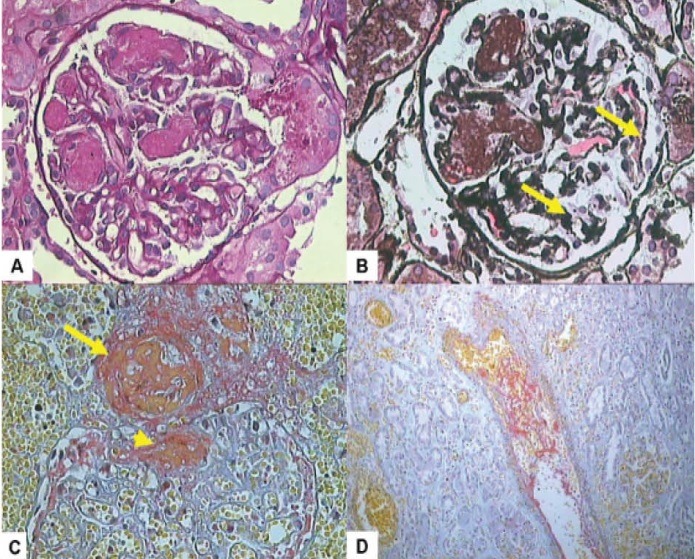
The main morphological changes seen in acute antiphospholipid syndrome nephropathy (APSN). A. High-power view of a glomerulus showing fibrin thrombi occluding many glomerular lumena. This lesion is termed as thrombotic microangiopathy (TMA). (H & E, ×200). B. Another glomerulus showing fibrin thrombi filling some of the capillary lumina. The remaining glomerular capillaries show wrinkling of basement membranes (arrows) suggestive of acute ischemic changes. (Silver stain, ×200). C. One arteriole shows a fresh thrombus in the lumen (arrow). The glomerulus also shows thrombi in arterioles near the hilar pole (arrow head). (MSB stain, ×200). D. One interlobular artery shows a partially occluding fresh thrombus with entrapped blood cells in the lumen. (MSB stain, ×200).
Figure 2.
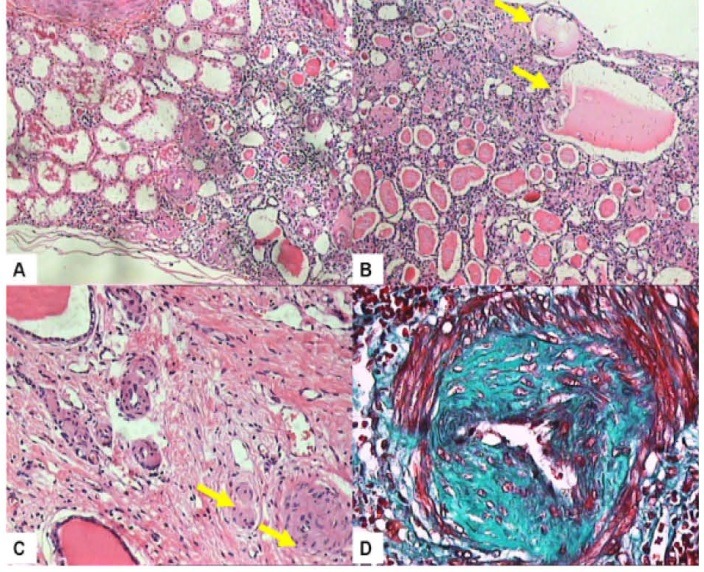
The typical morphological changes seen in chronic antiphospholipid syndrome nephropathy (APSN). A. Subcapsular focal cortical atrophy (FCA) sharply separated from the normal parenchyma on the right. Renal capsule is located on the lower aspect of the section (H & E, ×200). B. This section shows the typical features of FCA which include pseudocystic glomeruli, tubular thyroidization, tubular atrophy and interstitial fibrosis. Many glomeruli in this field are globally sclerosed and clustered closely together (H & E, ×200). C. Fibrous occlusion of an arteriole (arrow) and marked reduction of the lumen of one artery (arrow). (H & E, ×200). D. One interlobular artery shows cellular fibrous intimal hyperplasia and stellate shaped lumen (Masson’s trichrome stain, ×200).
Now that the morphological lesions of APSN are sufficiently well characterized, it is prime time to devise a classification which is of diagnostic and prognostic utility in this disease. This will not only draw the attention of nephropathologists and nephrologists to the disease but will also help increase awareness of all concerned health care providers for its better and early recognition and proper management. This is more so in the setting of lupus nephritis (LN), where there is greater possibility to overlook the lesions of APSN in the face of lupus glomerular and vascular lesions. Thus, in case of concurrence of LN and APSN, this classification can be used together with LN classification, to avoid under-diagnosis of APSN. It is however, very crucial to rule out other causes of vasculopathy before assigning these lesions to APS-associated aPLs. In addition, and particularly if the above call is premature, we think that it will be of immense help, if an international registry for APSN is established along similar lines as for catastrophic APS (CAPS). This will help better understand the full spectrum of the epidemiological, clinical and histopathological features of this disease and open up avenues for international collaboration and consensus classification in future. In the meantime, we also suggest to include APSN as one of the clinical criteria for a definite diagnosis of APS. It is currently considered an associated or non-criteria feature (19,20).
In accomplishing the above mentioned task, several points need to be kept in mind. What will be the minimum number of histopathological features to establish the diagnosis of APSN apart from the serological and clinical criteria? Which type of renal biopsy, surgical or percutaneous, to obtain to adequately address the diagnostic and prognostic criteria? What will be the adequacy criteria for the renal biopsy for proper classification of the disease? Which of the lesions will be most important for diagnostic or prognostic determinations? One possible approach may be to categorize all the lesions involving the glomeruli, the vessels and tubulointerstitial component, according to their activity or chronicity, severity and extent in a manner similar to that employed in the Oxford classification of IgA nephropathy (IgAN), rather than to create artificial classes and try to fit the lesions into those classes (21). Strict definitions of the lesions should be devised and tested for interobserver reproducibility before their incorporation into the final classification. All lesions may be classified as acute, chronic, and acute on chronic, with variable degree of severity. At least a beginning of the process can be initiated which will evolve in future as more evidence accumulates.
4. Conclusions
There is sufficient epidemiological, clinical and histopathological evidence to show that APSN is a distinctive lesion caused by aPLs in patients with different forms of APS. It is now time to devise a classification for an accurate diagnosis and prognostication of the disease.
Authors’ contributions
MM wrote the manuscript. HN made substantial contributions to conception and design of the manuscript. MM prepared the final manuscript.
Conflict of interests
The authors declare no conflict of interests.
Funding/Support
None declared.
Implication for health policy/practice/research/medical education:
There is sufficient epidemiological, clinical and histopathological evidence to show that antiphospholipid syndrome is a distinctive lesion caused by antiphospholipid antibodies in patients with different forms of antiphospholipid syndrome. It is now time to devise a classification for an accurate diagnosis and prognostication of the disease.
Please cite this paper as: Mubarak M, Nasri H.What nephrolopathologists need to know about antiphospholipid syndrome-associated nephropathy: Is it time for formulating a classification for renal morphologic lesions? J Nephropathol. 2014; 3(1): 4-8. DOI: 10.12860/jnp.2014.02
References
- 1.Fischer MJ, Rauch J, Levine JS. The antiphospholipid syndrome. Semin Nephrol. 2007;27(1):35–46. doi: 10.1016/j.semnephrol.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alchi B, Griffiths M, Jayne D. What nephrologists need to know about antiphospholipid syndrome? Nephrol Dial Transplant. 2010;25(10):3147–54. doi: 10.1093/ndt/gfq356. [DOI] [PubMed] [Google Scholar]
- 3.Cervera R, Piette JC, Font J, Khamashta MA, Shoenfeld Y, Camps MT. et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46(4):1019–1027. doi: 10.1002/art.10187. [DOI] [PubMed] [Google Scholar]
- 4.Uthman AT, Khamashta M. Antiphospholipid syndrome and the kidneys. Semin Arthritis Rheum. 2006;35(6):360–7. doi: 10.1016/j.semarthrit.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Amigo MC, Garcia-Torres R, Robles M, Bochicchio T, Reyes PA. Renal involvement in primary antiphospholipid syndrome. J Rheumatol. 1992;19(8):1181–5. [PubMed] [Google Scholar]
- 6.Cacoub P, Wechsler B, Piette JC, Beaufils H, Herreman G, Bletry O. et al. Malignant hypertension in antiphospholipid syndrome without overt lupus nephritis. Clin Exp Rheumatol. 1993;11(5):479–85. [PubMed] [Google Scholar]
- 7.Nochy D, Daugas E, Droz D, Beaufils H, Grünfeld JP, Piette JC. et al. The intrarenal vascular lesions associated with primary antiphospholipid syndrome. J Am Soc Nephrol. 1999;10(3):507–18. doi: 10.1681/ASN.V103507. [DOI] [PubMed] [Google Scholar]
- 8.Cheunsuchon B, Rungkaew P, Chawanasuntorapoj R, Pattaragarn A, Parichatikanond P. Prevalence and clinicopathologic findings of antiphospholipid syndrome nephropathy in Thai systemic lupus erythematosus patients who underwent renal biopsies. Nephrology. 2007;12(5):474–80. doi: 10.1111/j.1440-1797.2007.00792.x. [DOI] [PubMed] [Google Scholar]
- 9.Griffiths MH, Papadaki L, Neild GH. The renal pathology of primary antiphospholipid syndrome: a distinctive form of endothelial injury. Q J Med. 2000;93(7):457–67. doi: 10.1093/qjmed/93.7.457. [DOI] [PubMed] [Google Scholar]
- 10.D’Acruz DP. Renal manifestations of the antiphospholipid syndrome. Lupus. 2005;14(1):45–48. doi: 10.1191/0961203305lu2058oa. [DOI] [PubMed] [Google Scholar]
- 11.Moss KE, Isenberg DA. Comparison of renal disease severity and outcome in patients with primary antiphospholipid syndrome, antiphospholipid syndrome secondary to systemic lupus erythematosus (SLE) and SLE alone. Rheumatology (Oxford) 2001;40(8):863–7. doi: 10.1093/rheumatology/40.8.863. [DOI] [PubMed] [Google Scholar]
- 12.Tektonidou MG. Renal involvement in the antiphospholipid syndrome (APS)-APS nephropathy. Clin Rev Allergy Immunol. 2009;36(2-3):131–40. doi: 10.1007/s12016-008-8112-z. [DOI] [PubMed] [Google Scholar]
- 13.Tektonidou MG, Sotsiou F, Moutsopoulos HM. Antiphospholipid syndrome (APS) nephropathy in catastrophic, primary, and systemic lupus erythematosus-related APS. J Rheumatol. 2008;35(10):1983–8. [PubMed] [Google Scholar]
- 14.Daugas E, Nochy D, Huong DL, Duhaut P, Beaufils H, Caudwell V. et al. Antiphospholipid syndrome nephropathy in systemic lupus erythematosus. J Am Soc Nephrol. 2002;13(1):42–52. doi: 10.1681/ASN.V13142. [DOI] [PubMed] [Google Scholar]
- 15.Fakhouri F, Noël LH, Zuber J, Beaufils H, Martinez F, Lebon P. et al. The expanding spectrum of renal diseases associated with antiphospholipid syndrome. Am J Kidney Dis. 2003;41(6):1205–11. doi: 10.1016/s0272-6386(03)00352-4. [DOI] [PubMed] [Google Scholar]
- 16.Gigante A, Gasperini ML, Cianci R, Barbano B, Giannakakis K, Di Donato D. et al. Antiphospholipid antibodies and renal involvement. Am J Nephrol. 2009;30(5):405–12. doi: 10.1159/000235941. [DOI] [PubMed] [Google Scholar]
- 17.Kleinknecht D, Bobrie G, Meyer O, Noel LH, Callard P, Ramdane M. Recurrent thrombosis and renal vascular disease in patients with a lupus anticoagulant. Nephrol Dial Transplant. 1989;4(10):854–8. doi: 10.1093/ndt/4.10.854. [DOI] [PubMed] [Google Scholar]
- 18.Rossinol T, Cervera R, López C, Sole M, Ramos-Casals M, Font J. Antiphospholipid syndrome and minimal change nephropathy. Lupus. 2006;15(8):547. doi: 10.1191/0961203306lu2343xx. [DOI] [PubMed] [Google Scholar]
- 19.Asherson RA, Klumb EM. Antiphospholipid syndrome nephropathy in different scenarios. J Rheumatol. 2008;35(10):1909–11. [PubMed] [Google Scholar]
- 20.Myakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R. et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 21.Mubarak M. The Oxford classification of IgA nephropathy: a role model for classifying other renal glomerular diseases. Saudi J Kidney Dis Transplant. 2011;22(5):897–900. [PubMed] [Google Scholar]


