Abstract
Since its discovery in the 19th century, the complement system has developed into a clinically significant entity. The complement system has been implicated in a variety of clinical conditions, from autoimmune diseases to ischemia–reperfusion injury in transplantation. This article charts the historical progress of our understanding of the complement system and provides a synopsis on the activation pathways and its inherent regulators.
Keywords: complement activation, complement cascade, complement history, complement inhibitors, complement system
Introduction
The complement system is an integral part of the innate immune response and acts as a bridge between innate and acquired immunity. It consists of a series of proteins that are mostly (although not exclusively) synthesised in the liver, and exist in the plasma and on cell surfaces as inactive precursors (zymogens). Complement mediates responses to inflammatory triggers through a co-ordinated sequential enzyme cascade leading to clearance of foreign cells through pathogen recognition, opsonisation and lysis [1]. Complement also possesses anti-inflammatory functions: it binds to immune complexes and apoptotic cells, and assists in their removal from the circulation and damaged tissues [2, 3]. The complement proteins are activated by, and work with IgG and IgM antibodies, hence the name ‘complement’. Many complement proteins exist in a ‘precursor’ form and are activated at the site of inflammation. The complement system is more complex than many enzymatic cascades as it requires the formation of sequential non-covalently associated activated protein fragments. These in turn become convertases and cleave components for the next enzymatic complex in the cascade, and the rapid dissociation of these complexes (and loss of enzymatic activity) forms an integral part of the elegant regulation of complement activity.
History
In the late 19th century, the focus of scientific research was on the human body’s defence against microbial infection. The ‘Theory of Metchnikoff’ proposed that phagocytes in the blood were capable of ingesting and destroying the invading bacteria, thus providing the basis of innate cellular immunity. This phagocytic theory was challenged by many pathologists initially on the basis that the phagocytic leucocytes were ‘truly causal in the successful response to infection’ [4]. Buchner and colleagues (1891) found a heat labile factor in blood that was capable of killing bacteria, and named it ‘alexin’ (in Greek, means ‘to ward off’) [5, 6]. Jules Bordet supported this ‘humoral theory’ (immunity conferred due to antitoxic and bactericidal substances in body fluids) by demonstrating that immune lysis required the presence of two factors: a heat-labile lytic factor (similar to alexin) and a heat-stable factor, which he termed sensitiser (which we now know was antibody) [7]. Paul Ehrlich described the side-chain theory of antibody formation, especially the mechanisms of antibody neutralisation by toxins that induced bacterial lysis with the help of complement (which has replaced the historical term alexin). According to his theory, the immune cells contained receptors that could recognise antigens, and following immunisation, these receptors multiplied and were shed into the circulation as ‘amboceptors’ (now called antibodies). These antibodies attached not only to specific antigens but also to a heat-labile antimicrobial component called ‘complement’ [8, 9]. Ehrlich’s theory proposed that the antibody and complement combined to form a complex enzyme capable of attacking and killing cells and micro-organisms. In the ensuing years, this concept had a protagonist in the form of Bordet who argued that the antigen-antibody union was reversible, contradicting Ehrlich’s view that the antigen-antibody union was a firm and based on stereo chemical specificity [10]. Ehrlich’s concept emphasised the presence of multiple antigens and complements in the serum, while Bordet’s view revolved around a ‘single complement’ component that bound non-specifically to the antigen.
The concept that complement was not a single substance was provided by Ferrata and Brand, who demonstrated separation of complement into two fractions: midpiece (renamed as C1) and endpiece (renamed as C2) [11, 12]. They observed that the bactericidal activity of complement was only possible when both fragments were present. Von Dungern described a phenomenon whereby complement was inactivated by yeast cells, and a similar inactivation phenomenon was observed using cobra venom by Braun and Omorokow (both now known to activate the alternative activation pathway) [13, 14]. Coca demonstrated that the inactivation of the complement by yeast cells was due to the removal of a heat labile component, and yeast-treated complement activity could be reconstituted by the addition of normal guinea-pig serum which had been inactivated by heating for 30 minutes at 56 °C [15]. This heat-stable component of the complement system was referred to as C3. Inactivation of another complement by ammonia led to isolation and characterisation of a new component referred to as C4 [16]. The complement components C1 to C4 were initially assigned names in order of their discovery, and not according to their role in the activation sequence.
Improved electrophoretic and ultracentrifugation techniques over the next few decades enabled characterisation of complement as ‘proteins’, contrary to the prevailing opinion that complement was serum lipoid/soap complex [7]. C3, originally described in the 1920s, was now shown to be made of six proteins and was initially termed C’3a–C’3f in order of their discovery. In the same era, other studies focused on the reaction sequence of complement components. Ueno and Mayer showed that it was possible to ‘build up’ the complement system by using purified components [7]. Mayer [17, 18] proposed the ‘one-hit’ theory that suggested a single ‘complement hit’ could cause lysis of an erythrocyte. This was later supported by Inoue et al. who showed that the complement could kill a bacterium by a single hit [19]. Using a reconstitution assay, Mayer and colleagues added partially purified components to antibody-sensitised sheep erythrocytes to unravel the reaction sequence of the classical pathway [20, 21]. Work by eminent scientists like Nilsson, Muller-Eberhard and colleagues led to isolation and characterisation of the various components of the complement system: C4 [22], C5 [23], C6 and C7 [24], C8 [25] and C9 [26]. Nelson and colleagues were able to isolate complement components in animals as well [27, 28]. These researchers determined the sequence of component activation for what we now refer to as the classical activation pathway as: C’1 bound first followed sequentially by C’4, C’2, C’3a, C’3b, C’3e, C’3f, C’3c and C’3d. In 1968, the WHO Committee modified these nomenclatures and the new terminology being, in order of activation C1, C4, C2, C3, C5, C6, C7, C8 and C9.
Pathways of activation
There are three known pathways for complement activation: Classical, Alternative and Lectin pathway.
Classical pathway
The classical pathway is initiated by IgM or IgG antigen/antibody complexes binding to C1q (first protein of the cascade) leading to activation of C1r, which in turn cleaves C1s. This in turn activates the serine proteases that lead to cleaving of C4 and C2, leading to formation of C4b2a (C3 convertase), which in turn cleaves C3 into C3a and C3b [29]. While C3a acts as a recruiter of inflammatory cells (anaphylatoxin), C3b binds to the C4b2a complex to form C5 convertase (C4b2a3b). The C5 convertase initiates the formation of the Membrane Attack Complex (MAC), that inserts into membrane creating functional pores in bacterial membranes leading to its lysis [30]. The classical pathway can also be activated by other danger signals like C-reactive protein, viral proteins, polyanions, apoptotic cells and amyloid, thus providing evidence that classical pathway could be activated independent of antibodies [31–34].
Alternative pathway
Fifty years after the discovery of the classical activation pathway, Pillemer et al. [35, 36] proposed a highly controversial alternative activation pathway. Initially, this was rejected by the scientific community and only substantiated and accepted more than a decade later. Pillemer’s hypothesis was based on observations that the complement system could be activated by direct binding of bacteria and yeast independent of antibody interaction [37]. It was originally named the ‘properdin pathway’ and is now known as the alternative pathway [33]. The alternative pathway is not so much an activation pathway, as it is a failure to regulate the low level continuous formation of a soluble C3 convertase. The internal thioester bond of C3 is highly reactive and undergoes spontaneous hydrolysis resulting in a molecule known as C3 (H2O) which resembles C3b. This can then bind to factor B, and be processed into a short lived soluble C3 convertase that can generate more C3b. If this C3b binds to a nearby surface that is incapable of inactivating it (such as bacteria/yeast cells or damaged host tissues), this then leads to amplification of the alternative pathway [38–40]. The presence of complement regulators in healthy cells ensures the spontaneous hydrolysis of C3 is kept in check. C3 activation takes place when C3b binds to factor B and is then cleaved by factor D (a process which is stabilised by magnesium ions and properdin) [41]. The enzymatic action of factor D acts as the rate limiting step of the alternative pathway and cleaves factor B, the larger fragment of which remains bound to C3b to form the alternative pathway C3 convertase–C3bBb [29, 42]. C3b is able to create new C3 convertase in the presence of Factors B and D, thus acting as an ‘amplification loop’ for other pathways, as well as the alternative pathway [33]. The alternative pathway omits the components C1, C2 and C4.
Lectin pathway
Forty years after the proposal of the alternative pathway, the MBL (mannose-binding lectin)/MASP (MBL-associated serine protease) pathway was discovered. This pathway was characterised by using proteins isolated from rabbit liver and serum, but its function remained unclear initially [43, 44]. Two forms of MBL (MBL-A and -C) are present in rodents compared to a single form in the humans. Studies linking the deficiency of MBL protein to immunodeficiencies in children led to its recognition as an important activator of the complement system [45, 46]. The initiating molecules for this pathway are collectins (MBL and ficolin), which are multimeric lectin complexes. These bind to specific carbohydrate patterns uncommon in the host, leading to activation of the pathway through enzymatic activity of MASP [41]. There are structural similarities shared between MBL and C1 complexes (MBL- with C1q-associated serine proteases, MASP-1 and MASP-2 with C1r and C1s, respectively), leading to the belief that complement activation by MBL and C1 complexes are similar [47]. MASP-2 cleaves C4 and C2 to form C3 convertase, while MASP-1 may cleave C3 directly bypassing the C4b2a complex, albeit at a very slow rate [48, 49]. Another serine protease, MASP-3 was shown to down-regulate the C4 and C2 cleaving activity of MASP-2 [50]. Following the initial characterisation of MBL, 3 other lectins (known as ficolins) have been shown to interact with MASP: ficolin-1 (or M-ficolin), ficolin-2 (or L-ficolin) and ficolin-3 (or H-ficolin or Hakata antigen). The ficolins activate the lectin pathway by forming active complexes with MASP [51, 52]. More recently, a new C-type lectin (CL-11) was shown to interact with MASP-1 and/or MASP-3 and could activate the lectin pathway [53].
Other activators of the complement system
Various serine proteases belonging to the coagulation system have also been shown to activate the complement cascade independent of the established pathways. In vitro findings suggested that the coagulation factors FXa, FXIa and plasmin can cleave both C5 and C3, leading to generation of anaphylatoxins C5a and C3a [54]. Studies have documented FVIII and von Willebrand factor to possess lectin activity [55]. Vice versa, complement factors are also known to interact with the coagulation system. C1 inhibitor was shown to block the endogenous coagulation pathway [56], while C5a was shown to induce tissue factor (membrane glycoprotein that serves as a cofactor for blood coagulation factor VIIa) activity on endothelial cells [57]. Individual cells have also been implicated in activating certain elements of complement pathway. Huber-Lang et al. showed that phagocytic cells, especially lung macrophages could generate C5a from C5 independent of the plasma complement system using cell bound serine proteases [58]. C-reactive protein is an acute phase reactant that can activate the classical pathway of the complement system, and its role in the complement led ischemia–reperfusion injury (IRI) has been shown in intestinal and myocardial animal IRI models [34, 59]. Similarly, cross-talk between complement and toll-like receptors has shown to be possible due to mitogen activated protein kinases in renal IRI setting [60]. Cross-talk between complement system and other systems will exist, and future research will be aimed at evaluating these ‘communicators’ between systems.
Complement cascade
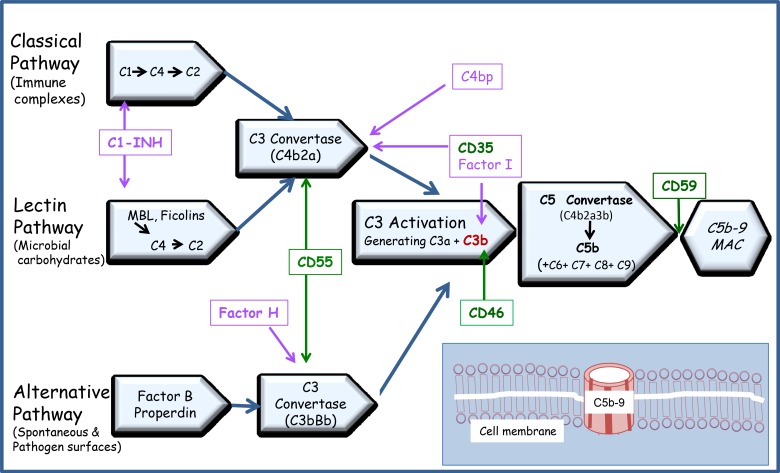
The principal function of the complement system is protection of the host from infection/inflammation by recruiting (chemotaxis) and enhancing phagocytosis by innate immune cells (opsonisation), leading to lysis of the target cells. All three pathways lead to the generation of C3 convertase that cleaves the C3 protein into C3a and C3b. While C3a acts as an anaphylatoxin, C3b covalently binds to the activating surface and participates in the self-activation loop of complement activation via the alternate pathway. C3b also associates with C3 convertases (C4b2a or C3bBb) to form the C5 convertase, which cleaves C5 complement into C5a and C5b [61]. Interaction of C5b with C6, C7, C8 and C9 leads to formation of C5b–9/MAC, a multimolecular structure that inserts into the membrane creating a functional pore leading to cell lysis [30]. MAC can cause lysis of some cells (e.g. erythrocytes) with a single hit, but some nucleated cells required multiple hits, or rather, multiple channel formation to cause cell lysis [62, 63]. However, studies have shown that when the number of channels assembled on the cells is limited, sublytic C5b–9 can activate transcription factors and signal transduction, leading to inhibition of apoptosis and cell homeostasis [64, 65]. The complement cascade with the inherent inhibitors is shown in Figure 1.
Fig. 1.
Pathways of complement activation: classical, alternative and lectin pathway: IgM or IgG antigen/antibody complexes binding to C1q, the first protein of the cascade, initiates the classical pathway. The alternative pathway is not so much an activation pathway, as it is a failure to regulate the low level continuous formation of a soluble C3 convertase. The third pathway is known as MBL (Mannose-binding lectin)/MASP (MBL associated Serine Protease) pathway. The initiating molecules for the MBL pathway are multimeric lectin complexes that bind to specific carbohydrate patterns uncommon in the host, leading to activation of the pathway through enzymatic activity of MASP. The sites of action of the membrane bound complement regulators–CD35, CD46, CD55 & CD59 (green boxes) and the fluid phase regulators – C1-INH, Factor H, Factor I and C4bp (violet boxes) are represented with arrows. Insert: Membrane Attack Complex (MAC). The interaction of C5b with C6, C7, C8 and C9 leads to formation of C5b–9 or Membrane Attack Complex (MAC), a multimolecular structure that inserts into the membrane creating a functional pore leading to cell lysis
The anaphylatoxins (C3a and C5a) are key players in the recruitment of inflammatory cells and release of mediators that amplify the inflammatory response. C5a is probably the principal anaphylatoxin mediating inflammation. C5a binds to C5a receptor (C5aR or CD88) that is widely present on inflammatory and non-inflammatory cells [66, 67]. Apart from recruiting the neutrophils, C5a also increases neutrophil adhesiveness and aggregation. C5a causes secretion of pro-inflammatory cytokines and lysosomal enzymes from the macrophages and monocytes, thus leading to chemotaxis [29, 68, 69]. C5a also up-regulates adhesion molecules such as α-integrin and β 2-integrin; in particular, Mac-1, in polymorphonuclear leukocytes [70, 71]. C5a was shown to be an important inflammatory mediator for the early adhesive interactions between neutrophils and endothelial cells in the acute inflammatory response [71]. It is responsible for up-regulation of vascular adhesion molecules such as P-selectin, E-selectin, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) [29, 72].
C3a does not act as a chemoattractant for neutrophils, but aids migration of eosinophils and mast cells [73, 74]. C3a and C5a also act on their receptors expressed on innate immune cells such as dendritic cells, thus playing a role in initiating and regulating T cell responses [75]. In the IRI setting, MAC has been shown to mediate IR injury, and its inhibition was shown to attenuate the IRI effect [76, 77].
Inherent regulation of pathways
To prevent inadvertent injury by activated complement, the host tissues have developed intricate and elaborate mechanisms in the form of soluble and membrane bound complement regulators that inhibit complement activation. The two main regulation mechanisms are: decay-acceleration activity (DAA) which increases the rate of dissociation of (C4b2a and C3bBb) C3 convertases, and factor I cofactor activity (CA), which results in the factor I-mediated cleavage of covalently bound C3b and C4b into inactive fragments incapable of reforming the C3 convertases [78, 79]. The pathways are regulated by both membrane-bound and fluid phase complement regulators that keep the complement system in check.
Membrane bound complement regulators
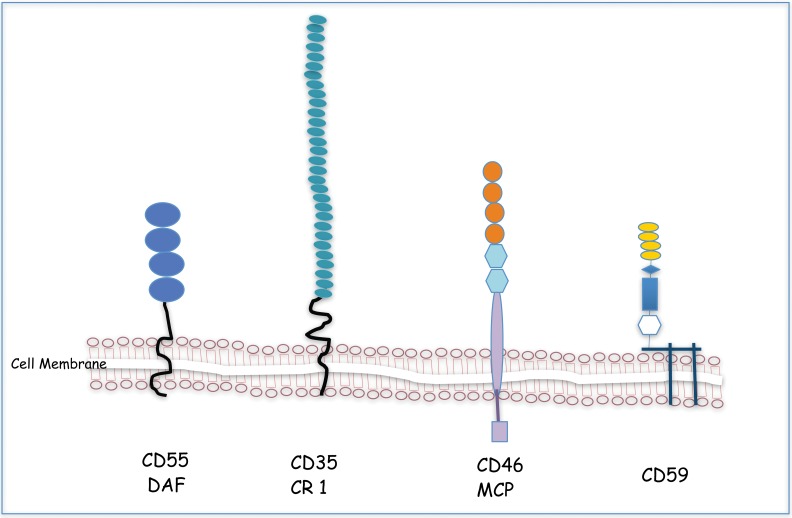
The membrane bound regulators–DAF, CR1 and MCP belong to a gene super family called as ‘regulators of complement activation’ (RCA)/Complement control proteins (CCP) and share a common structural motif called short consensus repeat (SCR). The SCR structure consists of around 60 amino acids held together by two disulfide bridges formed by cysteine residues [80]. The structural moiety of the membrane bound complement regulators are depicted in Figure 2.
Fig. 2.
Membrane Bound Complement Regulators: DAF, CR1 and MCP belong to a gene super family called as ‘regulators of complement activation’ (RCA)/complement control proteins (CCP) and share a common structural motif called short consensus repeat (SCR). The SCR structure (circles) consists of around 60 amino acids held together by two disulfide bridges formed by cysteine residues. CD59 is a GPI-anchored membrane complement that is expressed on almost all cells in the body. CD59 is the only well-characterised membrane inhibitor acting at the terminal step and prevents the assembly of the MAC
CD35 {complement receptor 1 (CR1)}: classical, lectin and alternative pathway
CD35 is a transmembrane glycoprotein that facilitates the decay of C3/C5 convertase in both the classical and alternate pathways and acts as a co-factor for factor I in the degradation of C3b and C4b [81]. Human CR1 is found on B cells, follicular dendritic cells, erythrocytes, polymorphonuclear cells, phagocytic macrophages and on podocytes in the glomerular of the kidney [82]. Expression of CD35 on erythrocytes is believed to be crucial in handling circulating immune complexes and abating the development of autoimmunity.
CD46 {membrane cofactor protein (MCP)}: classical, lectin and alternative pathway
CD46 acts as a cofactor for factor I mediated cleavage of C3b and C4b. It is widely expressed in humans apart from the erythrocytes, while in the rodents, it is expressed only in the testis [83]. By regulating the production of interferon (IFN)-γ and interleukin (IL)-10 in the T helper cells, it is involved in the down-modulation of adaptive T helper type 1 immune responses [84]. Deficiency of CD46 is a predisposing factor for numerous disease conditions arising from complement-mediated ‘self-attack’.
CD55 {decay accelerating factor (DAF)}: classical and alternative pathway
CD55 is a glycosyl-phosphatidyl-inositol (GPI) anchored membrane protein that is widely expressed on vascular and non-vascular tissue cells. The main role of CD55 is the inhibition and acceleration of the decay of classical and alternative pathways C3 convertase [85]. Apart from complement regulation, human DAF is known to act as a receptor for infection by certain viruses (echovirus and coxsackie B virus) and serves as a ligand for activation-associated leukocyte antigen CD97 [82]. Like rodent CD46, rodent CD55 is limited in its tissue expression.
CD59 (protectin): membrane attack complex
CD59 is a GPI-anchored membrane protein that is expressed on almost all cells in the body [86]. CD59 is the only well-characterised membrane inhibitor acting at the terminal step, and prevents the assembly of the MAC by inhibiting the C5b-8 catalysed insertion of C9 into the lipid bilayer [87].
CrrY {Complement receptor 1-related gene/protein Y}
CrrY is a transmembrane protein specific to rodents and is widely expressed on rat and mouse cells to compensate for the lack of rodent CD55 and CD46 expression. CrrY possesses both DAA and CA properties and mimics the activities of the human DAF and MCP which regulate C3 deposition on host cells [88].
Fluid phase or ‘soluble’ regulators: C1-Inhibitor (C1-INH): classical and lectin pathway
In the fluid phase, the best-known regulatory protein is C1-Inhibitor (C1-INH), which is synthesised in the liver and by monocytes. It forms an irreversible complex with the serine proteases C1r and C1s, typical of serpin regulation, and inactivates them. This leads to the disassociation of C1r and C1s from C1q in the complex. C1-INH can also bind to MASP-1 and MASP-2 and inactivate them leading to disruption of the lectin pathway [89, 90]. Under physiologic conditions, activated C1 has a half-life of only 13 seconds in the presence of C1-INH (regulates nonspecific complement activation) [91].
C1-INH inhibits other serine proteases, like kallikrein, along with coagulation factors (XIa, XIIa and plasmin), thus playing a role in coagulation regulation [92]. Absence or low levels of C1-INH results in conditions like hereditary angioneurotic oedema where spontaneous activation of C1 and kallikrein leads to the manifestation of symptoms [93]. Various animal IRI models have shown that C1-INH can protect liver, intestine, heart and brain tissue from ischemia–reperfusion damage [94].
Factor I: classical, lectin and alternative pathway
Factor I cleaves C3b and C4b to form C3 and C4 fragments (iC3b, C3c, C3dg and C4c and C4d, respectively), thus blocking the formation of C3 and C5 convertase enzymes [89]. The cofactors supporting factor I cleavage are factor H, CD35, CD46 and C4b-binding protein [95]. Factor I is secreted by cells such as hepatocytes, macrophages, lymphocytes, endothelial cells and fibroblasts. The outcome after renal transplantation is poor in patients known to have either a complement factor H or complement factor I mutation, with approximately 80% of patients losing the graft to recurrent disease within 2 years [96]. Mutations in factor I are linked with occurrence of atypical hemolytic uremic syndrome (HUS) [97] in human and increases the susceptibility to pyogenic infections like meningitis and upper respiratory tract infections [98].
Factor H: alternative and classical pathway
Factor H possesses multiple binding sites for C3b and accelerates the decay of the alternative C3 convertase through ‘competitive binding’ for factor B [99]. It also facilitates the cleavage of C3b by supporting factor I activity. It plays an essential role in controlling the alternative pathway in blood and on cell surfaces. Impaired recognition of factor H by host cell surfaces due to mutations and polymorphisms can lead to complement-mediated tissue damage and disease [100]. It is mainly synthesised in the liver, with minimal contributions from fibroblasts and endothelial cells. Genetic changes in factor H are linked to clinical conditions like HUS, membranoproliferative glomerulonephritis (dense deposit disease) and age-related macular degeneration [101].
C4bp (C4b-binding protein): classical and lectin pathway
C4bp is a regulator of the classical and lectin complement pathways. It binds to C4b and accelerates the decay of the C3 convertase [102, 103]. It also acts as a cofactor for the cleavage of C4b by factor I. It is predominantly synthesised in the liver and to a lesser extent by activated monocytes. C4bp has a complex structure, mainly composed of alpha-chains with a single copy of a beta-chain. C4bp possesses binding sites for heparin and C-reactive protein as well [104]. C4bp is up regulated in certain autoimmune diseases like lupus, but true deficiency state associated clinical conditions are yet to be reported.
Carboxypeptidase N: anaphylatoxin inactivator
Carboxypeptidase N was found to abolish the activity of the anaphylatoxins C3a and C5a and also those derived from bradykinin [105]. It is synthesised in the liver and cleaves carboxy-terminal arginines and lysines from peptides (such as complement anaphylatoxins, kinins and creatine kinase MM-skeletal muscle) found in the bloodstream [106]. Removal of the terminal arginine from C3a and C5a results in formation of C3a (desArg) and C5a (desArg), both of which have markedly lower ability to signal through receptor binding. Carboxypeptidase N plays an important role in protecting the body from excessive build up of potentially deleterious peptides that can act as local autocrine or paracrine hormones [107].
Conclusion
As evident by the historical progress, our understanding of the complement system is expanding. The complement system has been implicated in a variety of conditions: autoimmune diseases, sepsis, transplantation, ischemia-reperfusion injuries, traumatic brain injury, infections and bone biology [33]. Target areas for complement immunomodulation include blocking the activation pathways and developing specific complement inhibitors. Presently, rodent models dominate the complement research field, but whether findings from animal models can be potentially translated into human clinical application remains to be seen.
Contributor Information
P. N. Nesargikar, 1Cardiff Transplant Unit, University Hospital of Wales, Cardiff, UK.
B. Spiller, 2Institute of Molecular and Experimental Medicine, School of Medicine, Cardiff University, Cardiff, UK.
R. Chavez, 1Cardiff Transplant Unit, University Hospital of Wales, Cardiff, UK.
References
- 1.Schifferli JA, Ng YC, Peters DK. The role of complement and its receptor in the elimination of immune complexes. N Engl J Med. 1986 Aug 21;315(8):488–495. doi: 10.1056/NEJM198608213150805. [DOI] [PubMed] [Google Scholar]
- 2.Davies KA, Schifferli JA, Walport MJ. Complement deficiency and immune complex disease. Springer Semin Immunopathol. 1994;15(4):397–416. doi: 10.1007/BF01837367. [DOI] [PubMed] [Google Scholar]
- 3.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998 Dec 21;188(12):2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tauber AI, Chernyak L. The birth of immunology. II. Metchnikoff and his critics. Cell Immunol. 1989 Jul;121(2):447–473. doi: 10.1016/0008-8749(89)90043-9. [DOI] [PubMed] [Google Scholar]
- 5.Buchner H. Zur Nomenklatur der schutzenden Eiweisskorper. Centr Bakteriol Parasitenk. 1891;10:699–701. [Google Scholar]
- 6.Skarnes RC, Watson DW. Antimicrobial factors of normal tissues and fluids. Bacteriol Rev. 1957 Dec;21(4):273–294. doi: 10.1128/br.21.4.273-294.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan BP. Complement: Clinical Aspects and Relevance to Disease. UK: Academic Press; 1990. [Google Scholar]
- 8.Kaufmann SH. Immunology's foundation: the 100-year anniversary of the Nobel Prize to Paul Ehrlich and Elie Metchnikoff. Nat Immunol. 2008 Jul;9(7):705–712. doi: 10.1038/ni0708-705. [DOI] [PubMed] [Google Scholar]
- 9.Ehrlich P, et al. Zur Theorie der Lysenwirkung. Berlin Klin Woch. 1899;36:6. [in German] [Google Scholar]
- 10.Witesbsky E. Ehrlich's side-chain theory in the light of present immunology. Ann N Y Acad Sci. 1954 Sep;59(2):168–181. doi: 10.1111/j.1749-6632.1954.tb45929.x. [DOI] [PubMed] [Google Scholar]
- 11.Ferrata A. Berlin Klin Woch. 1907;44:366. [Google Scholar]
- 12.Brand E. Berlin Klin Woch. 1907;46:1075. [Google Scholar]
- 13.Omorokow Z. Immunitätsforsh. 1911;10:285. [Google Scholar]
- 14.Whitehead HR, Gordon J, Wormall A. The "Third Component" or Heat-Stable Factor of Complement. Biochem J. 1925;19(4):618–625. doi: 10.1042/bj0190618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coca AF. Z Immunitätsforsh. 1914;21:604. [Google Scholar]
- 16.Gordon J, Whitehead HR, Wormall A. The Fourth Component of Complement and its Relation to Opsonin. Biochem J. 1926;20(5):1044–1045. doi: 10.1042/bj0201044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer MM. Development of the one-hit theory of immune hemolysis. New Brunswick, NJ: Rutgers University Press; 1961. [Google Scholar]
- 18.Mayer MM, Miller JA, Shin HS. A specific method for purification of the second component of guinea pig complement and a chemical evaluation of the one-hit theory. J Immunol. 1970 Aug;105(2):327–341. [PubMed] [Google Scholar]
- 19.Inoue K, Akiyama Y, Kinoshita T, Higashi Y, Amano T. Evidence for a one-hit theory in the immune bactericidal reaction and demonstration of a multi-hit response for hemolysis by streptolysin O and Clostridium perfringens theta-toxin. Infect Immun. 1976 Feb;13(2):337–344. doi: 10.1128/iai.13.2.337-344.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morley BJ, et al. The complement facts book. UK: Academic Press; 2000. [Google Scholar]
- 21.Mayer MM. Studies on the mechanism of hemolysis by antibody and complement. Prog Allergy. 1958;5:215–270. [PubMed] [Google Scholar]
- 22.Mueller-Eberhard HJ, Biro CE. Isolation and description of the fourth component of human complement. J Exp Med. 1963 Sep 1;118:447–466. doi: 10.1084/jem.118.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilsson UR, Mueller-Eberhard HJ. Isolation of beta IF-globulin from human serum and its characterization as the fifth component of complement. J Exp Med. 1965 Aug 1;122:277–298. doi: 10.1084/jem.122.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilsson U. Separation and partial purification of the sixth, seventh and eighth components of human haemolytic complement. Acta Pathol Microbiol Scand. 1967;70(3):469–480. doi: 10.1111/j.1699-0463.1967.tb01315.x. [DOI] [PubMed] [Google Scholar]
- 25.Manni JA, Müller-Eberhard HJ. The eighth component of human complement (C8): isolation, characterization, and hemolytic efficiency. J Exp Med. 1969 Nov 1;130(5):1145–1160. doi: 10.1084/jem.130.5.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hadding U, Müller-Eberhard HJ. The ninth component of human complement: isolation, description and mode of action. Immunology. 1969 Jun;16(6):719–735. [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson RA, Jr., Jensen J, Gigli I, Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- 28.Nelson RA, Jr., Biro CE. Complement components of a haemolytically deficient strain of rabbits. Immunology. 1968 Apr;14(4):527–540. [PMC free article] [PubMed] [Google Scholar]
- 29.Arumugam TV, Magnus T, Woodruff TM, Proctor LM, Shiels IA, Taylor SM. Complement mediators in ischemia-reperfusion injury. Clin Chim Acta. 2006 Dec;374(1-2):33–45. doi: 10.1016/j.cca.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Morgan BP. Regulation of the complement membrane attack pathway. Crit Rev Immunol. 1999;19(3):173–198. [PubMed] [Google Scholar]
- 31.Barrington R, Zhang M, Fischer M, Carroll MC. The role of complement in inflammation and adaptive immunity. Immunol Rev. 2001 Apr;180:5–15. doi: 10.1034/j.1600-065x.2001.1800101.x. [DOI] [PubMed] [Google Scholar]
- 32.Gasque P. Complement: a unique innate immune sensor for danger signals. Mol Immunol. 2004 Nov;41(11):1089–1098. doi: 10.1016/j.molimm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Ehrnthaller C, Ignatius A, Gebhard F, Huber-Lang M. New insights of an old defense system: structure, function, and clinical relevance of the complement system. Mol Med. 2011 Mar-Apr;17(3-4):317–329. doi: 10.2119/molmed.2010.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Padilla ND, van Vliet AK, Schoots IG, Valls Seron M, Maas MA, Peltenburg EE, de Vries A, Niessen HW, Hack CE, van Gulik TM. C-reactive protein and natural IgM antibodies are activators of complement in a rat model of intestinal ischemia and reperfusion. Surgery. 2007 Nov;142(5):722–733. doi: 10.1016/j.surg.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 35.Pillemer L, Blum L, Lepow IH, Ross OA, Todd EW, Wardlaw AC. The properdin system and immunity. I. Demonstration and isolation of a new serum protein, properdin, and its role in immune phenomena. Science. 1954 Aug 20;120(3112):279–285. doi: 10.1126/science.120.3112.279. [DOI] [PubMed] [Google Scholar]
- 36.Pillemer L. The properdin system. Trans N Y Acad Sci. 1955 May;17(7):526–530. [PubMed] [Google Scholar]
- 37.Pillemer L, Blum L, Lepow IH, ross OA, Todd EW, Wardlaw AC. The properdin system and immunity. I. Demonstration and isolation of a new serum protein, properdin, and its role in immune phenomena. Science. 1954 Aug 20;120(3112):279–285. doi: 10.1126/science.120.3112.279. [DOI] [PubMed] [Google Scholar]
- 38.Arumugam TV, Shiels IA, Woodruff TM, Granger DN, Taylor SM. The role of the complement system in ischemia-reperfusion injury. Shock. 2004 May;21(5):401–409. doi: 10.1097/00024382-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. J Immunol. 2006 Feb 1;176(3):1305–1310. doi: 10.4049/jimmunol.176.3.1305. [DOI] [PubMed] [Google Scholar]
- 40.Ganter MT, Brohi K, Cohen MJ, Shaffer LA, Walsh MC, Stahl GL, Pittet JF. Role of the alternative pathway in the early complement activation following major trauma. Shock. 2007 Jul;28(1):29–34. doi: 10.1097/shk.0b013e3180342439. [DOI] [PubMed] [Google Scholar]
- 41.Diepenhorst GM, van Gulik TM, Hack CE. Complement-mediated ischemia-reperfusion injury: lessons learned from animal and clinical studies. Ann Surg. 2009 Jun;249(6):889–899. doi: 10.1097/SLA.0b013e3181a38f45. [DOI] [PubMed] [Google Scholar]
- 42.Stahl GL, Xu Y, Hao L, Miller M, Buras JA, Fung M, Zhao H. Role for the alternative complement pathway in ischemia/reperfusion injury. Am J Pathol. 2003 Feb;162(2):449–455. doi: 10.1016/S0002-9440(10)63839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawasaki T, Etoh R, Yamashina I. Isolation and characterization of a mannan-binding protein from rabbit liver. Biochem Biophys Res Commun. 1978 Apr 14;81(3):1018–1024. doi: 10.1016/0006-291x(78)91452-3. [DOI] [PubMed] [Google Scholar]
- 44.Kozutsumi Y, Kawasaki T, Yamashina I. Isolation and characterization of a mannan-binding protein from rabbit serum. Biochem Biophys Res Commun. 1980 Jul 31;95(2):658–664. doi: 10.1016/0006-291x(80)90836-0. [DOI] [PubMed] [Google Scholar]
- 45.Super M, Thiel S, Lu J, Levinsky RJ, Turner MW. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet. 1989 Nov 25;2(8674):1236–1239. doi: 10.1016/s0140-6736(89)91849-7. [DOI] [PubMed] [Google Scholar]
- 46.Garred P, Thiel S, Madsen HO, Ryder LP, Jensenius JC, Svejgaard A. [Deficiency of mannan-binding protein–a recently discovered complement defect syndrome] Ugeskr Laeger. 1993 Jan 4;155(1):25–29. [PubMed] [Google Scholar]
- 47.Vorup-Jensen T, Petersen SV, Hansen AG, Poulsen K, Schwaeble W, Sim RB, Reid KB, Davis SJ, Thiel S, Jensenius JC. Distinct pathways of mannan-binding lectin (MBL)- and C1-complex autoactivation revealed by reconstitution of MBL with recombinant MBL-associated serine protease-2. J Immunol. 2000 Aug 15;165(4):2093–2100. doi: 10.4049/jimmunol.165.4.2093. [DOI] [PubMed] [Google Scholar]
- 48.Matsushita M, Thiel S, Jensenius JC, Terai I, Fujita T. Proteolytic activities of two types of mannose-binding lectin-associated serine protease. J Immunol. 2000 Sep 1;165(5):2637–2642. doi: 10.4049/jimmunol.165.5.2637. [DOI] [PubMed] [Google Scholar]
- 49.Hajela K, Kojima M, Ambrus G, Wong KH, Moffatt BE, Ferluga J, Hajela S, Gál P, Sim RB. The biological functions of MBL-associated serine proteases (MASPs) Immunobiology. 2002 Sep;205(4-5):467–475. doi: 10.1078/0171-2985-00147. [DOI] [PubMed] [Google Scholar]
- 50.Dahl MR, Thiel S, Matsushita M, Fujita T, Willis AC, Christensen T, Vorup-Jensen T, Jensenius JC. MASP-3 and its association with distinct complexes of the mannan-binding lectin complement activation pathway. Immunity. 2001 Jul;15(1):127–135. doi: 10.1016/s1074-7613(01)00161-3. [DOI] [PubMed] [Google Scholar]
- 51.Matsushita M, Endo Y, Hamasaki N, Fujita T. Activation of the lectin complement pathway by ficolins. Int Immunopharmacol. 2001 Mar;1(3):359–363. doi: 10.1016/s1567-5769(00)00045-x. [DOI] [PubMed] [Google Scholar]
- 52.Matsushita M, Fujita T. Ficolins and the lectin complement pathway. Immunol Rev. 2001 Apr;180:78–85. doi: 10.1034/j.1600-065x.2001.1800107.x. [DOI] [PubMed] [Google Scholar]
- 53.Hansen S, Selman L, Palaniyar N, Ziegler K, Brandt J, Kliem A, Jonasson M, Skjoedt MO, Nielsen O, Hartshorn K, Jørgensen TJ, Skjødt K, Holmskov U. Collectin 11 (CL-11, CL-K1) is a MASP-1/3-associated plasma collectin with microbial-binding activity. J Immunol. 2010 Nov 15;185(10):6096–6104. doi: 10.4049/jimmunol.1002185. [DOI] [PubMed] [Google Scholar]
- 54.Amara U, Rittirsch D, Flierl M, Bruckner U, Klos A, Gebhard F, Lambris JD, Huber-Lang M. Interaction between the coagulation and complement system. Adv Exp Med Biol. 2008;632:71–79. doi: 10.1007/978-0-387-78952-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santizo F, Zenteno E, Pina-Canseco S, Hernandez-Cruz P, Cruz MM, Mayoral LP, Pérez-Campos E, Martínez-Cruz R. Lectin activity of the coagulation factor VIII/von Willebrand complex. Tohoku J Exp Med. 2009 Mar;217(3):209–215. doi: 10.1620/tjem.217.209. [DOI] [PubMed] [Google Scholar]
- 56.Davis AE., 3rd Biological effects of C1 inhibitor. Drug News Perspect. 2004 Sep;17(7):439–446. doi: 10.1358/dnp.2004.17.7.863703. [DOI] [PubMed] [Google Scholar]
- 57.Ikeda K, Nagasawa K, Horiuchi T, Tsuru T, Nishizaka H, Niho Y. C5a induces tissue factor activity on endothelial cells. Thromb Haemost. 1997 Feb;77(2):394–398. [PubMed] [Google Scholar]
- 58.Huber-Lang M, Younkin EM, Sarma JV, Riedemann N, McGuire SR, Lu KT, Kunkel R, Younger JG, Zetoune FS, Ward PA. Generation of C5a by phagocytic cells. Am J Pathol. 2002 Nov;161(5):1849–1859. doi: 10.1016/S0002-9440(10)64461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Griselli M, Herbert J, Hutchinson WL, Taylor KM, Sohail M, Krausz T, Pepys MB. C-reactive protein and complement are important mediators of tissue damage in acute myocardial infarction. J Exp Med. 1999 Dec 20;190(12):1733–1740. doi: 10.1084/jem.190.12.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Damman J, Daha MR, van Son WJ, Leuvenink HG, Ploeg RJ, Seelen MA. Crosstalk between complement and Toll-like receptor activation in relation to donor brain death and renal ischemia-reperfusion injury. Am J Transplant. 2011 Apr;11(4):660–669. doi: 10.1111/j.1600-6143.2011.03475.x. [DOI] [PubMed] [Google Scholar]
- 61.Song WC, Sarrias MR, Lambris JD. Complement and innate immunity. Immunopharmacology. 2000 Aug;49(1-2):187–198. doi: 10.1016/s0162-3109(00)80303-3. [DOI] [PubMed] [Google Scholar]
- 62.Koski CL, Ramm LE, Hammer CH, Mayer MM, Shin ML. Cytolysis of nucleated cells by complement: cell death displays multi-hit characteristics. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3816–3820. doi: 10.1073/pnas.80.12.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morgan BP. Complement membrane attack on nucleated cells: resistance, recovery and non-lethal effects. Biochem J. 1989 Nov 15;264(1):1–14. doi: 10.1042/bj2640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tegla CA, Cudrici C, Patel S, Trippe R, 3rd, Rus V, Niculescu F, Rus H. Membrane attack by complement: the assembly and biology of terminal complement complexes. Immunol Res. 2011 Oct;51(1):45–60. doi: 10.1007/s12026-011-8239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fosbrink M, Niculescu F, Rus H. The role of c5b-9 terminal complement complex in activation of the cell cycle and transcription. Immunol Res. 2005;31(1):37–46. doi: 10.1385/IR:31:1:37. [DOI] [PubMed] [Google Scholar]
- 66.Hugli TE. Biochemistry and biology of anaphylatoxins. Complement. 1986;3(3):111–127. doi: 10.1159/000467889. [DOI] [PubMed] [Google Scholar]
- 67.Gavrilyuk V, Kalinin S, Hilbush BS, Middlecamp A, McGuire S, Pelligrino D, Weinberg G, Feinstein DL. Identification of complement 5a-like receptor (C5L2) from astrocytes: characterization of anti-inflammatory properties. J Neurochem. 2005 Mar;92(5):1140–1149. doi: 10.1111/j.1471-4159.2004.02942.x. [DOI] [PubMed] [Google Scholar]
- 68.de Vries B, Köhl J, Leclercq WK, Wolfs TG, van Bijnen AA, Heeringa P, Buurman WA. Complement factor C5a mediates renal ischemia-reperfusion injury independent from neutrophils. J Immunol. 2003 Apr 1;170(7):3883–3889. doi: 10.4049/jimmunol.170.7.3883. [DOI] [PubMed] [Google Scholar]
- 69.Chenoweth DE, Hugli TE. Demonstration of specific C5a receptor on intact human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3943–3947. doi: 10.1073/pnas.75.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Banz Y, Rieben R. Role of complement and perspectives for intervention in ischemia-reperfusion damage. Ann Med. 2012 May;44(3):205–217. doi: 10.3109/07853890.2010.535556. [DOI] [PubMed] [Google Scholar]
- 71.Foreman KE, Vaporciyan AA, Bonish BK, Jones ML, Johnson KJ, Glovsky MM, Eddy SM, Ward PA. C5a-induced expression of P-selectin in endothelial cells. J Clin Invest. 1994 Sep;94(3):1147–1155. doi: 10.1172/JCI117430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Albrecht EA, Chinnaiyan AM, Varambally S, Kumar-Sinha C, Barrette TR, Sarma JV, Ward PA. C5a-induced gene expression in human umbilical vein endothelial cells. Am J Pathol. 2004 Mar;164(3):849–859. doi: 10.1016/S0002-9440(10)63173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hugli TE. The structural basis for anaphylatoxin and chemotactic functions of C3a, C4a, and C5a. Crit Rev Immunol. 1981 Feb;1(4):321–466. [PubMed] [Google Scholar]
- 74.Hugli TE. Structure and function of the anaphylatoxins. Springer Semin Immunopathol. 1984;7(2-3):193–219. doi: 10.1007/BF01893020. [DOI] [PubMed] [Google Scholar]
- 75.Zhou W. The new face of anaphylatoxins in immune regulation. Immunobiology. 2012 Feb;217(2):225–234. doi: 10.1016/j.imbio.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 76.Austen WG, Jr., Kyriakides C, Favuzza J, Wang Y, Kobzik L, Moore FD, Jr., Hechtman HB. Intestinal ischemia-reperfusion injury is mediated by the membrane attack complex. Surgery. 1999 Aug;126(2):343–348. [PubMed] [Google Scholar]
- 77.Fondevila C, Shen XD, Tsuchihashi S, Uchida Y, Freitas MC, Ke B, Busuttil RW, Kupiec-Weglinski JW. The membrane attack complex (C5b-9) in liver cold ischemia and reperfusion injury. Liver Transpl. 2008 Aug;14(8):1133–1141. doi: 10.1002/lt.21496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walport MJ. Complement. First of two parts. N Engl J Med. 2001 Apr 5;344(14):1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 79.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001 Apr 12;344(15):1140–1144. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 80.Nangaku M. Complement regulatory proteins in glomerular diseases. Kidney Int. 1998 Nov;54(5):1419–1428. doi: 10.1046/j.1523-1755.1998.00130.x. [DOI] [PubMed] [Google Scholar]
- 81.Ahearn JM, Fearon DT. Structure and function of the complement receptors, CR1 (CD35) and CR2 (CD21) Adv Immunol. 1989;46:183–219. doi: 10.1016/s0065-2776(08)60654-9. [DOI] [PubMed] [Google Scholar]
- 82.Miwa T, Song WC. Membrane complement regulatory proteins: insight from animal studies and relevance to human diseases. Int Immunopharmacol. 2001 Mar;1(3):445–459. doi: 10.1016/s1567-5769(00)00043-6. [DOI] [PubMed] [Google Scholar]
- 83.Miwa T, Nonaka M, Okada N, Wakana S, Shiroishi T, Okada H. Molecular cloning of rat and mouse membrane cofactor protein (MCP, CD46): preferential expression in testis and close linkage between the mouse Mcp and Cr2 genes on distal chromosome 1. Immunogenetics. 1998 Nov-Dec;48(6):363–371. doi: 10.1007/s002510050447. [DOI] [PubMed] [Google Scholar]
- 84.Cardone J, Le Friec G, Kemper C. CD46 in innate and adaptive immunity: an update. Clin Exp Immunol. 2011 Jun;164(3):301–311. doi: 10.1111/j.1365-2249.2011.04400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lublin DM, Atkinson JP. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu Rev Immunol. 1989;7:35–58. doi: 10.1146/annurev.iy.07.040189.000343. [DOI] [PubMed] [Google Scholar]
- 86.Davies A, Simmons DL, Hale G, Harrison RA, Tighe H, Lachmann PJ, Waldmann H. CD59, an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J Exp Med. 1989 Sep 1;170(3):637–654. doi: 10.1084/jem.170.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meri S, Morgan BP, Davies A, Daniels RH, Olavesen MG, Waldmann H, Lachmann PJ. Human protectin (CD59), an 18,000-20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology. 1990 Sep;71(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- 88.Li B, Sallee C, Dehoff M, Foley S, Molina H, Holers VM. Mouse Crry/p65. Characterization of monoclonal antibodies and the tissue distribution of a functional homologue of human MCP and DAF. J Immunol. 1993 Oct 15;151(8):4295–4305. [PubMed] [Google Scholar]
- 89.Fraczek LA, Martin BK. Transcriptional control of genes for soluble complement cascade regulatory proteins. Mol Immunol. 2010 Nov-Dec;48(1-3):9–13. doi: 10.1016/j.molimm.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 90.Vinci G, Lynch NJ, Duponchel C, Lebastard TM, Milon G, Stover C, Schwaeble W, Tosi M. In vivo biosynthesis of endogenous and of human C1 inhibitor in transgenic mice: tissue distribution and colocalization of their expression. J Immunol. 2002 Nov 15;169(10):5948–5954. doi: 10.4049/jimmunol.169.10.5948. [DOI] [PubMed] [Google Scholar]
- 91.Ziccardi RJ. The first component of human complement (C1): activation and control. Springer Semin Immunopathol. 1983;6(2-3):213–230. doi: 10.1007/BF00205874. [DOI] [PubMed] [Google Scholar]
- 92.Lappin DF, Guc D, Hill A, McShane T, Whaley K. Effect of interferon-gamma on complement gene expression in different cell types. Biochem J. 1992 Jan 15;281(Pt 2):437–442. doi: 10.1042/bj2810437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Al-Abdullah IH, Greally J. C1-inhibitor--biochemical properties and clinical applications. Crit Rev Immunol. 1985;5(4):317–330. [PubMed] [Google Scholar]
- 94.Davis AE, 3rd, Mejia P, Lu F. Biological activities of C1 inhibitor. Mol Immunol. 2008 Oct;45(16):4057–4063. doi: 10.1016/j.molimm.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Minta JO, Fung M, Turner S, Eren R, Zemach L, Rits M, Goldberger G. Cloning and characterization of the promoter for the human complement factor I (C3b/C4b inactivator) gene. Gene. 1998 Feb 16;208(1):17–24. doi: 10.1016/s0378-1119(97)00632-x. [DOI] [PubMed] [Google Scholar]
- 96.Kavanagh D, Goodship TH. Membrane cofactor protein and factor I: mutations and transplantation. Semin Thromb Hemost. 2006 Mar;32(2):155–159. doi: 10.1055/s-2006-939771. [DOI] [PubMed] [Google Scholar]
- 97.Kavanagh D, Kemp EJ, Mayland E, Winney RJ, Duffield JS, Warwick G, Richards A, Ward R, Goodship JA, Goodship TH. Mutations in complement factor I predispose to development of atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2005 Jul;16(7):2150–2155. doi: 10.1681/ASN.2005010103. [DOI] [PubMed] [Google Scholar]
- 98.Skattum L, van Deuren M, van der Poll T, Truedsson L. Complement deficiency states and associated infections. Mol Immunol. 2011 Aug;48(14):1643–1655. doi: 10.1016/j.molimm.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 99.Farries TC, Seya T, Harrison RA, Atkinson JP. Competition for binding sites on C3b by CR1, CR2, MCP, factor B and factor H. Complement Inflamm. 1990;7(1):30–41. doi: 10.1159/000463124. [DOI] [PubMed] [Google Scholar]
- 100.Ferreira VP, Pangburn MK, Cortés C. Complement control protein factor H: the good, the bad, and the inadequate. Mol Immunol. 2010 Aug;47(13):2187–2197. doi: 10.1016/j.molimm.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Atkinson JP, Goodship TH. Complement factor H and the hemolytic uremic syndrome. J Exp Med. 2007 Jun 11;204(6):1245–1248. doi: 10.1084/jem.20070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lappin DF, Whaley K. Interferon-induced transcriptional and post-transcriptional modulation of factor H and C4 binding-protein synthesis in human monocytes. Biochem J. 1990 Nov 1;271(3):767–772. doi: 10.1042/bj2710767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fujita T, Kamato T, Tamura N. Characterization of functional properties of C4-binding protein by monoclonal antibodies. J Immunol. 1985 May;134(5):3320–3324. [PubMed] [Google Scholar]
- 104.Wenderfer SE, Soimo K, Wetsel RA, Braun MC. Analysis of C4 and the C4 binding protein in the MRL/lpr mouse. Arthritis Res Ther. 2007;9(5):R114. doi: 10.1186/ar2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bokisch VA, Müller-Eberhard HJ. Anaphylatoxin inactivator of human plasma: its isolation and characterization as a carboxypeptidase. J Clin Invest. 1970 Dec;49(12):2427–2436. doi: 10.1172/JCI106462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Matthews KW, Mueller-Ortiz SL, Wetsel RA. Carboxypeptidase N: a pleiotropic regulator of inflammation. Mol Immunol. 2004 Jan;40(11):785–793. doi: 10.1016/j.molimm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 107.Skidgel RA, Erdös EG. Structure and function of human plasma carboxypeptidase N, the anaphylatoxin inactivator. Int Immunopharmacol. 2007 Dec 20;7(14):1888–1899. doi: 10.1016/j.intimp.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]