Abstract
This prospective, multicenter clinical trial was conducted to compare the performance of the cobas® 4800 CT/NG, APTIMA Combo 2®, and ProbeTec™ ET CT/GC assays for the detection of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) in Japan. From 1274 male and female patients, more than 1900 urine, endocervical and throat specimens were collected. Positive and negative concordance rates for CT and NG results obtained for urine and endocervical samples collected from the same patient were high in all three assays (range 96.0–99.6%). The accuracy of the cobas® 4800 CT/NG test did not differ significantly from that of the APTIMA Combo 2® and ProbeTec™ ET CT/GC assays. The accuracy of the assays did not change depending on the order of collection of endocervical specimens. Concordance rates for results obtained for throat swabs and mouthwashes in the ProbeTec™ ET CT/GC and cobas® 4800 CT/NG assays, respectively, were 98.8% for CT and 95.1% for NG. These data suggest that the cobas® 4800 CT/NG test is a reliable and highly accurate diagnostic tool for the detection of CT and NG in urine, genital, and oral specimens. Owing to the high correlation of urine and endocervical swab results and the ease of acquisition, urine samples are suggested as the specimen of choice for screening of CT and NG.
Keywords: Chlamydia trachomatis, Neisseria gonorrhoeae, nucleic acid amplification test, oral, urogenital
Introduction
Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) infections are increasing worldwide, even in developed countries such as Japan [1]. A study conducted in 2004 estimated that at least one million Japanese individuals (0.8% of total population) were infected with CT [2]. Since this earlier report, very few studies have been published on the prevalence of CT in the general population; therefore, this estimate is likely to have increased. In a more recent cross-sectional study, the CT prevalence rates were 9.5% and 6.7% in asymptomatic female and male colleges/university students in Japan, respectively [3].
NG is another common sexually transmitted disease (STD), which can cause a number of infections, including gonococcal urethritis and cervicitis [4]. The pharynx represents an important infection site for NG among commercial sex workers [4, 5]. Among 73 female and 46 male Japanese patients seeking medical attention for their urethritis and cervicitis, asymptomatic pharyngeal infection with NG or CT was found in 59% of females and 11% of males [4]. The asymptomatic nature of the infections, low rate of consistent condom use (ranging from 0% to 40% [3, 6]) among young, sexually active students, and the promiscuous behavior among commercial sex workers are believed to drive the rapidly increasing STD prevalence rates in Japan.
To address this growing health concern, diagnostic tools that are highly sensitive and specific for the detection of symptomatic and asymptomatic CT and NG infections in a variety of clinical specimens are needed. Molecular tests, such as nucleic acid amplification techniques (NAATs), have been used to analyze a variety of specimen types including male and female urine, male urethral swabs, and female endocervical/vaginal swabs; however, false-negative and false-positive results, partly due to the cross-reactivity of nongonococcal Neisseria species, remain a major issue for clinicians [4, 7]. Advances in NAATs have greatly improved the performance of commercially available assays, and NAATs are now broadly recommended as the test of choice (http://www.aphl.org/aphlprograms/infectious/std/Documents/CTGCLabGuidelinesMeetingReport.pdf).
Recently, the cobas® 4800 CT/NG test (Roche Molecular Diagnostics, Pleasanton, CA) has been described that allows highly sensitive, specific, and rapid automated detection of CT and NG from urine samples, endocervical and vaginal swabs, urethral swabs, and cervical specimens collected for liquid-based cytology [8].
In this study, we compared the performance of the cobas® 4800 CT/NG test with that of the APTIMA Combo 2® (TMA, Gen-Probe, San Diego, CA) and ProbeTec™ ET CT/GC (SDA, Becton Dickinson, Sparks, MD) assays in female and male urines, endocervical swabs, and throat samples. Results of this study highlight the appropriateness of urines for testing compared to endocervical swabs and demonstrate the excellent performance of the cobas® 4800 CT/NG test in the detection of CT and NG in different specimen types including throat swabs and throat washes.
Materials and methods
Overview
This prospective, multicenter study was performed in Japan between June and December 2009 to evaluate the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the cobas®4800 CT/NG test for the detection of CT and NG compared to those of the APTIMA Combo 2® and ProbeTec™ ET CT/GC assays.
Patient population
A total of 1274 male and female subjects (≥16 years) participated in this study and were recruited from sexually transmitted disease, obstetrics/gynecology, and family planning clinics at ten collection sites: Toho Obstetrics & Gynecology Hospital, Hokkaido (Kunihiro Minami); Iwasawa Clinic, Hokkaido (Akihiko Iwasawa); Yoshio Clinic, Hokkaido (Hiroshi Yoshio); Iesaka Clinic, Gunma (Kiyoko Iesaka); Miyamotochou Chuo Clinic, Kanagawa (Yasuhiko Onoe); Hoshina Clinic, Kyoto (Shinji Hoshina); Hara Genitourinary Hospital, Hyogo (Syouji Hara); Nishimura Urology Clinic, Fukuoka (Hirofumi Nishimura); Kawai Urology Clinic, Fukuoka (Syuichi Kawai); Ito Clinic, Fukuoka (Kenji Ito). A total of 106 subjects who had incomplete results for endocervical smears and 3 subjects who had invalid results for the cobas® 4800 CT/NG test were removed from the analysis.
Specimen collection, handling, and storage were performed according to the manufacturer’s instructions. Urine samples from 364 males and 244 females, and 404 endocervical swabs were collected; urine samples from 363 males and 241 females, as well as 402 endocervical swabs, were eligible for analysis. Urine specimens from male and female subjects were collected ≥1 h after the subject had previously voided. Three endocervical swabs were collected from females for analysis. Three subjects were removed from the analysis because of incomplete or invalid results and one female urine sample was removed because of an invalid SDA result. In addition, 445 throat swabs (400 from Hoshina Clinic and 45 from Miyamotochou) were collected according to the instructions provided with the ProbeTec™ ET CT/GC SDA assay; throat washes were obtained thereafter from the same patients by gargling with 15–20 ml saline solution for 10–20 s.
After specimens were collected, aliquoted, and labeled, they were transported to the three testing sites, Mitsubishi Chemical Medience Corporation (Itabashi-ku), SRL, Inc. (Tachikawa-shi), and RDKK (Minato-ku) for analysis.
Molecular testing for CT/NG
The cobas® 4800 CT/NG test (Roche Molecular Systems, Pleasanton, CA, USA) is a qualitative, multiplex in vitro NAAT designed to detect CT and/or NG from swab and urine specimens collected from symptomatic or asymptomatic subjects. The cobas® PCR Female Swab and Urine Sample Kits were used to collect and process samples according to the manufacturer’s package insert. For CT detection, the assay specifically targets the wild-type CT, Swedish variant (nvCT), and other Chlamydia strains that may contain deletions in the cryptic plasmid or those that do not carry the cryptic plasmid. DR-9 (direct repeat region) is the target for detection of NG. This repeat region enhances NG detection and eliminates cross-reactivity with other nongonococcal Neisseria and other bacterial species. Specimens were tested using the cobas® 4800 system. Briefly, samples (either 22 or 94 per run), controls, and reagents were loaded onto the cobas® 4800 system, which performed the DNA extraction using magnetic bead technology. Internal controls for CT and NG were added to each sample and control during extraction, controlling for extraction, amplification, inhibition, and competition. The instrument then loaded the extracted DNA and controls and amplification reagents into a 96-well amplification plate. The plate was covered and placed into the amplification instrument of the system. Results were described as positive, negative, or invalid by an algorithm within the cobas® 4800 software.
The APTIMA Combo 2® assay (Gen-Probe, San Diego, CA) and ProbeTec™ ET CT/GC assay (Becton Dickinson, Sparks, MD) were performed according to the assay package inserts.
Discrepancy analysis
To resolve discordant results obtained for endocervical samples, DNA was extracted from the cobas® 4800 CT/NG endocervical specimens using the Qiagen BioRobot Universal System (Qiagen, Valencia, CA). Two extractions were performed for each specimen. Hemi-nested PCR was performed to improve sequence quality. The target sequence is in the ORF-1 region of the CT cryptic plasmid. Forward and reverse primers for each round of PCR included M13 sequences to optimize sequencing results. The Chlamydia round 1 target amplicon (241 bp) was generated with forward primer sequence TGTAAAAGCACGGCCAGTGGGATTCCTGTAACAACAAGTCA and reverse primer sequence AGGAAACAGCTATGACCATCTTCCCCAGAACAATAAGAACAC. The Chlamydia round 2 target amplicon (201 bp) was generated with forward primer sequence TGTAAAACGACGGCCAGTCGCATAATTTTAGGCTTGCAG and reverse primer sequence AGGAAACAGCTATGACCACAATAAGAACACACACTTTGTCTCG. The first round of PCR was conducted in triplicate and followed by a QC analysis via agarose gel electrophoresis. If any of the triplicates produced a strong positive band, one of these positive replicates was taken through round 2 PCR. If none of the triplicate reactions produced a band or produced only weak bands in round 1, then all three reactions proceeded to the second round of PCR. The second round of PCR amplification was visualized by agarose gel electrophoresis once again. The amplicons from round 2 PCR were used as the templates for DNA sequencing using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA). The amplicons were purified (Exo-SAP treated) and sequenced with M13 forward and reverse primers on an Applied Biosystems 3730xl DNA Analyzer. The results were compared with reference sequences using Sequencher software version 4.9 (Gene Codes, Ann Arbor, MI). Trace scores were used to measure the quality of the DNA sequence. Verification also required a minimum match of 80 bases to the reference sequence on each strand of the sequenced amplicon.
Statistical analysis and result interpretation
Concordance percentage rates were calculated by taking the ratio of correctly identified positive and negative cases over the total number of cases analyzed. In the event of discordant results, the overall status of the sample (positive or negative for CT and/or NG) was based on the result demonstrated by the majority of the three assays.
Results
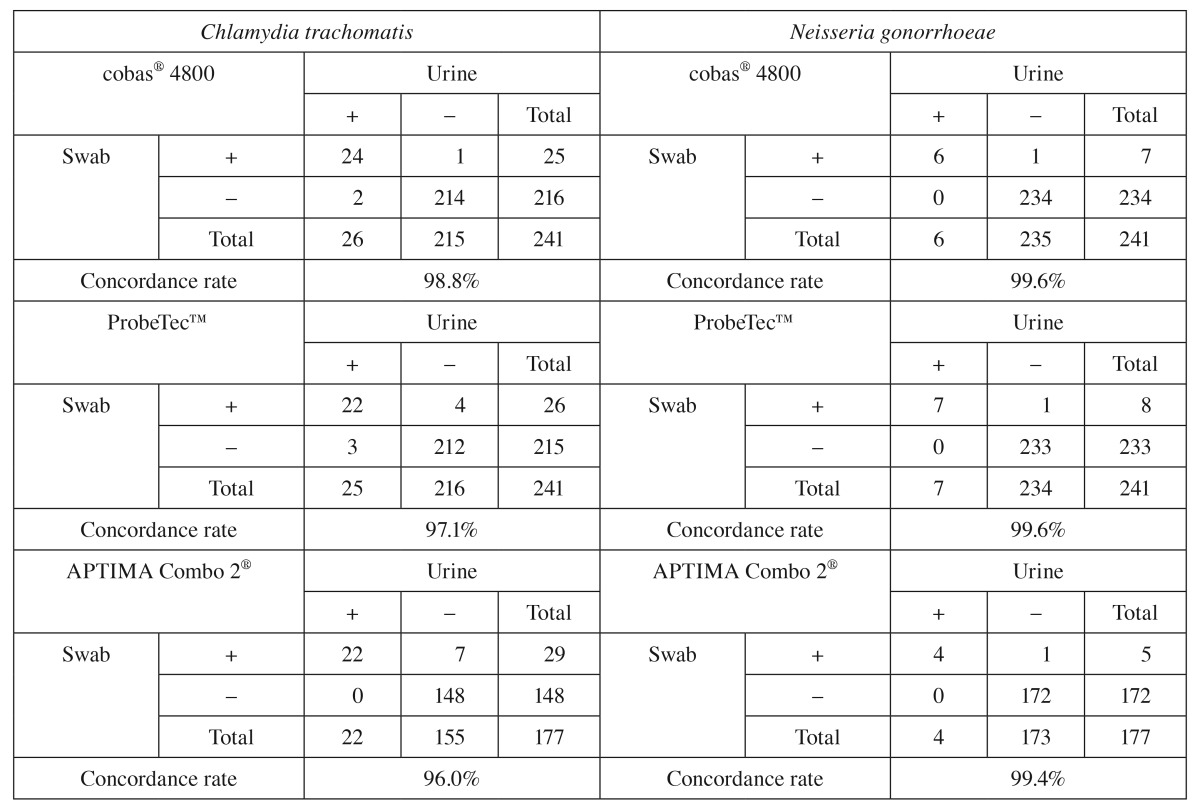
A total of 1902 specimens (urine, endocervical swabs, throat swabs, and throat washes) were collected from 1274 asymptomatic and symptomatic male and female subjects. First, concordance rates of results obtained from urine and endocervical samples were investigated. Table 1 demonstrates high concordance rates between results obtained for endocervical swabs and urine specimens from the same subjects in each of the three NAATs; concordance rates for CT and NG results obtained for these specimen types ranged between 98.8% and 99.6% for the cobas® 4800 CT/NG test, between 97.1% and 99.6% for the ProbeTec™ ET CT/GC SDA assay, and between 96.0% and 99.4% for the APTIMA Combo 2® TMA assay. Thus, results obtained for urine specimens highly agree with those obtained for endocervical specimens.
Table 1.
Concordance rates between Chlamydia trachomatis and Neisseria gonorrhoeae results obtained for urine and endocervical swabs using the cobas®4800, APTIMA Combo 2®, and ProbeTec™ ET CT/NG assays
|
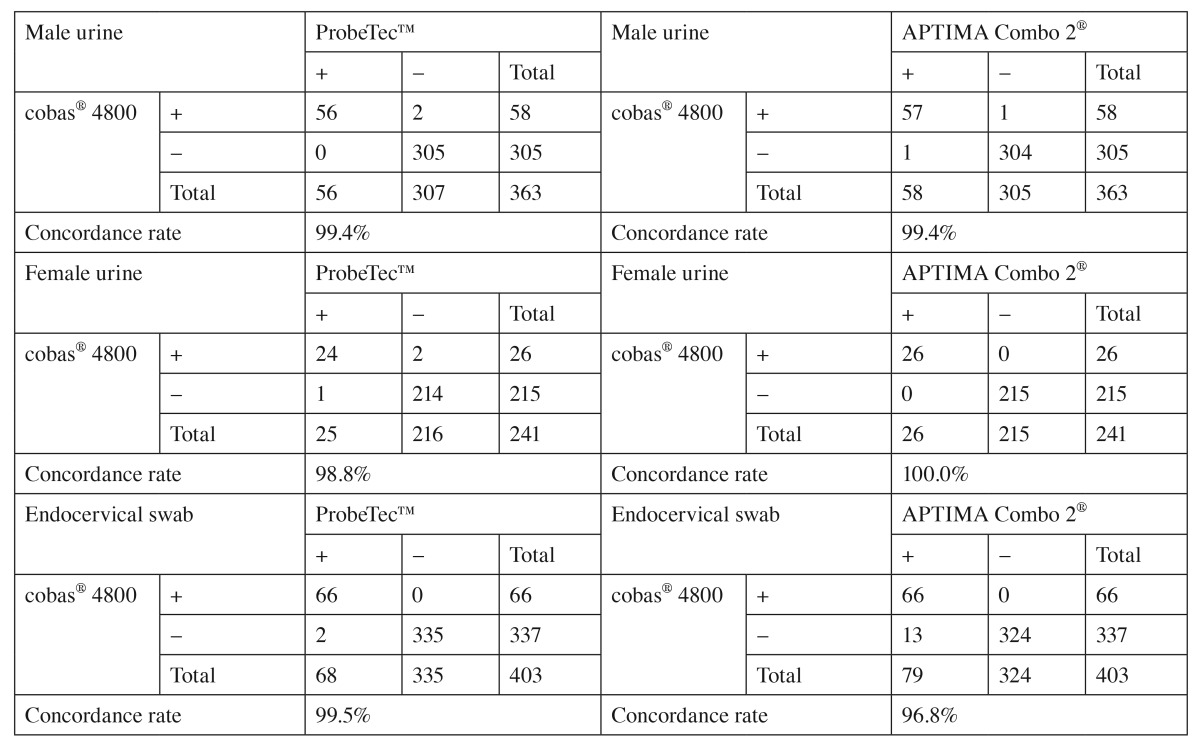
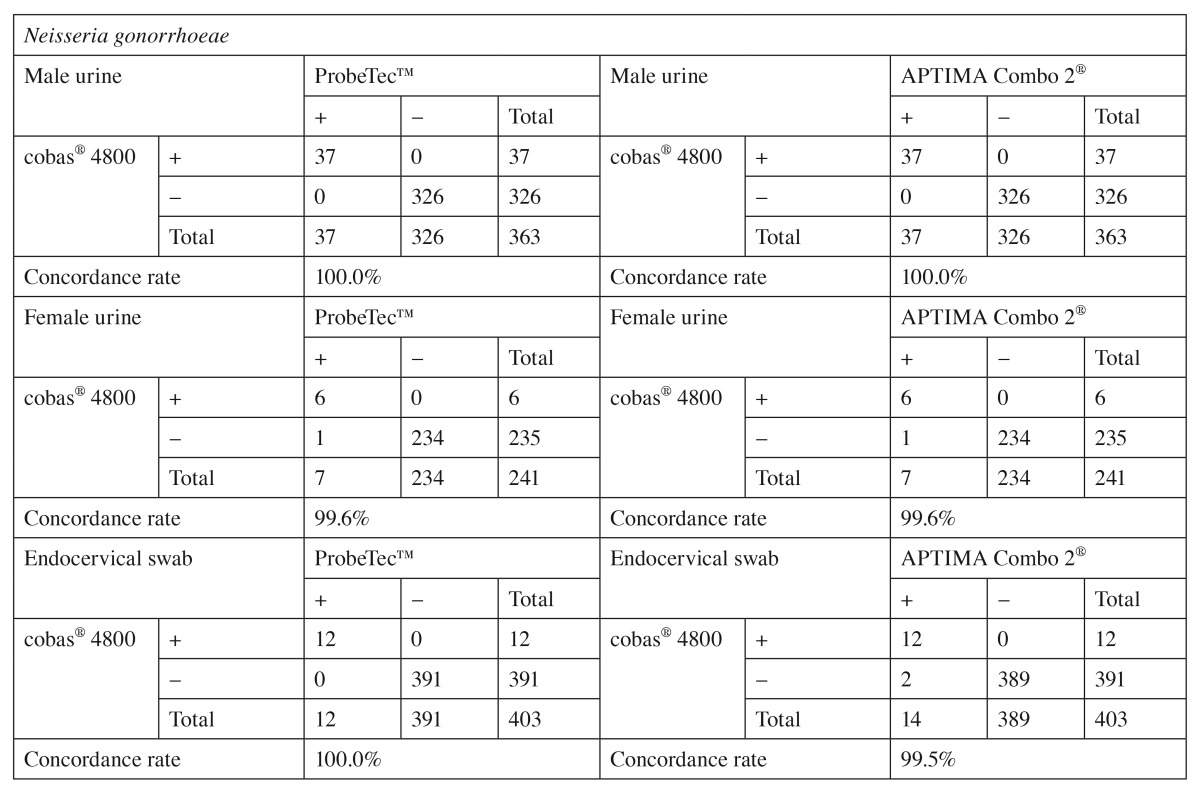
The prevalence of CT ranged from 8% to 47% and was 15% overall, the prevalence of NG ranged from 0 to 38% and was 6% overall (Table 2). Sensitivity, specificity, PPV, and NPV of the cobas® 4800 CT/NG test were 100% compared to the true infection status of the patient as determined by the rule of majority between the three NAATs (Table 2). Performance of the cobas® 4800 CT/NG test compared to that of the two comparator NAATs is presented stratified by specimen type for the detection of CT in Table 3 and for NG in Table 4. Concordance rates between the cobas® 4800 CT/NG test and the comparator NAATs ranged from 96.8% to 100.0% for male and female urine samples and for endocervical swabs. The highest number of discordant results (n=13) was observed between the cobas® 4800 CT/NG and APTIMA Combo 2® assays for endocervical swabs tested for the presence of CT (Table 3). In contrast, only two endocervical specimens gave discordant results for NG between the cobas® 4800 and APTIMA Combo 2® assays (Table 4). In all specimens that gave discordant results between the cobas® 4800 CT/NG test and either the APTIMA Combo 2® or ProbeTec™ ET CT/GC SDA assay, the cobas® 4800 CT/NG test result was in agreement with the result obtained with the other NAATs. Furthermore, discrepancy analysis was performed using a pre-validated sequencing approach. In six (50%) of 12 (one sample lost to follow-up) endocervical swab specimens, CT-specific sequences were detected.
Table 2.
Site-specific prevalences and performance characteristics of the cobas® 4800 CT/NG test
|
Chlamydia
trachomatis | ||||||||||
| TOH | IWA | YOS | IES | MIY | HAR | NIS | KAW | ITO | Overall | |
| No. of subjects | 345 | 32 | 178 | 120 | 45 | 14 | 17 | 50 | 29 | 830 |
| Prevalence (%) C. trachomatis |
8 | 25 | 8 | 11 | 44 | 29 | 47 | 42 | 34 | 15 |
| Sensitivity (%) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Specificity (%) | 100 | 96 | 100 | 100 | 100 | 100 | 89 | 100 | 95 | 100 |
| PPV (%) | 100 | 89 | 100 | 100 | 100 | 100 | 89 | 100 | 91 | 98 |
| NPV (%) | 100 | 96 | 100 | 100 | 100 | 100 | 89 | 100 | 95 | 100 |
|
Neisseria
gonorrhoeae | ||||||||||
| TOH | IWA | YOS | IES | MIY | HAR | NIS | KAW | ITO | Overall | |
| No. of subjects | 345 | 32 | 178 | 120 | 45 | 14 | 17 | 50 | 29 | 830 |
| Prevalence (%) N. gonorrhoeae |
1 | 13 | 2 | 0 | 16 | 36 | 35 | 22 | 38 | 6 |
| Sensitivity (%) | 100 | 100 | 100 | n.c. | 100 | 100 | 100 | 100 | 100 | 100 |
| Specificity (%) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| PPV (%) | 100 | 100 | 100 | n.c. | 100 | 100 | 100 | 100 | 100 | 100 |
| NPV (%) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| TOH, Toho Obstetrics & Gynecology Hospital; IWA, Iwasawa Clinic; YOS, Yoshio Clinic; IES, Iesaka Clinic; MIY, Miyamotochou Chuo Clinic; HAR, Hara Genitourinary Hospital; NIS, Nishimura Urology Clinic; KAW, Kawai Urology Clinic; ITO, Ito Clinic. n.c., not calculated due to zero value for N. gonorrhoeae. | ||||||||||
Table 3.
Comparison of results obtained in the cobas® 4800, APTIMA Combo 2®, and ProbeTec™ ET Chlamydia trachomatis assays in male and female urine and endocervical swabs
|
Table 4.
Comparison of results obtained from cobas® 4800, APTIMA Combo 2®, and ProbeTec™ ET NG assays performed in male and female urines and endocervical swabs
|
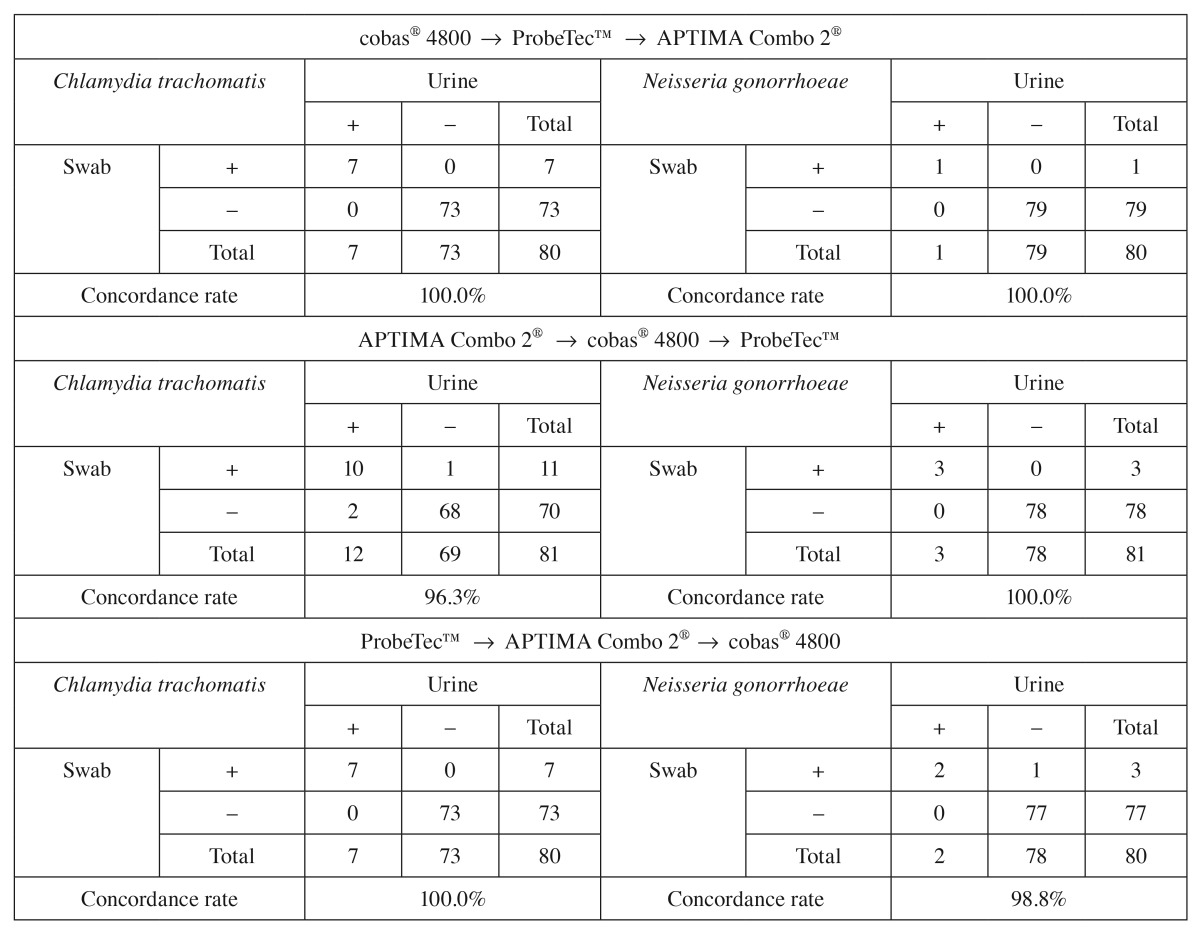
To determine whether the order of sample collection impacted concordance rates, we investigated specimen collection order. As shown in Table 5, concordance rates between the cobas® 4800 CT/NG test and the comparator assays were similar (96.3–100%) regardless of whether the swab for the cobas® 4800 CT/NG test was obtained first, second, or third in relation to the APTIMA Combo 2® and ProbeTec™ ET CT/GC SDA assays.
Table 5.
Impact of the order of specimen collection on the concordance rates between NAATs for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae
|
Last, we determined the agreement between results obtained for 445 throat swabs and throat washes using the ProbeTec™ ET CT/GC and cobas® 4800 CT/NG assays, respectively. Concordance rates for the two NAATs in the case of throat specimens were 98.2% for CT and 95.1% for NG. We observed 23 concordant-positive and 414 concordant-negative results for CT. There were 41 concordant positive and 382 concordant negative results for NG. The number of discordant results was markedly higher for NG (n=22) than that for CT (n=8).
Discussion
In this study, high concordance rates were observed in the performance of the cobas® 4800, APTIMA Combo 2®, and ProbeTec™ ET CT/GC assays for the detection of CT and NG in a Japanese population. Assay concordance rates were independent of the specimen type and order of specimen collection. Our results demonstrate that the cobas® 4800 CT/NG test is a highly sensitive and highly specific test for the detection of both CT and NG in urine samples and endocervical swab specimens.
The accuracy of the cobas® 4800 CT/NG test was comparable to or higher than that of the APTIMA Combo 2® and ProbeTec™ ET CT/GC assays [9–11]. Despite the advances in assay development, one of the challenges still facing NG testing is the occurrence of false positives mostly caused by cross-reactivity with other nongonococcal Neisseria species [7, 11, 12]. Using a large number of nongonococcal isolates, Tabrizi et al. [7] recently showed that the first generation cobas® Amplicor and the BD ProbeTec™ CT/NG assays display a higher number of false-positive NG results compared to other commercially available NAATs for the detection of NG including the APTIMA Combo 2® assay and the cobas® 4800 CT/NG test.
Overall, we found a higher agreement between results obtained in the cobas® 4800 CT/NG and ProbeTec™ ET CT/GC assays than that between the former tests and the APTIMA Combo 2® assay. In 12 of 13 endocervical specimens (one specimen lost to follow-up) for which the APTIMA Combo 2® and cobas® 4800 CT/NG assays gave discordant results for the detection of CT, results in the cobas® 4800 CT/NG test were concordant with those obtained in the ProbeTec™ ET CT/GC assay. Discrepancy analysis confirmed the cobas® 4800 CT/NG test results in 50% of these samples. It remains to be shown whether the samples showing positive results exclusively in the APTIMA Combo 2® assay indicate increased sensitivity or false positivity caused by reduced specificity or cross-contamination during the sample preparation or amplification processes.
The diagnostic yield in urine specimens was similar to the one in endocervical swabs. Therefore, urine samples can serve as the specimen of choice for the detection of CT and NG. While endocervical swabs have long been considered the specimen of choice, more recently, a number of reports confirmed the high accuracy of results in the case of urine specimens for the detection of CT and NG [9, 10]. Urine specimens are easy to obtain and their widespread use may increase the participation in screening programs (http://www.aphl.org/aphlprograms/infectious/std/Documents/CTGCLabGuidelinesMeetingReport.pdf).
Infection with NG can be localized in extragenital organs, i.e., the oral cavity [13, 14]. Therefore, throat swabs and/or throat washes are an important specimen type for the detection or oral infection with NG. Like urine samples, throat swabs and/or washes are easy to collect from patients, are noninvasive, and require minimal storage/handling procedures after collection. The rate of concordance between results obtained for throat swabs processed using the ProbeTec™ ET CT/GC assay and those obtained for throat washes processed using the cobas® 4800 CT/NG test was high. In this regard, Papp et al. [15] reported that study participants found oral-throat rinses acceptable, preferable, and feasible when compared with pharyngeal swabs. Future studies will have to determine the accuracy of testing these specimen types in more detail [16]. Interestingly, we also detected CT-specific DNA in oral samples from a significant number of subjects. It remains to be shown whether these reflect local infections with CT or rather indicate the colonization of the oral cavity with CT in the absence of local infection.
The strengths of this study lie in its large sample population consisting of asymptomatic and symptomatic subjects from STD, OB/GYN, and family planning clinics; the inclusion of two comparator assays in conjunction with sequencing to evaluate the performance of the cobas® 4800 CT/NG test; and the evaluation of throat swabs and throat washes. The most notable limitation of the study is the lack of additional information regarding the patient’s medical history.
In conclusion, results of this study provide strong support for the use of less invasive, i.e., urine, and extragenital, i.e., throat swabs and throat washes, specimen types for the detection of CT and NG. The cobas® 4800 CT/NG test is highly sensitive and highly specific for the detection of CT and NG.
Acknowledgments
We thank Kunihiro Minami, Akihiko Iwasawa, Hiroshi Yoshio, Kiyoko Iesaka, Yasuhiko Onoe, Shinji Hoshina, Syouji Hara, Hirofumi Nishimura, Syuichi Kawai, and Kenji Ito for providing the clinical specimens.
Contributor Information
Y. Kumamoto, 1Sapporo Medical University, Hokkaido, Japan.
T. Matsumoto, 2University of Occupational and Environmental Health, Fukuoka, Japan.
M. Fujisawa, 3Kobe University Hospital, Hyogo, Japan.
S. Arakawa, 3Kobe University Hospital, Hyogo, Japan.
References
- 1.Tanaka M. Recent trends of sexually transmitted diseases in Fukuoka city. Jpn Arch STD. 1993;4:39–46. [Google Scholar]
- 2.Japan SGfSsi STD surveillance 2001 in Japan. Jpn J Sex Transm Dis. 2002;13:147–167. [Google Scholar]
- 3.Imai H, Nakao H, Shinohara H, Fujii Y, Tsukino H, Hamasuna R, Osada Y, Fukushima K, Inamori M, Ikenoue T, Katoh T. Population-based study of asymptomatic infection with Chlamydia trachomatis among female and male students. Int J STD AIDS. 2010 May;21(5):362–366. doi: 10.1258/ijsa.2010.010026. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto T. Trends of sexually transmitted diseases and antimicrobial resistance in Neisseria gonorrhoeae. Int J Antimicrob Agents. 2008 Feb;31(Suppl 1):S35–S39. doi: 10.1016/j.ijantimicag.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 5.Tsunoe H, Tanaka M, Nakayama H, Sano M, Nakamura G, Shin T, Kanayama A, Kobayashi I, Mochida O, Kumazawa J, Naito S. High prevalence of Chlamydia trachomatis, Neisseria gonorrhoeae and Mycoplasma genitalium in female commercial sex workers in Japan. Int J STD AIDS. 2000 Dec;11(12):790–794. doi: 10.1258/0956462001915291. [DOI] [PubMed] [Google Scholar]
- 6.Imai H, Shinohara H, Nakao H, Tsukino H, Hamasuna R, Katoh T. Prevalence and risk factors of asymptomatic chlamydial infection among students in Japan. Int J STD AIDS. 2004 Jun;15(6):408–414. doi: 10.1258/095646204774195272. [DOI] [PubMed] [Google Scholar]
- 7.Tabrizi SN, Unemo M, Limnios AE, Hogan TR, Hjelmevoll SO, Garland SM, Tapsall J. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol. 2011 Oct;49(10):3610–3615. doi: 10.1128/JCM.01217-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rockett R, Goire N, Limnios A, Turra M, Higgens G, Lambert SB, Bletchly C, Nissen MD, Sloots TP, Whiley DM. Evaluation of the cobas 4800 CT/NG test for detecting Chlamydia trachomatis and Neisseria gonorrhoeae. Sex Transm Infect. 2010 Nov;86(6):470–473. doi: 10.1136/sti.2010.042812. [DOI] [PubMed] [Google Scholar]
- 9.Gaydos CA, Cartwright CP, Colaninno P, Welsch J, Holden J, Ho SY, Webb EM, Anderson C, Bertuzis R, Zhang L, Miller T, Leckie G, Abravaya K, Robinson J. Performance of the Abbott RealTime CT/NG for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2010 Sep;48(9):3236–3243. doi: 10.1128/JCM.01019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor SN, Van Der Pol B, Lillis R, Hook EW, 3rd, Lebar W, Davis T, Fuller D, Mena L, Fine P, Gaydos CA, Martin DH. Clinical evaluation of the BD ProbeTec™ Chlamydia trachomatis Qx amplified DNA assay on the BD Viper™ system with XTR™ technology. Sex Transm Dis. 2011 Jul;38(7):603–609. doi: 10.1097/OLQ.0b013e31820a94d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaydos CA, Quinn TC, Willis D, Weissfeld A, Hook EW, Martin DH, Ferrero DV, Schachter J. Performance of the APTIMA Combo 2 assay for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in female urine and endocervical swab specimens. J Clin Microbiol. 2003 Jan;41(1):304–309. doi: 10.1128/JCM.41.1.304-309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schachter J, Chernesky MA, Willis DE, Fine PM, Martin DH, Fuller D, Jordan JA, Janda W, Hook EW., 3rd Vaginal swabs are the specimens of choice when screening for Chlamydia trachomatis and Neisseria gonorrhoeae: results from a multicenter evaluation of the APTIMA assays for both infections. Sex Transm Dis. 2005 Dec;32(12):725–728. doi: 10.1097/01.olq.0000190092.59482.96. [DOI] [PubMed] [Google Scholar]
- 13.Schachter J, Moncada J, Liska S, Shayevich C, Klausner JD. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis. 2008 Jul;35(7):637–642. doi: 10.1097/OLQ.0b013e31817bdd7e. [DOI] [PubMed] [Google Scholar]
- 14.Walker CK, Sweet RL. Gonorrhea infection in women: prevalence, effects, screening, and management. Int J Womens Health. 2011;3:197–206. doi: 10.2147/IJWH.S13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papp JR, Ahrens K, Phillips C, Kent CK, Philip S, Klausner JD. The use and performance of oral-throat rinses to detect pharyngeal Neisseria gonorrhoeae and Chlamydia trachomatis infections. Diagn Microbiol Infect Dis. 2007 Nov;59(3):259–264. doi: 10.1016/j.diagmicrobio.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Fairley CK, Chen MY, Bradshaw CS, Tabrizi SN. Is it time to move to nucleic acid amplification tests screening for pharyngeal and rectal gonorrhoea in men who have sex with men to improve gonorrhoea control? Sex Health. 2011 Mar;8(1):9–11. doi: 10.1071/SH10134. [DOI] [PubMed] [Google Scholar]


