Abstract
Immune responses to filarial parasites like the river blindness inducing Onchocerca volvulus are obscured by combined reactions to the filarial nematodes themselves and their endosymbiont bacteria Wolbachia. Overall, infection with filarial nematodes induces a strong Th2 response characterized by IL-5 production and to a lesser degree a Th1 response and IFNγ production. Neutrophil and eosinophil infiltration into the corneal stroma are hallmark features of Onchocerca volvulus stimulation in a mouse model of river blindness. To determine the splenic and corneal response to filarial antigens in the absence of Wolbachia, C57BL/6 mice were immunized subcutaneously with either endosymbiotic Wolbachia alone, a soluble extract from the filaria Acanthocheilonema viteae that does not contain Wolbachia, or both, and injected into the corneal stroma. Neutrophil and eosinophil infiltration into the cornea was assessed by immunohistochemistry. In addition, Th1- and Th2-associated responses to filaria or Wolbachia were investigated by determining IL-5 and IFN-γ production by splenocytes. We found that A. viteae in the absence of Wolbachia induced IL-5 production and eosinophil infiltration, but not IFN-γ. Conversely, Wolbachia induced IFN-γ production and no migration of eosinophils. There was no difference in neutrophil infiltration. Together, these findings demonstrate a distinct Th-associated phenotype induced by filaria and Wolbachia.
Keywords: Acanthocheilonema, cornea, eosinophils, filaria, IFNγ, IL-5, neutrophils, Onchocerca, river blindness, Wolbachia
Introduction
Infection with the filarial nematode Onchocerca volvulus causes onchocerciasis, a disease characterized by ocular and dermal inflammation, leading to blindness and various forms of skin affection. WHO currently estimates about 37 million people to be infected with O. volvulus, mainly in sub-Saharan Africa, with more than 100 million people at risk of infection [1, 2]. There are two divergent types of immune responses: the hyperreactive sowda form with low worm burden due to an overwhelming immune response and strong symptoms, and the hyporesponsive generalized form with high worm burden but few symptoms [3].
Chronic infection with filarial nematodes induces production of IL-5 and IFNγ, and elevated blood eosinophilia. Generalized infection is associated with low levels of IL-5 while putatively immune patients show elevated levels of both IFNγ and mainly IL-5 [4]. Previous studies have demonstrated that IL-5 is predominantly induced during murine filarial infection. Similarly, dead and degenerating microfilariae in the skin of O. volvulus-infected individuals after chemotherapy are associated with increase of eosinophil, neutrophil, and macrophage numbers indicating a mixed Th1/Th2 response [4]. As filarial nematodes contain endosymbiotic Wolbachia bacteria that are associated with a pro-inflammatory host response, it has been difficult to discern the relative contribution of filarial and bacterial antigens to the host immune response.
We therefore utilized a murine model of ocular onchocerciasis in which mice are immunized subcutaneously and injected into the corneal stroma with either filarial antigens in the absence of Wolbachia or with isolated Wolbachia, or a combination of both. Previous studies showed that following immunization with O. volvulus extract (containing Wolbachia bacteria), mice develop an adaptive immune response characterized by high levels of IL-5 production and lower levels of IFNγ, and a predominant IgG1 antibody response. Injections of worm extract into the cornea then induce an early neutrophil infiltration at 24 h followed at 72 h by a predominant eosinophil infiltration [5]. In the current study, we used the Wolbachia-free filarial worm Acanthocheilonema viteae [6] as source for filarial extracts and insect-cell-derived Wolbachia.
Methods
Parasites and bacteria
A. viteae worms were purchased from TRS laboratories (Athens, GA, United States), and worm extract was prepared as described previously [7]. Briefly, worms were washed extensively, kept in culture for 14 days in antibiotic-supplemented medium, and the absence of bacterial growth was monitored. Worms were then homogenized in HBSS, sonicated, the insoluble fraction was removed by centrifugation, and the soluble fraction was used for all studies.
Aa23 insect cells containing Wolbachia were provided by Dr. Mark Taylor (Liverpool School of Tropical Medicine, Great Britain). Cells were grown at 27°C with no CO2 in 50% Mitsuhashi Maramorosh/50% Schneider’s Insect Cell Medium, supplemented with 10% insect cell grade FBS and 1% Pen/Strep. Cells were harvested and sonicated twice for 1 min. Cells were then centrifuged at 600 g for 5 min. Supernatants were pooled and centrifuged again for a total of five times. Finally, the supernatants were centrifuged at 12,100 g for 10 min, and the pellet containing Wolbachia was resuspended in HBSS.
Mice
C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME, United States) and kept under pathogen-free conditions at the local animal facility. Mice were immunized subcutaneously three times at weekly intervals with either 10 µg A. viteae in squalene adjuvant [7], Wolbachia, or with A. viteae and Wolbachia. Squalene adjuvant contained 10% squalene (Sigma Aldrich, St. Louis, MO, United States), 0.4% Tween 80 (Sigma Aldrich, St. Louis, United States) and 1% Pluronic L (BASF, Ludwigshafen, Germany) in PBS. One week after the last immunization, corneas were injected with A. viteae, Wolbachia, or a mixture of both [8, 9].
Immunohistochemistry
After 24 h, mice were sacrificed, eyes were snap frozen in OCT, and 5 µm sections were stained for neutrophils using NIMP/R14 or for eosinophils using anti-murine MBP (JJ Lee, Mayo Clinic AZ, United States). Cells were directly counted by fluorescence microscopy.
ELISA
Spleens from immunized mice were removed following three subcutaneous immunizations in weekly intervals. Single-cell suspensions were prepared and stimulated in triplicate with A. viteae, Wolbachia, A. viteae + Wolbachia, or anti-CD3 as positive control for 72 h at 37 °C. IL-5 and IFNγ production was measured by ELISA (R&D Systems). Limit of detection was 30 pg/ml for IL-5 and 10 pg/ml for IFNγ.
Statistics
GraphPad Prism™ was used to analyze the data by Student’s t-test, and p<0.05 was considered significant.
Results
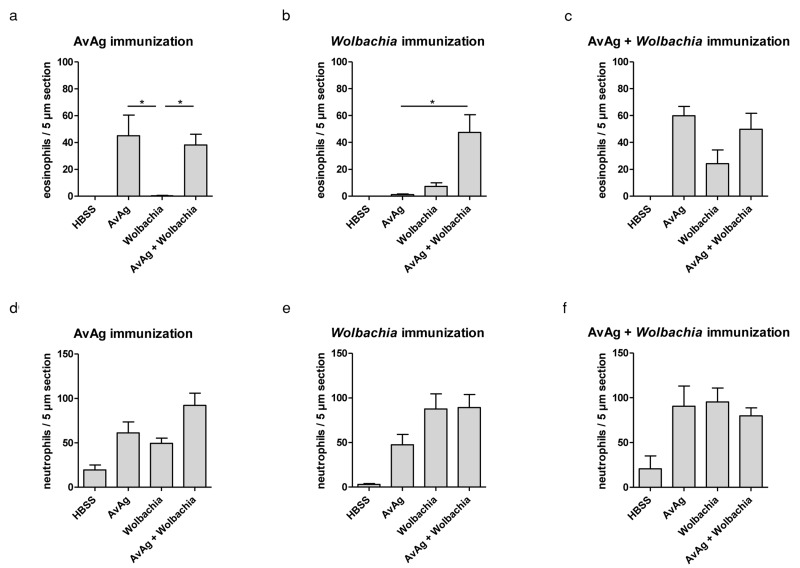
Eosinophilia is a hallmark feature of helminth infection, and these cells migrate to the corneal stroma in a murine model of ocular onchocerciasis using O. volvulus soluble extracts containing Wolbachia [5]. To determine if Wolbachia or filaria are essential for eosinophil infiltration, mice were immunized subcutaneously and injected intrastromally with extract from A. viteae worms that naturally do not harbor Wolbachia. Additionally, corneas were injected with Wolbachia alone or a combination of A. viteae and Wolbachia. As shown in Figure 1a, injection of A. viteae and A. viteae/Wolbachia induces eosinophil infiltration while injection of Wolbachia alone does not. When mice were immunized with Wolbachia alone, only the intrastromal challenge with A. viteae/Wolbachia could induce eosinophil infiltration, indicating that A. viteae is required for eosinophil migration, but Wolbachia is required for generation of an adaptive immune response that allows A. viteae-induced eosinophil migration (Fig. 1b). When mice were immunized with a combination of A. viteae and Wolbachia, filaria and Wolbachia could induce eosinophil migration separately, although filariae were required for efficient eosinophil infiltration (Fig. 1c).
Fig. 1.
C57BL/6 mice were immunized with AvAg (a, d), Wolbachia (b, e), or a mixture of AvAg and Wolbachia (c, f). Corneas were injected with HBSS as negative control, AvAg, Wolbachia, or AvAg + Wolbachia. Eosinophil (a–c) and neutrophil (d–f) infiltrations were determined 24 h post injection. The X-axis indicates the agent used for intrastromal injections. Data shown are combined results of two experiments as mean ± SEM with two corneas for HBSS injection, six corneas for AvAg and Wolbachia injection, and seven corneas for AvAg + Wolbachia. Statistical testing used the Kruskal-Wallis test followed by Dunn’s multiple comparison test. * indicates p<0.05
Previous studies in our lab showed neutrophil infiltration after intrastromal injection of filarial extract even in the absence of an adaptive immune response. After immunization with A. viteae, Wolbachia, or A. viteae and Wolbachia, we show neutrophil infiltration after challenge with any extract. This demonstrates that neutrophil infiltration is indeed independent of immunization status. Neutrophil infiltration is also observed in the presence of filarial extract only as well as in the presence of Wolbachia only (Fig. 1d–f).
As eosinophil release from the bone marrow is largely dependent on IL-5, and we found that A. viteae, but not Wolbachia, is important for eosinophil migration into the corneal stroma, we next examined if A. viteae induces IL-5 production by splenocytes in the absence of Wolbachia.
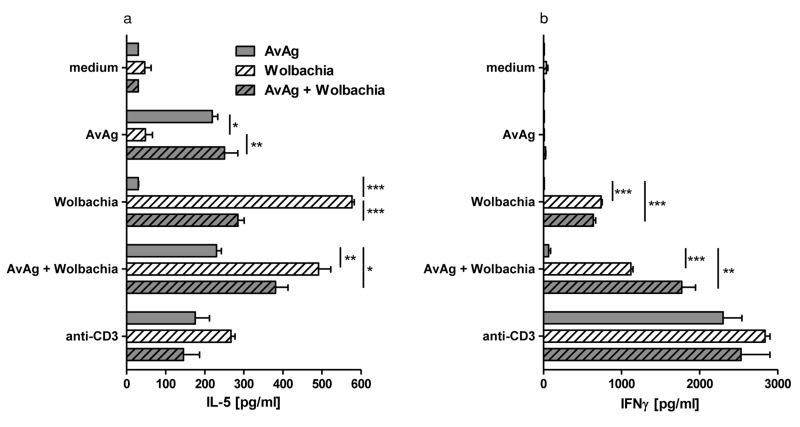
Figure 2a shows that IL-5 was produced by splenocytes from A. viteae-immunized mice stimulated with A. viteae or A. viteae/Wolbachia. This finding indicates that filarial antigens induce IL-5, and is consistent with Figure 1a showing eosinophils in the corneas of these mice. Surprisingly, we found that spleen cells from Wolbachia-immunized mice that were stimulated in vitro with Wolbachia only also produced IL-5.
Fig. 2.
C57BL/6 mice were immunized with AvAg, Wolbachia, or a mixture of AvAg and Wolbachia, and single cell splenocyte cultures were stimulated with medium, AvAg, Wolbachia, AvAg + Wolbachia, or anti-CD3. IL-5 and IFNγ levels were determined by ELISA. Limit of detection was 10 pg/ml for IFNγ and 30 pg/ml for IL-5. Data shown are representative of two experiments with three mice per group and is shown as mean ± SEM. *, **, *** represent statistical significance as calculated by the student’s t-test corresponding to p<0.05, p<0.01 and p<0.0001, respectively
Mice co-immunized with A. viteae/Wolbachia produced IL-5 after in vitro stimulation with either A. viteae or Wolbachia alone, or both. Together, these results demonstrate that for the in vitro production of IL-5, the presence of the same antigens during priming and challenge is required.
In contrast to IL-5 production by A. viteae-immunized and stimulated samples, these cells did not produce IFNγ (Fig. 2b). Also, IFNγ was not induced by A. viteae stimulation in splenocytes from Wolbachia immunized mice. IFNγ production was not induced in the converse experiment, with A. viteae-immunized and Wolbachia-stimulated cells. Even splenocytes from double-immunized mice did not induce IFNγ when A. viteae only was used as in vitro stimulus. These findings indicate that A. viteae does not induce IFNγ when used in either priming or recall stimulation. Conversely, Wolbachia induced IFNγ when used for priming and stimulation. We conclude that Wolbachia is primarily required for IFNγ production by splenocytes, whereas A. viteae induces IL-5 but not IFNγ production.
Of note, IFNγ levels in these experiments were higher than IL-5 levels following splenocyte stimulation. We hypothesize that this is due to adjuvant-mediated Th1 induction during the immunization phase.
Discussion
Onchocerciasis patients experience severe skin disease and visual impairment, often resulting in blindness. Studying human onchocerciasis is mostly limited to the analysis of activation of peripheral blood mononuclear cells or skin responsiveness, and the analysis of onchocercal nodules. Ocular studies in human patients are scarce. Our laboratory previously established a mouse model of Onchocerca keratitis where repeat injections of filarial antigens induce the generation of an adaptive immune response similar to that of patients with generalized onchocerciasis [10]. The subsequent injection of filarial antigens into the corneal stroma induces migration of neutrophils and eosinophils, with lower numbers of macrophages and T cells infiltrating the corneal stroma. Homologies to human onchocercal keratitis may only be hypothesized as human corneal samples for the investigation of pathology are scarce for obvious reasons.
Previous studies using this murine model of Onchocerca keratitis demonstrated that (1) there is not only a predominant Th2 response, with elevated IL-4, IL-5, IgE, IgG1, and eosinophilia, but also an induction of IFNγ [10]; (2) the presence of endosymbiotic Wolbachia is essential for neutrophil recruitment to the corneal stroma and development of corneal haze [7]; (3) Wolbachia-induced corneal inflammation is TLR2 and MyD88 dependent [11, 12]; and (4) dendritic cell activation and IFNγ production are dependent on TLR2, whereas Th2-associated responses (IL-5 production, eosinophil infiltration into the cornea) are TLR2 independent [13]. These findings are consistent with the hypothesis that Wolbachia induce Th1-associated responses and neutrophil recruitment through TLR2/MyD88-dependent responses, whereas Th2 responses are Wolbachia independent and may be induced directly by filarial antigens. The relative contribution of filaria and Wolbachia in the generation of adaptive immune responses was investigated in this study. To address the relative role of bacterial vs. filarial antigens in the generation of immune responses, we immunized wildtype C57BL/6 mice subcutaneously and injected the corneal stroma with Wolbachia alone, filarial antigens in the absence of Wolbachia (A. viteae), or a combination of both antigens.
Results from the current study show that production of the Th1 cytokine IFNγ completely depends on the presence of Wolbachia. Wolbachia are required during the priming and challenging phase. Stimulation with filarial proteins alone (A. viteae extract) was not sufficient for IFNγ induction. This confirms the hypothesis that Th1 responses are primarily Wolbachia and thus TLR2 dependent [13].
The major transcription factor regulating the generation of IFNγ-producing Th1 cells is T-box expressed in T cells (T-bet) [14] whereas Th1 induction is strongly down-regulated by the expression of GATA-3 [15, 16]. It was found in filaria-infected patients that T-bet expression in T cells is down-regulated following stimulation with filarial proteins and subsequently decreased IFNγ production [17]. In contrast, stimulation with live larvae decreased the expression of both T-bet and GATA-3 as well as production of Th1 and Th2 cytokines [18]. Expression of T-bet also negatively regulates generation of Th17 cells as shown by overwhelming IL-17 production in T-bet−/− mice following immunization with soluble schistosome egg antigen [19]. IL-17 expression was also found increased in filarial-infected patients compared with uninfected patients [20]; however, the role of IL17 was not subject of this study.
TLRs and T-cell responses are also linked by thymic stromal lymphopoietin (TSLP). Activation of dendritic cells via TLRs usually results in IL-12 production and induction of Th1 responses by T cells [21]. However, if dendritic cells are activated by TSLP, Th2 responses are induced as indicated by the production of Eotaxin-2, TARC, and MDC, and by the lack of IFNγ and IL-12 production [22], although the authors refer to those Th2 cells as inflammatory Th2 cells due to their production of TNFα and their lack in producing IL-10. TSLP can be induced by IL-1β and TNFα [23], and the production of those cytokines following stimulation of murine corneal epithelial cells with filarial extracts has been demonstrated [24]. Interestingly, corneal epithelial cells were also shown to produce TSLP in response to the TLR2/6 ligand FSL1 [25]. Thus, filaria-dependent IL-5 induction might occur in a TLR2-independent pathway requiring TSLP; however, Wolbachia-induced IL-5 production is more likely to involve TLR2 as this is the major known receptor for these bacteria. To confirm TLR2-dependent IL-5 production, future experiments could use TLR2−/− and MyD88−/− mice in comparison to C57BL/6 mice. The role of TSLP in the induction of Th2 responses might be further investigated by determining TSLP concentration in murine corneal lysates and by blocking TSLP in the cornea by injection of anti-TSLP.
In contrast to IFNγ production only being induced by Wolbachia, IL-5 production did require priming, but could be induced by both filaria and Wolbachia. The presence of identical antigens was required during priming and challenge stimulation. This is unexpected as the production of IL-5 was previously found to be TLR2 independent and thus thought to be Wolbachia independent [13]. This finding might indicate that Wolbachia is not only activating the immune system via TLR2, but other Toll-like receptors like TLR4 that have been previously implicated in the pathogenesis of filariasis might also be involved [7, 26].
In addition to this generalized immune response, we also analyzed the local infiltration of neutrophils and eosinophils into the corneal stroma of immunized mice. We found, as has been demonstrated previously, that neutrophil migration does not require immunization [11]. Filarial antigens and Wolbachia both efficiently induced neutrophil migration into the corneal stroma, independent of the immunization status. It has been previously shown that not only are Wolbachia sufficient for induction of neutrophil migration but also that wolbachial lipoproteins can induce neutrophil infiltration into the corneal stroma [27, 28].
Eosinophil infiltration into the mouse cornea was analyzed following the immunization of mice with different antigens. In contrast to neutrophil infiltration that does not require a filarial-specific adaptive immune response, eosinophil infiltration into the mouse cornea has been shown to only occur following immunization with filarial extracts [10]. This has been demonstrated for O. volvulus and B. malayi that both contain Wolbachia bacteria. It is presumed that the injection of AvAg + Wolbachia induces a similar cellular infiltration into the corneal stroma, thus there is no expected eosinophil infiltration into naive mouse corneas following AvAg, Wolbachia or AvAg + Wolbachia injection.
Presence of filarial antigens during priming and challenge sufficed to induce eosinophil migration whereas Wolbachia stimulation during priming and challenge did not result in eosinophil infiltration into the corneal stroma. However, when we immunized with Wolbachia, there appeared to be initiation of the adaptive immune response, and AvAg + Wolbachia injection into the corneal stroma was sufficient to induce eosinophil migration. Even though eosinophil numbers are usually increased during filarial infection, the mechanism by which eosinophils affect parasite survival remains unclear. Eosinophils and IgE antibodies have been implicated in the generation of protective immunity against O. volvulus [29]. Similarly, it has been shown in jirds that immunization with A. viteae induces eosinophil-mediated protective immunity [30]. This suggests that immunization using wolbachial products is probably much less efficient as it does not affect eosinophils as strongly as filarial proteins do.
Overall, we confirm that filarial worms themselves are important for the induction of an adaptive Th2 immune response characterized by IL-5 production and eosinophil migration whereas Wolbachia is required for IFNγ production. Surprisingly, Wolbachia could also induce IL-5 production, however, without subsequent eosinophil migration into the cornea. Both filaria and Wolbachia suffice to induce neutrophil migration.
Acknowledgments
We thank Eugenia Diaconu for technical support and Dr. Marc Hübner for critical reading of the manuscript.
Contributor Information
K. Gentil, 1Department of Ophthalmology and Visual Sciences, Case Western Reserve University Case Medical Center, Cleveland, Ohio, USA; 2Institute for Medical Microbiology, Immunology and Parasitology, University Hospital Bonn, Bonn, Germany.
A. Hoerauf, 2Institute for Medical Microbiology, Immunology and Parasitology, University Hospital Bonn, Bonn, Germany.
E. Pearlman, 1Department of Ophthalmology and Visual Sciences, Case Western Reserve University Case Medical Center, Cleveland, Ohio, USA.
References
- 1.WHO Managing morbidity and preventing disability in the Global Programme to Eliminate Lymphatic Filariasis: WHO position statement. Wkly Epidemiol Rec. 2011 Dec 16;86(51-52):581–585. [PubMed] [Google Scholar]
- 2.CDC, 2010. http://www.cdc.gov/parasites/onchocerciasis/epi.html [Google Scholar]
- 3.Hoerauf A, Pfarr K, Mand S, Debrah AY, Specht S. Filariasis in Africa–treatment challenges and prospects. Clin Microbiol Infect. 2011 Jul;17(7):977–985. doi: 10.1111/j.1469-0691.2011.03586.x. [DOI] [PubMed] [Google Scholar]
- 4.Hoerauf A, Brattig N. Resistance and susceptibility in human onchocerciasis–beyond Th1 vs. Th2. Trends Parasitol. 2002 Jan;18(1):25–31. doi: 10.1016/s1471-4922(01)02173-0. [DOI] [PubMed] [Google Scholar]
- 5.Pearlman E, Hall LR, Higgins AW, Bardenstein DS, Diaconu E, Hazlett FE, Albright J, Kazura JW, Lass JH. The role of eosinophils and neutrophils in helminth-induced keratitis. Invest Ophthalmol Vis Sci. 1998 Jun;39(7):1176–1182. [PubMed] [Google Scholar]
- 6.Hartmann N, Stuckas H, Lucius R, Bleiss W, Theuring F, Kalinna BH. Trans-species transfer of Wolbachia: microinjection of Wolbachia from litomosoides sigmodontis into Acanthocheilonema viteae. Parasitology. 2003 Jun;126(Pt 6):503–511. [PubMed] [Google Scholar]
- 7.Saint André Av, Blackwell NM, Hall LR, Hoerauf A, Brattig NW, Volkmann L, Taylor MJ, Ford L, Hise AG, Lass JH, Diaconu E, Pearlman E. The role of endosymbiotic Wolbachia bacteria in the pathogenesis of river blindness. Science. 2002 Mar 8;295(5561):1892–1895. doi: 10.1126/science.1068732. [DOI] [PubMed] [Google Scholar]
- 8.Hall LR, Diaconu E, Pearlman E. A dominant role for Fc gamma receptors in antibody-dependent corneal inflammation. J Immunol. 2001 Jul 15;167(2):919–925. doi: 10.4049/jimmunol.167.2.919. [DOI] [PubMed] [Google Scholar]
- 9.Kaifi JT, Diaconu E, Pearlman E. Distinct roles for PECAM-1, ICAM-1, and VCAM-1 in recruitment of neutrophils and eosinophils to the cornea in ocular onchocerciasis (river blindness) J Immunol. 2001 Jun 1;166(11):6795–6801. doi: 10.4049/jimmunol.166.11.6795. [DOI] [PubMed] [Google Scholar]
- 10.Pearlman E, Lass JH, Bardenstein DS, Kopf M, Hazlett FE, Jr., Diaconu E, Kazura JW. Interleukin 4 and T helper type 2 cells are required for development of experimental onchocercal keratitis (river blindness) J Exp Med. 1995 Oct 1;182(4):931–940. doi: 10.1084/jem.182.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillette-Ferguson I, Daehnel K, Hise AG, Sun Y, Carlson E, Diaconu E, McGarry HF, Taylor MJ, Pearlman E. Toll-like receptor 2 regulates CXC chemokine production and neutrophil recruitment to the cornea in Onchocerca volvulus/Wolbachia-induced keratitis. Infect Immun. 2007 Dec;75(12):5908–5915. doi: 10.1128/IAI.00991-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillette-Ferguson I, Hise AG, Sun Y, Diaconu E, McGarry HF, Taylor MJ, Pearlman E. Wolbachia- and Onchocerca volvulus-induced keratitis (river blindness) is dependent on myeloid differentiation factor 88. Infect Immun. 2006 Apr;74(4):2442–2445. doi: 10.1128/IAI.74.4.2442-2445.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daehnel K, Gillette-Ferguson I, Hise AG, Diaconu E, Harling MJ, Heinzel FP, Pearlman E. Filaria/Wolbachia activation of dendritic cells and development of Th1-associated responses is dependent on Toll-like receptor 2 in a mouse model of ocular onchocerciasis (river blindness) Parasite Immunol. 2007 Sep;29(9):455–465. doi: 10.1111/j.1365-3024.2007.00962.x. [DOI] [PubMed] [Google Scholar]
- 14.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000 Mar 17;100(6):655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 15.Ferber IA, Lee HJ, Zonin F, Heath V, Mui A, Arai N, O'Garra A. GATA-3 significantly downregulates IFN-gamma production from developing Th1 cells in addition to inducing IL-4 and IL-5 levels. Clin Immunol. 1999 May;91(2):134–144. doi: 10.1006/clim.1999.4718. [DOI] [PubMed] [Google Scholar]
- 16.Ouyang W, Ranganath SH, Weindel K, Bhattacharya D, Murphy TL, Sha WC, Murphy KM. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998 Nov;9(5):745–755. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 17.Babu S, Kumaraswami V, Nutman TB. Transcriptional control of impaired Th1 responses in patent lymphatic filariasis by T-box expressed in T cells and suppressor of cytokine signaling genes. Infect Immun. 2005 Jun;73(6):3394–3401. doi: 10.1128/IAI.73.6.3394-3401.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. J Immunol. 2006 Mar 1;176(5):3248–3256. doi: 10.4049/jimmunol.176.5.3248. [DOI] [PubMed] [Google Scholar]
- 19.Rutitzky LI, Smith PM, Stadecker MJ. T-bet protects against exacerbation of schistosome egg-induced immunopathology by regulating Th17-mediated inflammation. Eur J Immunol. 2009 Sep;39(9):2470–2481. doi: 10.1002/eji.200939325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metenou S, Dembele B, Konate S, Dolo H, Coulibaly YI, Diallo AA, Soumaoro L, Coulibaly ME, Coulibaly SY, Sanogo D, Doumbia SS, Traoré SF, Mahanty S, Klion A, Nutman TB. Filarial infection suppresses malaria-specific multifunctional Th1 and Th17 responses in malaria and filarial coinfections. J Immunol. 2011 Apr 15;186(8):4725–4733. doi: 10.4049/jimmunol.1003778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt Rde W, Omori M, Zhou B, Ziegler SF. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 22.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, de Waal-Malefyt Rd R, Bazan F, Kastelein RA, Liu YJ. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002 Jul;3(7):673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 23.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci U S A. 2007 Jan 16;104(3):914–919. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gentil K, Pearlman E. Gamma interferon and interleukin-1 receptor 1 regulate neutrophil recruitment to the corneal stroma in a murine model of Onchocerca volvulus keratitis. Infect Immun. 2009 Apr;77(4):1606–1612. doi: 10.1128/IAI.00671-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma P, Bian F, Wang Z, Zheng X, Chotikavanich S, Pflugfelder SC, Li DQ. Human corneal epithelium-derived thymic stromal lymphopoietin links the innate and adaptive immune responses via TLRs and Th2 cytokines. Invest Ophthalmol Vis Sci. 2009 Jun;50(6):2702–2709. doi: 10.1167/iovs.08-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brattig NW, Bazzocchi C, Kirschning CJ, Reiling N, Büttner DW, Ceciliani F, Geisinger F, Hochrein H, Ernst M, Wagner H, Bandi C, Hoerauf A. The major surface protein of Wolbachia endosymbionts in filarial nematodes elicits immune responses through TLR2 and TLR4. J Immunol. 2004 Jul 1;173(1):437–445. doi: 10.4049/jimmunol.173.1.437. [DOI] [PubMed] [Google Scholar]
- 27.Brattig NW, Büttner DW, Hoerauf A. Neutrophil accumulation around Onchocerca worms and chemotaxis of neutrophils are dependent on Wolbachia endobacteria. Microbes Infect. 2001 May;3(6):439–446. doi: 10.1016/s1286-4579(01)01399-5. [DOI] [PubMed] [Google Scholar]
- 28.Turner JD, Langley RS, Johnston KL, Gentil K, Ford L, Wu B, Graham M, Sharpley F, Slatko B, Pearlman E, Taylor MJ. Wolbachia lipoprotein stimulates innate and adaptive immunity through Toll-like receptors 2 and 6 to induce disease manifestations of filariasis. J Biol Chem. 2009 Aug 14;284(33):22364–22378. doi: 10.1074/jbc.M901528200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abraham D, Leon O, Schnyder-Candrian S, Wang CC, Galioto AM, Kerepesi LA, Lee JJ, Lustigman S. Immunoglobulin E and eosinophil-dependent protective immunity to larval Onchocerca volvulus in mice immunized with irradiated larvae. Infect Immun. 2004 Feb;72(2):810–817. doi: 10.1128/IAI.72.2.810-817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bleiss W, Oberländer U, Hartmann S, Adam R, Marko A, Schönemeyer A, Lucius R. Protective immunity induced by irradiated third-stage larvae of the filaria Acanthocheilonema viteae is directed against challenge third-stage larvae before molting. J Parasitol. 2002 Apr;88(2):264–270. doi: 10.1645/0022-3395(2002)088[0264:PIIBIT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]