Abstract
Dermatomyositis is an idiopathic inflammatory myopathy with typical cutaneous manifestations. It has been proposed that dermatomyositis may be caused by autoimmune responses to viral infections. Previous studies have shown an association between dermatomyositis and malignant tumors such as ovarian cancer, lung cancer, and colorectal cancer. However, a chronic hepatitis B virus (HBV) infection associated with dermatomyositis and hepatocellular carcinoma (HCC) has been very rarely reported. Here, we report a rare case of dermatomyositis coinciding with HBV-associated HCC. A 55-year-old male was confirmed to have HCC and dermatomyositis based on proximal muscle weakness, typical skin manifestations, elevated muscle enzyme levels, and muscle biopsy findings. This case suggests that HCC and/or a chronic HBV infection may be factors in the pathogenesis of dermatomyositis through a paraneoplastic mechanism.
Keywords: Dermatomyositis, Hepatitis B virus, Hepatocellular carcinoma
INTRODUCTION
Dermatomyositis (DM) is an idiopathic inflammatory myopathy with progressive, symmetrical weakness of the proximal muscles and characteristic cutaneous manifestations such as poikiloderma [1]. The etiology and pathogenesis of DM remain unknown; however, in recent years, some researchers have shown that the cause of DM may be related to an autoimmune response induced by a viral infection or a paraneoplastic syndrome. In particular, DM has been strongly associated with breast, ovarian, lung, pancreatic, gastric, and colorectal cancer, as well as non-Hodgkin's lymphoma [2].
However, DM associated with hepatocellular carcinoma (HCC) has rarely been reported [2-7]. Here, we report a case of DM associated with hepatitis B virus (HBV)-related liver cirrhosis and HCC with a review of the current literature.
CASE REPORT
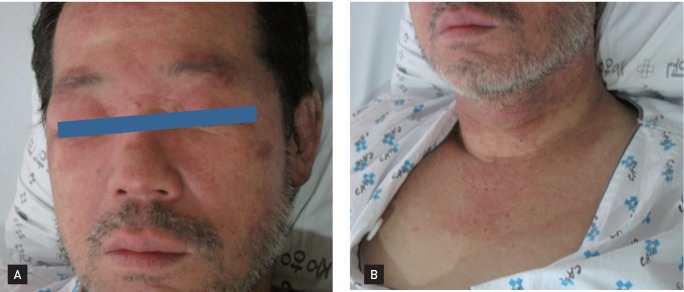
A 55-year-old male visited our hospital complaining of progressive weakness in his bilateral arms, hips, and thighs. He had difficulty combing his hair and was experiencing dysphagia and erythematous changes in the skin (his face and a 'V' on his upper chest and neck that had developed 3 weeks earlier) (Fig. 1).
Figure 1.
Skin manifestations of a 55-year-old man with dermatomyositis. (A) Characteristic purplish heliotrope rash over the upper eyelids. (B) 'V'-shaped purplish rash on the neck.
A physical examination revealed a heliotrope rash with bilateral periorbital edema and poikiloderma on the upper chest and neck. A musculoskeletal examination showed marked weakness of all proximal muscles in the symmetric extremities. Serum laboratory tests revealed the following: white blood cells 9,720/mm3, hemoglobin 13.6 g/dL, platelets 98,000/mm3, creatine kinase 16,440 IU/L (normal range, 0 to 190), lactate dehydrogenase 994 IU/L, aspartate aminotransferase 717 IU/L, alanine transaminase 139 IU/L, total bilirubin 1.4 mg/dL, albumin 2.2 g/dL, alkaline phosphatase 119 IU/L, and prothrombin time (activity) 55%. HBV surface antigen was detected, and the HBV DNA level was 7,380 IU/mL. No antibodies against hepatitis C virus were detected.
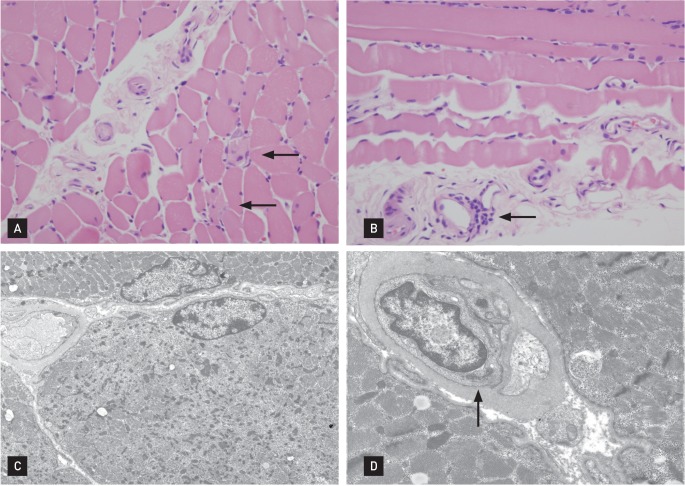
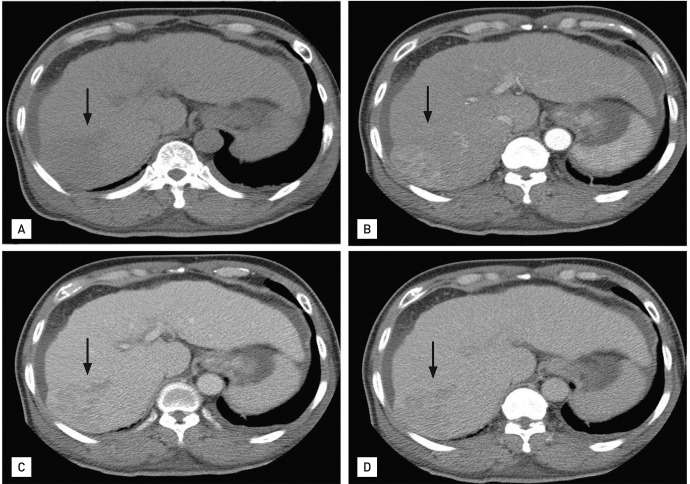
IgM antibodies against Epstein-Barr virus, cytomegalovirus, and herpes simplex virus were not detected. There was no other evidence of infectious mononucleosis. Antinuclear antibody and anti-SS-Ro tests were both positive. Tests for other autoantibodies, including anti-dsDNA, anti-ribonucleoprotein, anti-Sm, anti-Jo-1, and anti-Scl70, were all negative. Electromyography showed a small number of polyphasic waves with a normal interference pattern in all muscles tested. A muscle biopsy of the right upper arm showed a sparse lymphocytic infiltrate around the blood vessels and negative immunofluorescence pathologic findings, compatible with DM (Fig. 2). The patient's levels of carcinoembryonic antigen, carbohydrate antigen 19-9, and prostate-specific antigen were normal, but he had elevated levels of α-fetoprotein (AFP; 448.8 ng/mL) and proteins induced by vitamin K absence-II (> 500 mAU/mL). Liver dynamic computed tomography (CT) revealed a 6.5-cm sized arterial enhancing mass in hepatic segment 7/6 (Fig. 3).
Figure 2.
(A) Under light microscopy, rare degenerating cells (arrows) were present (H&E, ×200). (B) Mild perivascular mononuclear cell infiltration (arrow) was found (H&E, ×200). (C) Ultrastructurally, degenerating cells were scattered and exhibited rarefaction of myofilaments and Z-band streaming (uranyl acetate lead citrate, ×3,500). (D) The endothelial cells had a prominent tubuloreticular body (arrow) (uranyl acetate lead citrate, ×9,000). These findings are compatible with dermatomyositis.
Figure 3.
Liver dynamic computed tomography showed a 6.5 × 6-cm arterial enhancing mass (arrows) in hepatic segment 7/6, suggesting hepatocellular carcinoma. The nodular surfaced liver with perihepatic fluid collection suggested liver cirrhosis (A, precontrast; B, arterial phase; C, portal phase; D, delayed phase).
We diagnosed the patient with HBV-related HCC based on his status as an HBV carrier, elevated AFP level (> 200 ng/mL), and typical radiographic findings (e.g., arterial enhancing features on liver dynamic CT).
Optimal management procedures for HCC such as transcatheter arterial chemoembolization could not be performed because of the patient's poor general condition. We started prophylactic antiviral therapy with lamivudine for the prevention of viral reactivation. A total of 60 mg of oral prednisolone was administered daily. After steroid therapy, the patient's skin rash, muscle strength, and dysphagia showed slight improvement, and his elevated muscle enzyme levels returned to the near-normal range. However, his underlying HCC progressed rapidly: the size of the cancer grew up to 11 cm with portal vein thrombosis and multiple lymph node metastasis. The patient ultimately died due to hepatic failure after 4 months.
DISCUSSION
An association between malignancy and DM has been widely reported in the literature with incidences ranging from 3% to 35% [1]. Possible mechanisms include paraneoplastic syndrome, a compromised immune system, common carcinogenic environmental factors, and cross-activity of immune reactions against the tumor, all of which transform into an autoimmune syndrome as a consequence of cross-reactivity with skin and muscle antigens [8].
Levine [9] suggested a model of paraneoplasia focusing on common autoantigen expression and immune targeting between cancer tissues and muscle tissue in myositis. One of these so-called myositis-specific autoantibodies recognizes Mi-2 antigen, a component of the nucleosome remodeling deacetylase complex. Recent data examining the immune recognition of Mi-2 antigen suggest that patients with cancer-associated myositis develop humoral immune responses against different regions of the molecule, as compared to the responses of those with myositis alone [10]. Toshikuni et al. [6] proposed that anti-Mi-2 antibodies may be cross-reactive with HCC. In our case, there was another possibility-that the patient's DM was caused by other infectious agents. Therefore, we tested for various infectious agents, including echovirus, adenovirus, coxsackie virus, influenza, human immunodeficiency virus, and human T cell leukemia/lymphoma virus, which are known to cause myositis, and all tests were negative.
Antibodies that attack a virus or virus-enzyme complex could cross-react with a homologous area of host proteins and result in autoantibody production (i.e., a cross-reactive phenomenon). A test for anti-Jo-1 antibodies was negative, but anti-Mi-2 antibodies were not examined in this case.
Six patients with DM associated with HCC have been described in the literature including the present case (Table 1) [2-7]. These results suggest that DM in patients with HCC is a risk factor for advanced HCC.
Table 1.
A review of cases of dermatomyositis associated with hepatocellular carcinoma
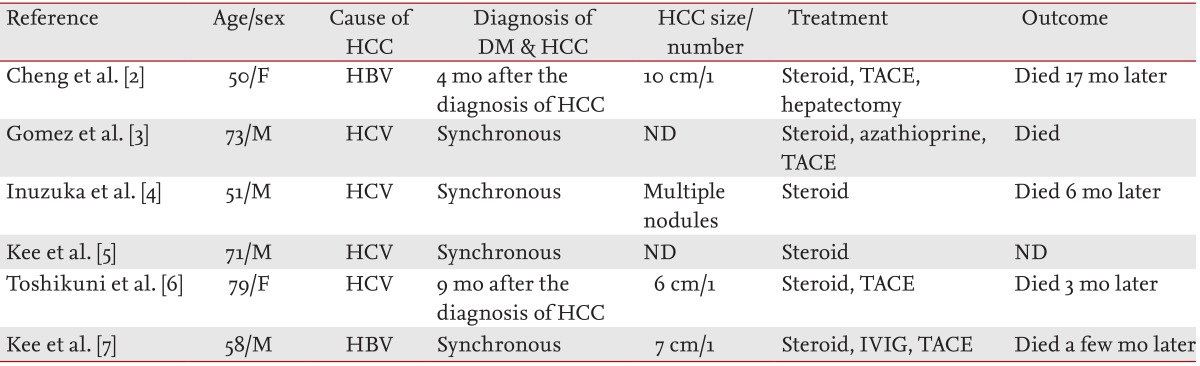
HCC, hepatocellular carcinoma; DM, dermatomyositis; F, female; HBV, hepatitis B virus; TACE, transarterial chemoembolization; M, male; HCV, hepatitis C virus; ND, not described; IVIG, intravenous immunoglobulin.
DM has been noted to improve after the treatment of cancer, with recurrences of muscle weakness taking place after relapse of the malignancy, further suggesting a paraneoplastic origin. Unfortunately, we could not manage the HCC in our patient because of his poor general condition and hepatic function. There was a previous report of improvement in DM without corticosteroids after the resection of HCC [7]. In our case DM was improved by oral prednisolone.
In conclusion, DM may be caused by HCC, especially advanced HCC, and/or a chronic HBV infection. Therefore, HCC might be considered a cause of DM if no other etiology can be determined.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Iorizzo LJ, 3rd, Jorizzo JL. The treatment and prognosis of dermatomyositis: an updated review. J Am Acad Dermatol. 2008;59:99–112. doi: 10.1016/j.jaad.2008.02.043. [DOI] [PubMed] [Google Scholar]
- 2.Cheng TI, Tsou MH, Yang PS, Sung SM, Chuang VP, Sung JL. Dermatomyositis and erythrocytosis associated with hepatocellular carcinoma. J Gastroenterol Hepatol. 2002;17:1239–1240. doi: 10.1046/j.1440-1746.2002.t01-1-02851.x. [DOI] [PubMed] [Google Scholar]
- 3.Gomez A, Solans R, Simeon CP, et al. Dermatomyositis, hepatocarcinoma, and hepatitis C: comment on the article by Weidensaul et al. Arthritis Rheum. 1997;40:394–395. doi: 10.1002/art.1780400232. [DOI] [PubMed] [Google Scholar]
- 4.Inuzuka M, Tomita K, Tokura Y, Takigawa M. Acquired ichthyosis associated with dermatomyositis in a patient with hepatocellular carcinoma. Br J Dermatol. 2001;144:416–417. doi: 10.1046/j.1365-2133.2001.04040.x. [DOI] [PubMed] [Google Scholar]
- 5.Kee KM, Wang JH, Lee CM, Changchien CS, Eng HL. Chronic hepatitis C virus infection associated with dermatomyositis and hepatocellular carcinoma. Chang Gung Med J. 2004;27:834–839. [PubMed] [Google Scholar]
- 6.Toshikuni N, Torigoe R, Mitsunaga M, Omoto A, Nakashima K. Dermatomyositis associated with hepatocellular carcinoma in an elderly female patient with hepatitis C virus-related liver cirrhosis. World J Gastroenterol. 2006;12:1641–1644. doi: 10.3748/wjg.v12.i10.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kee SJ, Kim TJ, Lee SJ, et al. Dermatomyositis associated with hepatitis B virus-related hepatocellular carcinoma. Rheumatol Int. 2009;29:595–599. doi: 10.1007/s00296-008-0718-1. [DOI] [PubMed] [Google Scholar]
- 8.Andras C, Ponyi A, Constantin T, et al. Dermatomyositis and polymyositis associated with malignancy: a 21-year retrospective study. J Rheumatol. 2008;35:438–444. [PubMed] [Google Scholar]
- 9.Levine SM. Cancer and myositis: new insights into an old association. Curr Opin Rheumatol. 2006;18:620–624. doi: 10.1097/01.bor.0000245721.02512.77. [DOI] [PubMed] [Google Scholar]
- 10.Hengstman GJ, Vree Egberts WT, Seelig HP, et al. Clinical characteristics of patients with myositis and autoantibodies to different fragments of the Mi-2 beta antigen. Ann Rheum Dis. 2006;65:242–245. doi: 10.1136/ard.2005.040717. [DOI] [PMC free article] [PubMed] [Google Scholar]