Abstract
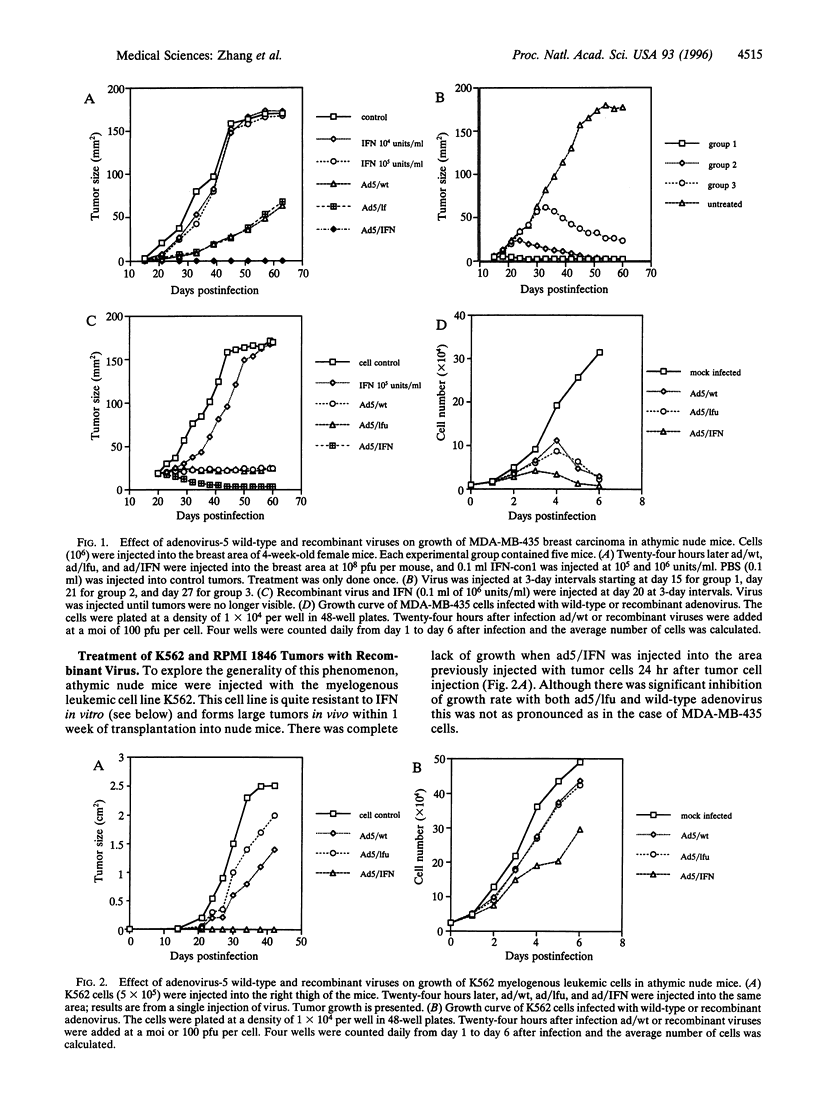
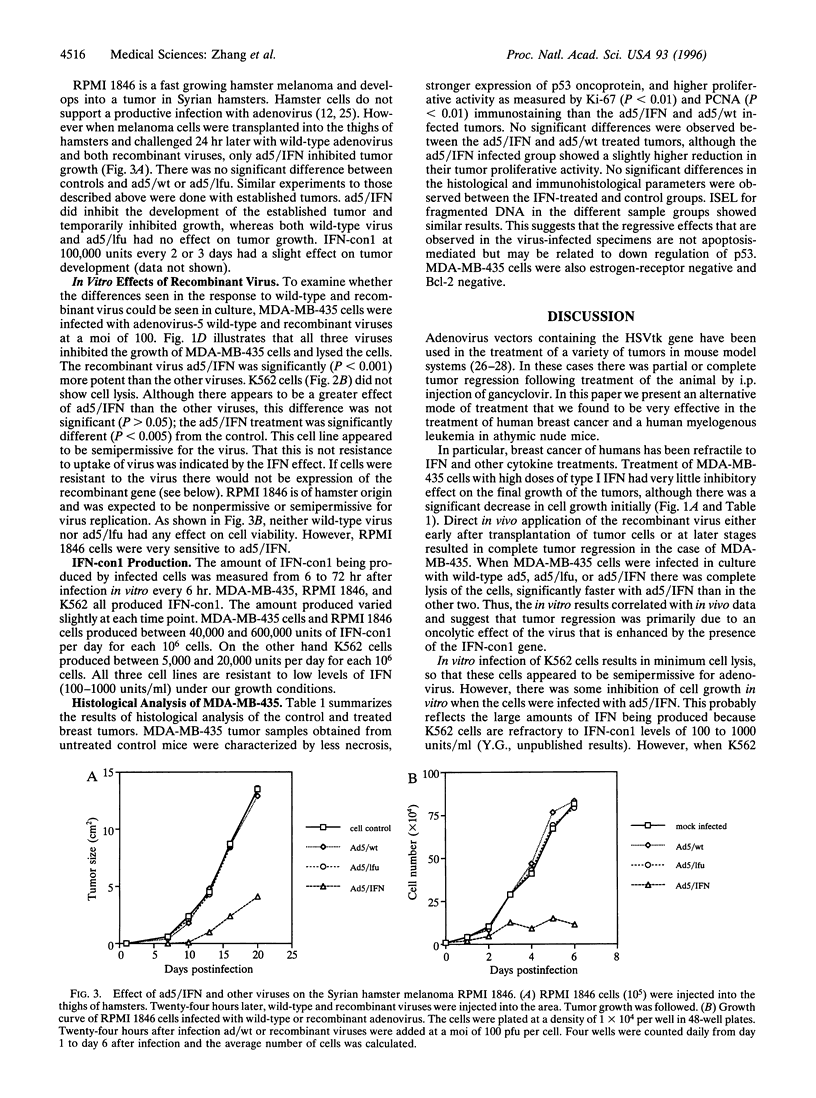
Treatment of a human breast cancer cell line (MDA-MB-435) in nude mice with a recombinant adenovirus containing the human interferon (IFN) consensus gene, IFN-con1 (ad5/IFN), resulted in tumor regression in 100% of the animals. Tumor regression occurred when virus was injected either within 24 hr of tumor cell implantation or with established tumors. However, regression of the tumor was also observed in controls in which either the wild-type virus or a recombinant virus containing the luciferase gene was used, although tumor growth was not completely suppressed. Tumor regression was accompanied by a decrease in p53 expression. Two other tumors, the human myelogenous leukemic cell line K562 and the hamster melanoma tumor RPMI 1846, also responded to treatment but only with ad5/IFN. In the case of K562 tumors, there was complete regression of the tumor, and tumors derived from RPMI 1846 showed partial regression. We propose that the complete regression of the breast cancer with the recombinant virus ad5/IFN was the result of two events: viral oncolysis in which tumor cells are being selectively lysed by the replication-competent virus and the enhanced effect of expression of the IFN-con1 gene. K562 and RPMI 1846 tumors regressed only as a result of IFN gene therapy. This was confirmed by in vitro analysis. Our results indicate that a combination of viral oncolysis with a virus of low pathogenicity, itself resistant to the effects of IFN and IFN gene therapy, might be a fruitful approach to the treatment of a variety of different tumors, in particular breast cancers.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnekoh B., Greenhalgh D. A., Bundman D. S., Eckhardt J. N., Longley M. A., Chen S. H., Woo S. L., Roop D. R. Inhibition of melanoma growth by adenoviral-mediated HSV thymidine kinase gene transfer in vivo. J Invest Dermatol. 1995 Mar;104(3):313–317. doi: 10.1111/1523-1747.ep12664614. [DOI] [PubMed] [Google Scholar]
- Bártek J., Bártková J., Lukás J., Stasková Z., Vojtesek B., Lane D. P. Immunohistochemical analysis of the p53 oncoprotein on paraffin sections using a series of novel monoclonal antibodies. J Pathol. 1993 Jan;169(1):27–34. doi: 10.1002/path.1711690106. [DOI] [PubMed] [Google Scholar]
- Caillaud C., Akli S., Vigne E., Koulakoff A., Perricaudet M., Poenaru L., Kahn A., Berwald-Netter Y. Adenoviral vector as a gene delivery system into cultured rat neuronal and glial cells. Eur J Neurosci. 1993 Oct 1;5(10):1287–1291. doi: 10.1111/j.1460-9568.1993.tb00914.x. [DOI] [PubMed] [Google Scholar]
- Cattoretti G., Becker M. H., Key G., Duchrow M., Schlüter C., Galle J., Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992 Dec;168(4):357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- Daidone M. G., Silvestrini R., Luisi A., Mastore M., Benini E., Veneroni S., Brambilla C., Ferrari L., Greco M., Andreola S. Changes in biological markers after primary chemotherapy for breast cancers. Int J Cancer. 1995 May 4;61(3):301–305. doi: 10.1002/ijc.2910610304. [DOI] [PubMed] [Google Scholar]
- Dykins R., Corbett I. P., Henry J. A., Wright C., Yuan J., Hennessy C., Lennard T. J., Angus B., Horne C. H. Long-term survival in breast cancer related to overexpression of the c-erbB-2 oncoprotein: an immunohistochemical study using monoclonal antibody NCL-CB11. J Pathol. 1991 Feb;163(2):105–110. doi: 10.1002/path.1711630205. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y., Yu D., Blatt L. M., Taylor M. W. Tumor suppressor activity of the human consensus type I interferon gene. Cytokines Mol Ther. 1995 Dec;1(4):289–300. [PubMed] [Google Scholar]
- Hall P. A., Levison D. A., Woods A. L., Yu C. C., Kellock D. B., Watkins J. A., Barnes D. M., Gillett C. E., Camplejohn R., Dover R. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol. 1990 Dec;162(4):285–294. doi: 10.1002/path.1711620403. [DOI] [PubMed] [Google Scholar]
- Hendricks J. B., Wilkinson E. J. Comparison of two antibodies for evaluation of estrogen receptors in paraffin-embedded tumors. Mod Pathol. 1993 Nov;6(6):765–770. [PubMed] [Google Scholar]
- Hu C. J., Ozes O. N., Klein S. B., Blatt L. M., Taylor M. W. Comparison of the in vitro host range of recombinant met-interferon-con1, interferon-alpha 2b, and interferon-beta [corrected]. J Interferon Cytokine Res. 1995 Mar;15(3):231–234. doi: 10.1089/jir.1995.15.231. [DOI] [PubMed] [Google Scholar]
- Kitajewski J., Schneider R. J., Safer B., Munemitsu S. M., Samuel C. E., Thimmappaya B., Shenk T. Adenovirus VAI RNA antagonizes the antiviral action of interferon by preventing activation of the interferon-induced eIF-2 alpha kinase. Cell. 1986 Apr 25;45(2):195–200. doi: 10.1016/0092-8674(86)90383-1. [DOI] [PubMed] [Google Scholar]
- Longiaru M., Horwitz M. S. Chinese hamster ovary cells replicate adenovirus deoxyribonucleic acid. Mol Cell Biol. 1981 Mar;1(3):208–215. doi: 10.1128/mcb.1.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews T., Boehme R. Antiviral activity and mechanism of action of ganciclovir. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 3):S490–S494. doi: 10.1093/clinids/10.supplement_3.s490. [DOI] [PubMed] [Google Scholar]
- Naso C., Simoni C., Merlini L., Rosso M., Pronzato P., Repetto L., Gardin G., Rosso R. Lymphoblastoid interferon in advanced breast cancer: a phase II study. J Chemother. 1993 Aug;5(4):258–261. doi: 10.1080/1120009x.1993.11739241. [DOI] [PubMed] [Google Scholar]
- Oldham R. K., Blumenschein G., Schwartzberg L., Birch R., Arnold J. Combination biotherapy utilizing interleukin-2 and alpha interferon in patients with advanced cancer: a National Biotherapy Study Group Trial. Mol Biother. 1992 Mar;4(1):4–9. [PubMed] [Google Scholar]
- Orazi A., Cattoretti G., Heerema N. A., Sozzi G., John K., Neiman R. S. Frequent p53 overexpression in therapy related myelodysplastic syndromes and acute myeloid leukemias: an immunohistochemical study of bone marrow biopsies. Mod Pathol. 1993 Sep;6(5):521–525. [PubMed] [Google Scholar]
- Repetto L., Venturino A., Simoni C., Rosso M., Melioli G., Rosso R. Interferons in the treatment of advanced breast cancer. J Biol Regul Homeost Agents. 1993 Oct-Dec;7(4):109–114. [PubMed] [Google Scholar]
- Rosenfeld M. A., Yoshimura K., Trapnell B. C., Yoneyama K., Rosenthal E. R., Dalemans W., Fukayama M., Bargon J., Stier L. E., Stratford-Perricaudet L. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992 Jan 10;68(1):143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- Saccani Jotti G., Johnston S. R., Salter J., Detre S., Dowsett M. Comparison of new immunohistochemical assay for oestrogen receptor in paraffin wax embedded breast carcinoma tissue with quantitative enzyme immunoassay. J Clin Pathol. 1994 Oct;47(10):900–905. doi: 10.1136/jcp.47.10.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz G. G., Walter T., Seibl R., Kessler C. Nonradioactive labeling of oligonucleotides in vitro with the hapten digoxigenin by tailing with terminal transferase. Anal Biochem. 1991 Jan;192(1):222–231. doi: 10.1016/0003-2697(91)90212-c. [DOI] [PubMed] [Google Scholar]
- Siekierka J., Mariano T. M., Reichel P. A., Mathews M. B. Translational control by adenovirus: lack of virus-associated RNAI during adenovirus infection results in phosphorylation of initiation factor eIF-2 and inhibition of protein synthesis. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1959–1963. doi: 10.1073/pnas.82.7.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkovics J., Horvath J. New developments in the virus therapy of cancer: a historical review. Intervirology. 1993;36(4):193–214. doi: 10.1159/000150339. [DOI] [PubMed] [Google Scholar]
- Smith T. A., Mehaffey M. G., Kayda D. B., Saunders J. M., Yei S., Trapnell B. C., McClelland A., Kaleko M. Adenovirus mediated expression of therapeutic plasma levels of human factor IX in mice. Nat Genet. 1993 Dec;5(4):397–402. doi: 10.1038/ng1293-397. [DOI] [PubMed] [Google Scholar]
- Stratford-Perricaudet L. D., Levrero M., Chasse J. F., Perricaudet M., Briand P. Evaluation of the transfer and expression in mice of an enzyme-encoding gene using a human adenovirus vector. Hum Gene Ther. 1990 Fall;1(3):241–256. doi: 10.1089/hum.1990.1.3-241. [DOI] [PubMed] [Google Scholar]
- Taylor M. W., Cordell B., Souhrada M., Prather S. Viruses as an aid to cancer therapy: regression of solid and ascites tumors in rodents after treatment with bovine enterovirus. Proc Natl Acad Sci U S A. 1971 Apr;68(4):836–840. doi: 10.1073/pnas.68.4.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbright T. M., Orazi A., de Riese W., de Riese C., Messemer J. E., Foster R. S., Donohue J. P., Eble J. N. The correlation of P53 protein expression with proliferative activity and occult metastases in clinical stage I non-seminomatous germ cell tumors of the testis. Mod Pathol. 1994 Jan;7(1):64–68. [PubMed] [Google Scholar]
- WHEELOCK E. F., DINGLE J. H. OBSERVATIONS ON THE REPEATED ADMINISTRATION OF VIRUSES TO A PATIENT WITH ACUTE LEUKEMIA. A PRELIMINARY REPORT. N Engl J Med. 1964 Sep 24;271:645–651. doi: 10.1056/NEJM196409242711302. [DOI] [PubMed] [Google Scholar]
- Wang Q., Taylor M. W. Correction of a deletion mutant by gene targeting with an adenovirus vector. Mol Cell Biol. 1993 Feb;13(2):918–927. doi: 10.1128/mcb.13.2.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijsman J. H., Jonker R. R., Keijzer R., van de Velde C. J., Cornelisse C. J., van Dierendonck J. H. A new method to detect apoptosis in paraffin sections: in situ end-labeling of fragmented DNA. J Histochem Cytochem. 1993 Jan;41(1):7–12. doi: 10.1177/41.1.7678025. [DOI] [PubMed] [Google Scholar]
- Yei S., Bachurski C. J., Weaver T. E., Wert S. E., Trapnell B. C., Whitsett J. A. Adenoviral-mediated gene transfer of human surfactant protein B to respiratory epithelial cells. Am J Respir Cell Mol Biol. 1994 Sep;11(3):329–336. doi: 10.1165/ajrcmb.11.3.8086169. [DOI] [PubMed] [Google Scholar]
- Zabner J., Petersen D. M., Puga A. P., Graham S. M., Couture L. A., Keyes L. D., Lukason M. J., St George J. A., Gregory R. J., Smith A. E. Safety and efficacy of repetitive adenovirus-mediated transfer of CFTR cDNA to airway epithelia of primates and cotton rats. Nat Genet. 1994 Jan;6(1):75–83. doi: 10.1038/ng0194-75. [DOI] [PubMed] [Google Scholar]
- Zhang J. F., Hu C., Geng Y., Blatt L. M., Taylor M. W. Gene therapy with an adeno-associated virus carrying an interferon gene results in tumor growth suppression and regression. Cancer Gene Ther. 1996 Jan-Feb;3(1):31–38. [PubMed] [Google Scholar]



