Abstract
In humans, theta phase (4–8 Hz) synchronization observed on electroencephalography (EEG) plays an important role in the manipulation of mental representations during working memory (WM) tasks; fronto-temporal synchronization is involved in auditory-verbal WM tasks and fronto-parietal synchronization is involved in visual WM tasks. However, whether or not theta phase synchronization is able to select the to-be-manipulated modalities is uncertain. To address the issue, we recorded EEG data from subjects who were performing auditory-verbal and visual WM tasks; we compared the theta synchronizations when subjects performed either auditory-verbal or visual manipulations in separate WM tasks, or performed both two manipulations in the same WM task. The auditory-verbal WM task required subjects to calculate numbers presented by an auditory-verbal stimulus, whereas the visual WM task required subjects to move a spatial location in a mental representation in response to a visual stimulus. The dual WM task required subjects to manipulate auditory-verbal, visual, or both auditory-verbal and visual representations while maintaining auditory-verbal and visual representations. Our time-frequency EEG analyses revealed significant fronto-temporal theta phase synchronization during auditory-verbal manipulation in both auditory-verbal and auditory-verbal/visual WM tasks, but not during visual manipulation tasks. Similarly, we observed significant fronto-parietal theta phase synchronization during visual manipulation tasks, but not during auditory-verbal manipulation tasks. Moreover, we observed significant synchronization in both the fronto-temporal and fronto-parietal theta signals during simultaneous auditory-verbal/visual manipulations. These findings suggest that theta synchronization seems to flexibly connect the brain areas that manipulate WM.
Keywords: working memory, electroencephalogram, synchronization, visual, auditory
Introduction
Working memory (WM) consists of not only a short-term maintenance system, but also a central executive system that manipulates the maintained representation (Baddeley, 2007). Previous studies have proposed that the executive system is associated with the prefrontal area, whereas the maintenance system is distributed in more posterior sensory areas, such as occipito/parietal areas for visual WM and temporal areas for auditory-verbal WM (Smith and Jonides, 1999; Rowe et al., 2000; Wager and Smith, 2003; Sakai and Passingham, 2004). Recent human electroencephalography (EEG) studies have demonstrated an important role for large-scale phase synchronization in WM (Fries, 2005; Klimesch et al., 2008), as such synchronous neural oscillations are thought to link multiple brain regions dynamically (Engel and Singer, 2001; Varela et al., 2001; Ward, 2003). In fact, the theta-range (4–8 Hz) phase synchronizations between the prefrontal cortex and the relevant cortical areas form the executive functions that link the storage systems during the manipulation of mental representations (Kawasaki et al., 2010). However, there remain open questions regarding how relevant information is flexibly selected for manipulation in the mind and how irrelevant information is stored during the mental manipulations that are required to perform multiple tasks simultaneously.
To investigate brain-network dynamics, we measured EEG signals during a single or dual task for two sensory modalities and analyzed phase synchronization between distant cortical areas. Here, we used two types of WM manipulation tasks: an auditory-verbal WM task, which required the mental calculation of numbers presented through an auditory-verbal stimulus; and a visual WM task, which required the participants to move a spatial location in a mental representation in accordance with a visual stimulus. In addition to the two single WM tasks, each subject performed the two tasks sequentially or simultaneously, as dual tasks.
We conducted region-of-interest analyses, because we had previously identified the representative electrodes of prefrontal, auditory-verbal, and visual areas by analyzing EEG data from the same two single WM tasks (Kawasaki et al., 2010). The theta amplitudes on the frontal and visual electrodes and on the frontal and auditory-verbal electrodes were enhanced during the manipulation of visual and auditory-verbal representations, respectively. Moreover, theta phase synchronization between the electrodes was observed for the relevant WM tasks.
Materials and methods
Subjects
Fourteen healthy volunteers (10 males and 4 females; mean age = 27.92 ± 6.76 years, range, 21–41 years; 13 right-handed individuals) participated in this experiment. The subjects reported via subjective questionnaires regarding having normal visual acuity (with or without correction), hearing, and motor abilities. All subjects gave written informed consent prior to participation in this study. The study was approved by the RIKEN Ethics Committee (in accordance with the Declaration of Helsinki).
Experimental procedure
Each subject completed five separate sessions in a random order among subjects: one auditory-verbal WM condition, one visual WM condition, two sequential dual WM condition, and one simultaneous dual WM condition. Throughout the sessions, the subjects faced a computer screen with headphones.
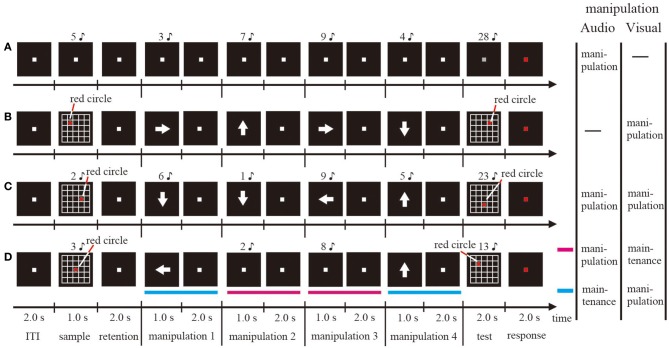
For the auditory-verbal WM condition (Figure 1A), at the beginning of each trial, a word indicating a 1-digit number was presented as the auditory-verbal stimulus to both ears for 1 s via the headphones (sample stimulus). Subjects were required to memorize and maintain the presented number with rehearsal in their minds. After a 2-s retention interval, another 1-digit number was presented as the audio stimulus for 1 s, and then the subjects were asked to update and maintain the number in their minds by adding the presented number and the maintained number for 2 s. Subjects were required to repeat the mental addition four times, and then determine whether the total number that they calculated mentally matched a probe audio stimulus presented after a white fixation point on a gray PC display (test stimulus). In half of the trials, the test stimulus matched the total number. In the remaining trials, the wrong total number was presented as the probe stimulus by replacing one of the four numbers presented with a different number.
Figure 1.
Schematic illustrations of one trial sequence for the visual single WM (A), the auditory-verbal single WM (B), the simultaneous WM (C), and the sequential WM (D) tasks.
For the visual WM condition (Figure 1B), at the beginning of each trial, 5 × 5 gridded squares and a red circle included within one of those squares were presented for 1 s on the computer screen as the visual stimulus (sample stimulus). Subjects were required to memorize and then maintain the position of the red circle for 2 s after the visual stimulus disappeared. Subsequently, a white arrow designating the direction toward which the subjects had to move the red circle in their minds was presented at the center of the screen for 1 s. The subjects manipulated the mental representations for 2 s. The direction of the arrow was upward, downward, rightward, or leftward. Similar to the audio WM condition, the subjects were required to repeat the mental manipulation four times, and then determine whether the position of the red circle that they moved mentally matched a probe visual stimulus (test stimulus). In half of the trials, the probe stimulus matched the mental representation. In the remaining trials, the wrong probe was presented by changing only the fourth direction of the moving from the actual direction. The size of the red circle and the gridded squares were 1 × 1° and 5 × 5° (1 × 1° per square), respectively.
For the sequential dual WM condition (Figure 1D), the visual and audio stimuli described above were presented simultaneously on the computer screen and via the headphones, respectively (sample stimulus). Subjects were required to memorize both the auditory-verbal and visual stimuli and, after the retention interval, manipulate either the auditory-verbal or the visual representation. If an arrow was presented on the screen, subjects performed the visual WM task; if a number was presented via the headphones, they performed the auditory-verbal WM task. Each task was performed randomly twice. The subjects were not aware of the sequences of auditory-verbal and visual WM tasks. After a total of four manipulations, the auditory-verbal and visual stimuli were presented simultaneously and subjects were required to judge whether they were identical to the manipulated mental representation for both auditory-verbal and visual WM tasks or not (test stimulus). In half of the trials, both the auditory-verbal and visual test stimuli matched the mental representations. In the remaining trials, the wrong probe for either auditory-verbal or visual stimuli was presented, similar to what was observed in the single auditory-verbal and visual WM conditions.
For the simultaneous dual WM condition (Figure 1C), the procedures of sample display, retention interval, and test stimulus were identical to those used in the sequential dual WM condition, with the exception that the auditory-verbal and visual stimuli were presented simultaneously and the participants were asked to perform both the auditory-verbal and visual WM tasks during each manipulation period.
In all conditions, subjects were asked to indicate, by pressing a button, whether the stimulus was correct or not while the fixation point was kept red for 2 s. Each session consisted of 24 trials. The duration of the inter-trial interval (ITI) was 2 s (Figure 1). The ITI was defined as the baseline period in this study. In a given session, one condition was being tested. All participants underwent a training session before the corresponding EEG measurement session. The stimulus was generated on a Windows computer using Matlab 7.5.0 (Mathworks, Inc., Natick, MA) with the Psychophysics Toolbox extension. The sound of each number was highly distinctive.
EEG recordings and analyses
EEG was recorded continuously from 62 scalp electrodes (Ag/AgCl) embedded in an electrode cap (Easy Cap; EASYCAP GmbH, Germany) and in accordance with the placement of the international 10/10 system. EEG signals were referenced digitally to the averaged recordings from the right and left earlobes. Electrode impedance was maintained below 14.6 kΩ. Electrooculography (EOG) was recorded from electrodes that were placed above and below the left eye, to monitor eye blinks or vertical eye movements. EOG electrodes placed 1 cm lateral from the right and left eyes monitored horizontal eye movements. The EEG and EOG signals were amplified using the Neuroscan system (Neuroscan, USA). The sampling rate was 500 Hz.
EEG data were preprocessed by first segmenting the EEG data into 5-s epochs (with 1-s pre-manipulation, 3-s manipulation, and 1-s post-manipulation periods; 2500 time points in total). Epochs containing artifacts caused by blinks or eye movements were detected from the EOG and EEG data using an amplitude criterion (±100 μV) and were excluded from further analyses.
Next, to identify cortical activity with reduced effects of volume conduction, we applied a current source density transformation to the voltage distribution on the surface of the scalp using the spherical Laplace operator (Perrin et al., 1989; Kayser and Tenke, 2006). Finally, to identify the time-frequency phases, we applied wavelet transforms using Morlet's wavelet function (Tallon-Baudry et al., 1997).
We used Morlet's wavelets for the high time and frequency resolutions, which allowed a better observation of transitions in both low- and high-frequency oscillations (Herrmann et al., 2005). The phase for each time point in each transcranial magnetic stimulation (TMS) application was the arctangent of the results of the convolution of the original EEG signal s(t) with a complex Morlet's wavelet function w(t, f):
where σt is the standard deviation of the Gaussian window. The wavelet used here was roughly characterized by the number of cycles nco within a 6σt interval (Lachaux et al., 2000), which contains about 99.7% of the power of the Gaussian window. We chose nco = 3 (= 6fσt), with f ranging from 1 to 20 Hz in 1-Hz steps.
Phase synchronization index (PSI)
To identify the phase relations between any two electrodes, the PSI for each time point and each electrode pair was defined by the following equation:
where Δ Φj k(t, f) is the phase difference between jth and kth electrodes and the number of time points N with an interval of 1 s is 500.
To evaluate the task-related PSI changes, we applied a bootstrap calculation to the PSIs of the individual subjects and compared the virtual PSI data during the tasks [φAj k (t, f)] and baseline data of the ITI period [φBj k (t, f)], as follows:
where φA*j k (t, f) and φB*j k (t, f) represent the original PSI, φ Aj k (f), φ Bj k (f), and φj k(f) represent the means of φAj k (t, f), φBj k (t, f), and all of the data, respectively. We performed a 2-sample t-test using the 2000 bootstrapped re-samples of each time point for individual subjects (Kawasaki et al., 2010).
We calculated the Z-values which mean “the degrees about whether differences are significant or not” by using the non-parametric Wilcoxon signed-rank test for the different normalized PSI values. The null hypothesis is that the difference of the representative PSI values between the events equals to zero. If the Z-value is near zero, the different PSI values between the events are not significant (the null hypothesis is not rejected). On the other hand, if the Z-value is larger than the statistical threshold, the difference in PSI values between the events is statistically significant (the null hypothesis is rejected). To test if the significance results from a chance, we repeated the Wilcoxon signed-rank tests for 2000 times with the bootstrapped resample data of each time point for individual subjects.
Finally, we tested whether the mean of the distribution of the 2000 resampled Z-values is zero or not by using the sign test against the null hypothesis that the mean of Z-values equals to zero. If the Z-value is near zero, the difference in PSI values between the events is not significant (the null hypothesis is not rejected). On the other hand, if the Z-value is larger than the significance threshold, it is no coincidence that the difference in PSI values between the events is statistically significant (the null hypothesis is rejected).
Results
Behavioral results
The subject-averaged accuracy rates were 95.2 ± 1.6, 97.3 ± 1.2, 89.9 ± 1.9, and 94.9 ± 1.1% (mean accuracy rate ± s.e.m.) for auditory-verbal (A), visual (V), simultaneous dual (Sim), and sequential dual (Seq) WM conditions, respectively (Table1).
Table 1.
Mean accuracy rate ± standard error mean (s.e.m.) for each condition.
| Audio | Visual | Sim. dual | Seq. dual | |
|---|---|---|---|---|
| Mean accuracy rate ± s.e.m. (%) | 95.2 ± 1.6 | 97.3 ± 1.2 | 89.9 ± 1.9 | 94.9 ± 1.1 |
A one-way repeated measures ANOVA revealed a significant main effect of the task conditions [F(3, 39) = 5.18, P < 0.005]. There was a significant difference between visual WM and sequential dual WM conditions but not among other combinations. The post-hoc analyses (Tukey HSD test) revealed HSD-values as follows: A vs. V, HSD = 0.021; A vs. Sim, HSD = −0.042; A vs. Seq, HSD = −0.003; V vs. Sim, HSD = −0.063, P < 0.01; V vs. Seq, HSD = −0024; Sim vs. Seq, HSD = 0.039.
PSI results
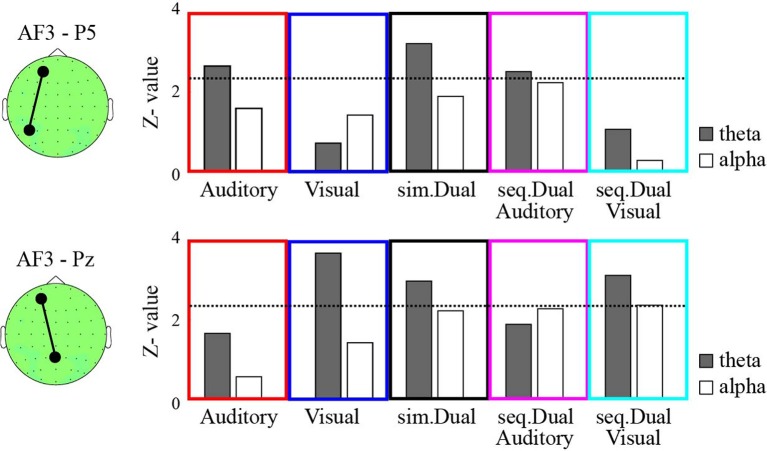
This study selected the AF3, P5, and Pz electrodes as representative frontal, temporal, and parietal electrodes, respectively, because the AF3-P5 and AF3-Pz pairs showed clear synchronizations during WM manipulation periods in our previous study (Kawasaki et al., 2010). We calculated the PSI between a pair of these electrodes at the theta (6 Hz) band during the manipulation periods (for 1 s after the offset of the manipulation cue) compared with the corresponding values recorded during the ITI under the four conditions.
The bootstrapped results revealed that the theta (6 Hz) PSIAF3−P5 was significant during the manipulation periods (for 1 s after the offset of the manipulation cue) compared with the corresponding values recorded during the ITI in the auditory-verbal WM, simultaneous WM, and auditory-verbal manipulation of sequential WM (P < 0.01; Figure 2). In contrast, the theta PSIAF3−Pz significantly increased in the visual WM, simultaneous WM, and visual manipulation of sequential WM (P < 0.01). No significant increases were observed in the theta PSIAF3−P5 for the visual WM and the PSIAF3−Pz for the auditory-verbal WM.
Figure 2.
The z-values of the phase synchronization index (PSI) between AF3 and P5 (top) and between AF3 and Pz (bottom) during the manipulation periods compared with the ITI under the auditory-verbal single WM (red), the visual single WM (blue), the simultaneous WM (black), the auditory-verbal manipulation in the sequential WM (magenta), and the auditory-verbal manipulation in the sequential WM (cyan) tasks. The gray and white bars indicate the z-values of the theta and alpha synchronizations, respectively. The dotted lines in each panel denote the threshold value (P < 0.01). The topographies on the left show the AF3, P5, and Pz electrodes on the recording montage.
Moreover, we analyzed the PSI of the higher-frequency bands (i.e., alpha, beta, and gamma); however, the PSIAF3−P5 and the PSIAF3−Pz observed during the auditory-verbal and visual WM conditions were not significantly different between the manipulation periods and the ITI. The PSI results of the alpha (12 Hz) band are shown in Figure 2, as examples.
Discussion
The present study demonstrated clearly that theta phase synchronization played an important role in selecting and connecting the relevant brain areas during WM manipulation. A previous study showed that the frontal area is significantly synchronized with the parietal areas (i.e., visual regions) or with the temporal areas (i.e., auditory-verbal regions) during the manipulation of visual or auditory-verbal representation, respectively (Kawasaki et al., 2010). In addition, the present study used sequential dual WM tasks, which require manipulation of one modality and maintenance of the other modality, and showed theta phase synchronization only between the relevant brain regions (e.g., fronto-parietal synchronization for the visual manipulation). In contrast, both fronto-parietal and fronto-temporal theta phase synchronizations were observed during the simultaneous dual WM, which requires the manipulation and maintenance of both modalities.
The slow-oscillatory (i.e., theta) synchronization reflects the dynamic, long-range linking of task-relevant brain areas within WM brain networks (Sauseng et al., 2005; Mizuhara and Yamaguchi, 2007; Klimesch et al., 2008; Kawasaki et al., 2010). Previous EEG studies in human have shown that theta phase synchronization between frontal and temporal regions is observed during several WM tasks including encoding, maintenance, and retrieval processes (Sarnthein et al., 1998; Sauseng et al., 2004; Serrien et al., 2004). Moreover, fronto-parietal theta synchronization is enhanced under WM tasks which required high WM load and complex manipulation (Sauseng et al., 2005; Kopp et al., 2006; Payne and Kounios, 2009). The present study also supports the hypothesis that theta synchronization is a requirement of executive function.
Functional theta synchronization is also observed within the resting-state brain network (Von Stein and Sarnthein, 2000; Buzsaki and Draguhn, 2004; Jensen and Colgin, 2007) and long-term memory (especially memory formation) brain networks (Summerfield and Mangels, 2005; Sato and Yamaguchi, 2007). For example, theta synchronization between frontal lobe and hippocampus appeared during long-term memory formation and stimulus encoding (Hasselmo et al., 2002). Moreover, a recent study based on TMS and EEG showed that the resetting of the theta phase propagates directionally from the TMS-targeted areas to the sensory-motor brain areas (Kawasaki et al., 2014). These findings suggest an important role for theta synchronization in large-scale communication and information transfer among distant “task-relevant” brain regions (Fries, 2005; Fell and Axmacher, 2011).
In contrast to global theta synchronization, higher-frequency oscillations would show local synchronizations within circumscribed brain areas during WM (Lutzenberger et al., 2002; Babiloni et al., 2004; Klimesch, 2012), although some studies have reported global beta or gamma phase synchronizations (Tallon-Baudry et al., 2001; Axmacher et al., 2008). Unlike low frequency, the higher-frequency phases are difficult to synchronize across global areas, since such synchronization requires accurate simultaneous activation of areas that are far apart. Indeed, the present study found no significant phase synchronization between brain regions in the high-frequency bands for any WM manipulation. Our previous study, which was performed using the same tasks, showed enhancements of the alpha oscillations in the local brain areas in WM maintenance periods. Moreover, many studies demonstrated the presence of amplitude modulations in the local relevant brain areas during several cognitive tasks (Klimesch et al., 2008), although some studies reported global gamma phase synchronization (Rodriguez et al., 1999; Doesburg et al., 2008). Such high oscillations are assumed to be functionally related to the slow oscillations, such as the coupling between theta phases and gamma amplitudes (Klimesch et al., 2008) and the coupling between theta and beta amplitudes (Kawasaki and Yamaguchi, 2013). The relationships between the global theta and high-frequency phase synchronization in WM should be clarified in future studies.
The finding of modality-specific posterior brain regions and modality-nonspecific frontal regions would be consistent with previous psychological WM models which propose the existence of modality-independent sensory storage buffers (e.g., phonological loop and visuo-spatial sketchpad) and a common central executive (e.g., Scarborough, 1972; Baddeley, 1986; Luck and Vogel, 1997). The overlapped prefrontal cortex are synchronized with not only the visual (parietal) areas but also the auditory-verbal (temporal) areas in the simultaneous dual WM task, which might lead to dual-task interference, that is, degraded performance relative to a single task when two tasks are being performed simultaneously (i.e., psychological refractory period) (Pashler, 1994; Logan and Gordon, 2001). Moreover, the enhancements of frontal activity during the shortened stimulus onset asynchrony between the two tasks in the dual WM tasks would then be the neural factor responsible for the bottleneck in executive functions (Herath et al., 2001; Jiang et al., 2004; Marois and Ivanoff, 2005).
The behavioral data showed that the accuracy rates under the simultaneous dual WM condition were lower than under the other three conditions. This might be consistent with previous findings, which suggest greater difficulty with greater dual-tasking (i.e., psychological refractory period) (Pashler, 1994; Logan and Gordon, 2001). However, it is difficult to compare among conditions, because the accuracy rate was very high, over 90%; that is to say, there is a ceiling effect in our behavioral data. Therefore, there might be a possibility that the results would be different under conditions of increased difficulty.
On the other hand, due to the high accuracy rates, we could accurately identify the theta synchronization by using EEG data from the many trials in which the subjects successfully manipulated the WM representations. However, on this point also, we have no findings on how prominent the synchronization would be at greater task difficulty.
In this study, the theta synchronization might be involved in the manipulation rather than in maintenance of representations. Although the one-modality manipulation periods under the sequential WM condition are defined as not only the manipulation of one modality but also the maintenance of the other, the theta synchronization was observed between only the to-be-manipulated modality-related brain areas (e.g., fronto-parietal theta synchronization under the visual manipulation periods). However, the subjects could have processed the other modality's executive function (e.g., selection of the to-be-manipulated modality and inhibition of the to-be-maintained modality). Moreover, there is a limitation on pure comparison between manipulation and maintenance in our tasks, since manipulation always included maintenance. To address this issue, future study should isolate these functions and compare among them clearly.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The research was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (21120005 and 25119512) and a Grant-in-Aid for Young Scientists (B) (23700328).
References
- Axmacher N., Schmitz D. P., Wagner T., Elger C. E., Fell J. (2008). Interactions between medial temporal lobe, prefrontal cortex, and inferior temporal regions during visual working memory, a combined intracranial EEG and functional magnetic resonance imaging study. J. Neurosci. 28, 7304–7312 10.1523/JNEUROSCI.1778-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C., Miniussi C., Moretti D. V., Vecchio F., Salinari S., Frisioni G., et al. (2004). Functional frontoparietal connectivity during short-term memory as revealed by high-resolution EEG coherence analysis. Behav. Neurosci. 118, 687–697 10.1037/0735-7044.118.4.687 [DOI] [PubMed] [Google Scholar]
- Baddeley A. D. (1986). Working Memory. Oxford: Oxford University Press [Google Scholar]
- Baddeley A. D. (2007). Working Memory, Thought and Action. Oxford: Oxford University Press; 10.1093/acprof:oso/9780198528012.001.0001 [DOI] [Google Scholar]
- Buzsaki G., Draguhn A. (2004). Neuronal oscillations in cortical networks. Science 304, 1926–1929 10.1126/science.1099745 [DOI] [PubMed] [Google Scholar]
- Doesburg S. M., Roggeveen A. B., Kitajo K., Ward L. M. (2008). Large-scale gamma-band phase synchronization and selective attention. Cereb. Cortex 18, 386–396 10.1093/cercor/bhm073 [DOI] [PubMed] [Google Scholar]
- Engel A., Singer W. (2001). Temporal binding and the neural correlates of sensory awareness. Trends Cogn. Sci. 5, 16–25 10.1016/S1364-6613(00)01568-0 [DOI] [PubMed] [Google Scholar]
- Fell J., Axmacher N. (2011). The role of phase synchronization in memory processes. Nat. Rev. Neurosci. 12, 105–118 10.1038/nrn2979 [DOI] [PubMed] [Google Scholar]
- Fries P. (2005). A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn. Sci. 9, 474–480 10.1016/j.tics.2005.08.011 [DOI] [PubMed] [Google Scholar]
- Hasselmo M. E., Bodelón C., Wyble B. P. (2002). A proposed function for hippocampal theta rhythm, separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 14, 793–817 10.1162/089976602317318965 [DOI] [PubMed] [Google Scholar]
- Herath P., Klingberg T., Young J., Amunts K., Roland P. (2001). Neural correlates of dual task interference can be dissociated from those of divided attention: an fMRI study. Cereb. Cortex 11, 796–805 10.1093/cercor/11.9.796 [DOI] [PubMed] [Google Scholar]
- Herrmann C. S., Grigutsch M., Busch N. A. (2005). EEG oscillations and wavelet analysis in Event-Related Potentials: a Methods Handbook, ed Handy T. C. (Cambridge, MA: MIT Press; ), 229–259 [Google Scholar]
- Jensen O., Colgin L. L. (2007). Cross-frequency coupling between neuronal oscillations. Trends Cogn. Sci. 11, 267–269 10.1016/j.tics.2007.05.003 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Saxe R., Kanwisher N. (2004). Functional magnetic resonance imaging provides new constraints on theories of the psychological refractory period. Psychol. Sci. 15, 390–396 10.1111/j.0956-7976.2004.00690.x [DOI] [PubMed] [Google Scholar]
- Kawasaki M., Kitajo K., Yamaguchi Y. (2010). Dynamic links between theta executive functions and alpha storage buffers in auditory and visual working memory. Eur. J. Neurol. 31, 1683–1689 10.1111/j.1460-9568.2010.07217.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki M., Uno Y., Mori J., Kobata K., Kitajo K. (2014). Transcranial magnetic stimulation-induced global propagation of transient phase resetting associated with directional information flow. Front. Hum. Neurosci. 8:173 10.3389/fnhum.2014.00173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki M., Yamaguchi Y. (2013). Frontal theta and beta synchronizations for monetary reward increase visual working memory capacity. Soc. Affect. Cogn. Neurosci. 8, 523–530 10.1093/scan/nss027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser J., Tenke C. E. (2006). Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clin. Neurophysiol. 117, 348–368 10.1016/j.clinph.2005.08.034 [DOI] [PubMed] [Google Scholar]
- Klimesch W. (2012). α-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci. 16, 606–617 10.1016/j.tics.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W., Freunberger R., Sauseng P., Gruber W. (2008). A short review of slow phase synchronization and memory: evidence for control processes in different memory systems? Brain Res. 1235, 31–44 10.1016/j.brainres.2008.06.049 [DOI] [PubMed] [Google Scholar]
- Kopp F., Schröger E., Lipka S. (2006). Synchronized brain activity during rehearsal and short-term memory disruption by irrelevant speech is affected by recall mode. Int. J. Psychophysiol. 61, 188–203 10.1016/j.ijpsycho.2005.10.001 [DOI] [PubMed] [Google Scholar]
- Lachaux J. P., Rodriguez E., Le Van Quyen M., Lutz A., Martinerie J., Varela F. J. (2000). Studying single-trials of phase synchronous activity in the brain. Int. J. Bifurcat. Chaos 10, 2429–2439 10.1142/S0218127400001560 [DOI] [Google Scholar]
- Logan G. D., Gordon R. D. (2001). Executive control of visual attention in dual-task situations. Psychol. Rev. 108, 393–434 10.1037/0033-295X.108.2.393 [DOI] [PubMed] [Google Scholar]
- Luck S. J., Vogel E. K. (1997). The capacity of visual working memory for features and conjunctions. Nature 390, 279–281 10.1038/36846 [DOI] [PubMed] [Google Scholar]
- Lutzenberger W., Ripper B., Busse L., Birbaumer N., Kaiser J. (2002). Dynamics of gamma-band activity during an audiospatial working memory task in humans. J. Neurosci. 22, 5630–5638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marois R., Ivanoff J. (2005). Capacity limits of information processing in the brain. Trends Cogn. Sci. 9, 296–305 10.1016/j.tics.2005.04.010 [DOI] [PubMed] [Google Scholar]
- Mizuhara H., Yamaguchi Y. (2007). Human cortical circuits for central executive function emerge by theta phase synchronization. Neuroimage 36, 232–244 10.1016/j.neuroimage.2007.02.026 [DOI] [PubMed] [Google Scholar]
- Pashler H. (1994). Dual-task interference in simple tasks: data and theory. Psychol. Bull. 116, 220–244 10.1037/0033-2909.116.2.220 [DOI] [PubMed] [Google Scholar]
- Payne L., Kounios J. (2009). Coherent oscillatory networks supporting short-term memory retention. Brain Res. 1247, 126–132 10.1016/j.brainres.2008.09.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin F., Pernier J., Bertrand O., Echallier J. F. (1989). Spherical splines for scalp potential and current density mapping. Electroencephalogr. Clin. Neurophysiol. 72, 184–187 10.1016/0013-4694(89)90180-6 [DOI] [PubMed] [Google Scholar]
- Rodriguez E., George N., Lachaux J. P., Martinerie J., Renault B., Varela F. J. (1999). Perception's shadow: long-distance synchronization of human brain activity. Nature 397, 430–433 10.1038/17120 [DOI] [PubMed] [Google Scholar]
- Rowe J. B., Toni I., Josephs O., Frackowiak R. S. J., Passingham R. E. (2000). The prefrontal cortex: response selection or maintenance within working memory? Science 288, 1656–1660 10.1126/science.288.5471.1656 [DOI] [PubMed] [Google Scholar]
- Sakai K., Passingham R. E. (2004). Prefrontal selection and medial temporal lobe reactivation in retrieval of short—term verbal information. Cereb. Cortex 14, 163–168 10.1093/cercor/bhh050 [DOI] [PubMed] [Google Scholar]
- Sarnthein J., Petsche H., Rappelsberger P., Shaw G. L., von Stein A. (1998). Synchronization between prefrontal and posterior association cortex during human working memory. Proc. Natl. Acad. Sci. U.S.A. 95, 7092–7096 10.1073/pnas.95.12.7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N., Yamaguchi Y. (2007). Theta synchronization networks emerge during human object-place memory encoding. Neuroreport 18, 419–424 10.1097/WNR.0b013e3280586760 [DOI] [PubMed] [Google Scholar]
- Sauseng P., Klimesch W., Doppelmayr M., Hanslmayr S., Schabus M., Gruber W. R. (2004). Theta coupling in the human electroencephalogram during a working memory task. Neurosci. Lett. 354, 123–126 10.1016/j.neulet.2003.10.002 [DOI] [PubMed] [Google Scholar]
- Sauseng P., Klimesch W., Schabus M., Doppelmayr M. (2005). Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. Int. J. Psychophysiol. 57, 97–103 10.1016/j.ijpsycho.2005.03.018 [DOI] [PubMed] [Google Scholar]
- Scarborough D. L. (1972). Memory for brief visual displays of symbols. Cogn.Psychol. 3, 408–429 10.1016/0010-0285(72)90015-1 [DOI] [Google Scholar]
- Serrien D. J., Pogosyan A. H., Brown P. (2004). Influence of working memory on patterns of motor related cortico-cortical coupling. Exp. Brain Res. 155, 204–210 10.1007/s00221-003-1720-1 [DOI] [PubMed] [Google Scholar]
- Smith E. E., Jonides J. (1999). Neuroscience - storage and executive processes in the frontal lobes. Science 283, 1657–1661 10.1126/science.283.5408.1657 [DOI] [PubMed] [Google Scholar]
- Summerfield C., Mangels J. A. (2005). Coherent theta-band EEG activity predicts item-context binding during encoding. Neuroimage 24, 692–703 10.1016/j.neuroimage.2004.09.012 [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C., Bertrand O., Delpuech C., Pernier J. (1997). Oscillatory gamma band activity (30-70 Hz) induced by a visual search task in human. J. Neurosci. 17, 722–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C., Bertrand O., Fischer C. (2001). Oscillatory synchrony between human extrastriate areas during visual short-term memory maintenance. J. Neurosci. 21, RC177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela F., Lachaux J. P., Rodriguez E., Martinerie J. (2001). The brainweb: phase synchronization and large-scale integration. Nat. Rev. Neurosci. 2, 229–239 10.1038/35067550 [DOI] [PubMed] [Google Scholar]
- Von Stein A., Sarnthein J. (2000). Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int. J. Psychophysiol. 38, 301–313 10.1016/S0167-8760(00)00172-0 [DOI] [PubMed] [Google Scholar]
- Wager T. D., Smith E. E. (2003). Neuroimaging studies of working memory: a meta-analysis. Cogn. Affect. Behav. Neurosci. 3, 255–274 10.3758/CABN.3.4.255 [DOI] [PubMed] [Google Scholar]
- Ward L. M. (2003). Synchronous neural oscillations and cognitive processes. Trends Cogn. Sci. 7, 553–559 10.1016/j.tics.2003.10.012 [DOI] [PubMed] [Google Scholar]