Abstract
On the basis of a recent epidemiological study it is hypothesized that pyrite content in coal is an important factor in coal workers' pneumoconiosis (CWP) pathogenesis. While the role of pyrite in pathogenesis remains to be resolved, the ability of the mineral to generate reactive oxygen species (ROS) through various mechanisms is likely a contributing factor. The aim of this study was to elucidate the importance of the pyrite content of coal in generating an inflammatory stress response (ISR), which is defined as the upregulation of ROS normalized by cell viability. The ISR of A549 human lung epithelial cells in the presence of natural coal samples with variable pyrite contents was measured. Normalized to surface area, five particle loadings for each coal reference standard were analyzed systematically for a total of 24 h. The ISR generated by coals containing 0.00, 0.01, and 0.49 wt.% pyritic sulfur is comparable to,though less than, the ISR generated by inert glass beads (299% of the control). The coals containing 0.52 and 1.15 wt.% pyritic sulfur generated the greatest ISR (798% and 1426% of the control, respectively).
Conclusions
While ISR does not increase proportionally to pyrite content in coal, the two coals with the highest pyritic sulfur and available iron contents generate the greatest ISR. Therefore, the present study indicates that coals with elevated pyrite contents are likely to induce a significant health burden by stimulating inflammation within the lungs, and may contribute to the development of CWP.
Keywords: Pyrite, Iron, Coal workers' pneumoconiosis, Inflammatory stress response
1. Introduction
Colloquially referred to as black lung disease, coal workers' pneumoconiosis (CWP)1 is one of the most prevalent occupational diseases in the world. In the last decade of the twentieth century CWP accounted for half of the pneumoconiosis deaths in the United States (NIOSH, 2003). Given that coal miners only constitute 0.02% of the total United States workforce, they represent an extraordinarily high susceptibility group. Continued mechanization of underground mining and a shift to western coal fields, where surface mining predominates, is likely to diminish the prevalence of the disease among coal miners in the United States. However, coal production is rapidly expanding worldwide to satisfy the increasing energy demand. A 49% increase in production is estimated to occur between 2006 and 2030 (USEIA, 2009). Most of this expanded production is in East Asia, where mechanizationand safety regulations are lagging those established in the United States. Hence, CWP prevalence is likely to increase worldwide.
Due to the biopersistence of quartz and the symptomatic similarities, CWP was originally classified as a variant of silicosis. However, a recent epidemiological study linked the prevalence of this disease to the presence of the mineral pyrite in the coal (Huang et al., 2005). By examining the occurrence of CWP throughout the United States and analyzing coal samples in respective locations, a strong positive correlation is seen between the incidence of CWP and content of bioavailable iron (BAI) in coal (predominately pyrite; correlation coefficient r = 0.94) (Huang et al., 2005). In a separate study, a similarly strong positive correlation is seen between the occurrence of CWP and the pyritic sulfur content of coal (correlation coefficient r = 0.91) (Cohn et al., 2006a). Furthermore, the ability of coal samples with variable pyrite contents to generate reactive oxygen species (ROS) in a phosphate buffered solution was examined in this earlier work. It was determined that not only does the amount of hydrogen peroxide increase with increasing pyritic sulfur content, but so does the coal's ability to generate hydroxyl radical (correlation coefficient r = 0.99) (Cohn et al., 2006a).
Pyrite generates ROS as a result of the incomplete reduction of molecular oxygen via multiple mechanisms, most notably Fenton chemistry (Schoonen et al., 2006, 2010; Cohn et al., 2006b; Harrington et al., 2012a), a process that can take place in solution or on the minerals surface (Biegler et al., 1975; Moses et al., 1987; Moses and Herman, 1991; Rosso et al., 1999a,b; Elsetinow et al., 2001). Most studies on the oxidative dissolution of pyrite have been conducted in water. However, a recent study shows that the oxidation of pyrite in simulated lung fluid (SLF) also generates ROS (Harrington et al., 2012a).
Besides pyrite-driven ROS formation, ROS concentrations will increase in human tissue as a normal cellular response to exposure to particulate matter, bacteria, and other foreign substances (Bleck et al., 2010; Rada and Leto, 2010). While alveolar macrophage cells specialize in protecting the body after inhalation exposures (Martin and Frevert, 2005), epithelial cells also upregulate ROS (pre-dominately hydrogen peroxide) when challenged (Rada and Leto, 2010). The degree of hydrogen peroxide upregulation is an indicator of cellular stress and can be quantified using the 2′,7′-dichloro-fluoroscein-diacetate (DCFH-DA) assay. Upon addition to the slurry, DCFH-DA is taken into a healthy cell and converted to DCFH. Once stressed, the cell generates ROS – hydrogen peroxide and hydroxyl radical – and DCFH ishydrolyzed to become fluorescent DCF (Huang et al., 1993).
Another measure of particle toxicity is the ensuing cellular death. There are a number of different assays that measure cell death or viability. For this study the cell viability assay 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulf-ophenyl)-2H-tetrazolium (MTS) is utilized. It works by quantifying the production of phenazine methosulfate (PMS), which generates formazan in the presence of tetrazolium. The concentration of formazan product in the system is directly proportional to the number of living cells. Since any living cell generates PMS regardless of its health status, high values in the MTS assay are only an indicator of immediate cell viability and do not predict future apoptosis.
A previously published protocol describes a new procedure for cellular toxicity determination by measuring the aforementioned assays in combination in A549 cells challenged by mineral particulates (Harrington et al., 2012b). Defined as the formation of ROS within the A549 epithelial cell normalized to cell viability measurements, the inflammatory stress response (ISR) allows for a meaningful comparison of ROS generation while also accounting for the presence of cell death as a result of the exposure. By measuring the upregulation of ROS and the viability of the cells, we gain insight into the immediate toxicity of the treatment on the cells as well as the state of the remaining viable cells.
The objective of this paper is to evaluate the hypothesis that the inhalation of coal containing pyrite leads to inflammation in the human lungs. This hypothesis builds on earlier work that shows that pyrite, by itself, can generate ROS in water and SLF (Schoonen et al., 2010; Harrington et al., 2012a) as well as upregulate ROS formation in epithelial cells (Harrington et al., 2012b). ROS upregulation in cells results in cellular oxidative stress (Fubini, 1997; Vallyathan et al., 1998; Fubini and Hubbard, 2003). Ultimately, chronic cellular stress can lead to tissue damage and diseases, such as CWP (Spector, 2000). In this study, the upregulation of ROS and viability of epithelial lung cells are determined after exposure to severalcoal reference materials with known amounts of pyrite.
2. Materials and methods
2.1. Mineral sample preparation and soil standards
Five coal standard reference materials (CSRM) were obtained from The National Institute of Standards and Technology (NIST). The CSRM (NIST numbers 1635, 2682b, 2692b, 2684b and 2685b) contain different amounts of pyrite (expressed as pyritic sulfur mass fractions), see Table 1. The specific surface area for each of the coal reference standards was determined using a Quanta-chrome NOVA 5-point BET analyzer using UHP N2 gas. The results are summarized in Table 1.
Table 1.
NIST coal standard reference material information.
| Standard reference material numbera | |||||
|---|---|---|---|---|---|
|
| |||||
| 1635 | 2682b | 2692b | 2684b | 2685b | |
| Origin | Erie, CO | Gillette, WY | Holden, WV | Marion, IL | Captina, WV |
| Coal type | Subbituminous | Subbituminous | Bituminous | Bituminous | Bituminous |
| Surface area (m2 g−1)b | 1.79 | 4.94 | 1.35 | 2.11 | 1.72 |
| Pyritic sulfur (mass fraction)c | 0.00 | 0.01 | 0.49 | 0.52 | 1.15 |
| Iron release (μM)d | 0.00 | 0.02 | 0.48 | 8.31 | 13.3 |
| Elemental analysise | Mass fraction (wt.%) | ||||
|
|
|||||
| Iron | 0.239 ± 0.005 | 0.24 | – | 1.5 | 3.9 |
| Sulfur | 0.3616 ± 0.0017 | 0.4917 ± 0.0079 | 1.170 | 3.076 ± 0.031 | 4.730 ± 0.068 |
| Mass fraction (μg g−1) | |||||
|
|
|||||
| Arsenic | 0.42 ± 0.15 | 1.0 | – | 3.9 | 12 |
| Chromium | 2.5 ± 0.3 | 15 | – | 17 | 22 |
| Manganese | 21.4 ± 1.5 | 26 | – | 36 | 41 |
| Mercury | 0.0109 ± 0.0010 | 0.1088 ± 0.0029 | 0.1333 ± 0.0041 | 97.4 ± 4.7 | 146.2 ± 10.6 |
| Zinc | 4.7 ± 0.5 | 8.6 | – | 110 | 17 |
National Institute of Standards and Technology standard reference material numbers.
BET surface area values are for coals as received and used in experiments, not desiccated.
Dry basis.
0.125 m2 L−1 coal loadings, total iron [i.e. Fe(II) and Fe(III)] after 7 h in 1.5 mg L−1 RNA solution (Cohn et al., 2006a).
National Institute of Standards and Technology – Certified Analysis (NIST, 2007b,c,a, 2008, 2010).
2.2. Culturing and plating the A549 human lung epithelial cell line
The A549 human lung epithelial cell line was cultured and plated using a previously published procedure (Harrington et al., 2012b). Briefly, cells were plated in Ham's F12K Media containing 10% Fetal Bovine Serum (FBS) and 1% 1× Penicillin/Streptomycin (cell growth media). Once confluence was reached, the cells were passaged at 1 × 105 cells mL−1 using trypsin with EDTA to detach the cells. The cells were then counted using the Trypan blue stain on a hemocytometer. Wells in columns 3 through 10 of the 96-well microplates were loaded with 8 × 104 cells mL−1, covered with a microplate lid, and allowed to incubate at 37°C in culturing media for approximately 2 d until confluent. Due to background signal generated by the assays, columns 1 and 2 as well as 11 and 12 were kept cell free for normalization. All work with cells took place in a sterile hood to avoid airborne bacteria that would affect the results. When not in the hood, the microplate lid was kept on at all times. Temperature, relative humidity and carbon dioxide (CO2) concentration in air were kept constant (37 °C, 95% and 5% CO2 in air, respectively) in the incubator.
2.3. Inflammatory stress response measurements
Cellular confluence was achieved after incubation in 96-well microplates for 2 d. The cell growth medium was discarded and a previously published protocol for determining ISR was employed (Harrington et al., 2012b). In brief, after all liquid contents were allowed to drain out (cells remain attached), columns 1 through 6 were filled with 200 μL of 50 μM DCFH-DA (from Sigma Aldrich) in Hank's Buffered Salt Solution (HBSS) and columns 7 through 12 were filled with 200 μL HBSS, and then placed in the incubator. During incubation, four serial dilutions of the contaminant slurries were made. All final dilutions were normalized to surface area. The stock solution contained 0.002 m2 contaminant mL−1 HBSS (see Table2 for select mg mL−1 loadings). After incubating for 20 min, all liquid contents of the microplate were again discarded. 200 μL of the contaminant slurry was added to the plates. Rows A and H were kept free of contaminants due to edge effects (Lundholt et al., 2003). Row B was kept free of particles for normalization (control). After slurry addition, 20 μL of MTS (from Promega) was added to all wells in columns 7 through 12. The plate was then placed in the incubator for 30 min before the first analysis. Since each 96-well microplate contained assays for both cellular generated ROS and cell viability, duplicating the plates allowed for 8 replicates for each loading.
Table 2.
Particle loading conversion.
| Standard reference material numbera | Particle loading (mg mL−1) | ||
|---|---|---|---|
|
| |||
| 0.002 m2 mL−1 | 0.001 m2 mL−1 | 0.000125 m2 mL−1 | |
| 1635 | 1.117 | 0.559 | 0.0698 |
| 2682b | 0.405 | 0.202 | 0.0253 |
| 2692b | 1.482 | 0.741 | 0.0926 |
| 2684b | 0.948 | 0.474 | 0.0593 |
| 2685b | 1.163 | 0.581 | 0.0727 |
National Institute of Standards and Technology standard reference material numbers.
DCFH-DA is a fluorometric probe used to determine cellularly derived ROS and was analyzed using Thermo Scientific's Fluoroskan Ascent. MTS is a colorometric probe used to determine cell viability and was analyzed using Molecular Devices' SpectraMax 340PC384. The microplates were kept in the incubator at all times other than for analyses. During periods before analysis and transport, microplates were kept in the dark using foil. Plates were analyzed at 30 min, 1 h, 2 h, 4 h, 8 h and 24 h.
3. Results
The design of the experiment allowed for six particle loadings (including a control) to be evaluated. In addition, by taking measurements at fixed times for up to 24 h we are able to record temporal changes in cell viability, ROS production, and, hence, ISR. In a separate study we applied this protocol to a number of minerals and standard soils (Harrington et al., 2012b). Those earlier results are useful in placing the cellular response to coal in context. For example, the ability of inert materials (i.e. glass bead and anatase) to generate a small ISR due to the presence of the foreign particles gives a lower range of ISR for a reactive particle (450% of the control) and experiments performed on the highly reactive pyrite gave what is anticipated to be the upper range (110,000% of the control).
CSRM number 1635, which contains no pyrite, generated a relatively linear increase in ISR with particle loading (Fig. 1). The peak ISR occurred with the highest particle loading and at the 30 min time point. This ISR maximum is more than threefold greater than the control. A common temporal trend is exhibited with an initial ISR followed by a drop and then a steady ISR increase (Harrington et al., 2012b). CSRM numbers 2682b and 2692b generated the lowest ISR (175% and 251% of the control, respectively). However, the temporal trends in ISR are different (Fig. 3). While CSRM number 2682b increased steadily with time, number 2692b reacts similar to CSRM number 1635 by first generating a higher ISR followed by a dip and a gradualand steady increase. Of the three coal reference materials, number 1635 is the only one that caused significant cell death (59% of the viable cells compared to a control at 30 min), which subsided with time, although it is responsible for the high ISR. While initially the ISR for CSRM number 1635 is driven by low cell viability, eventually it is driven by ROS upregulation.
Fig. 1.
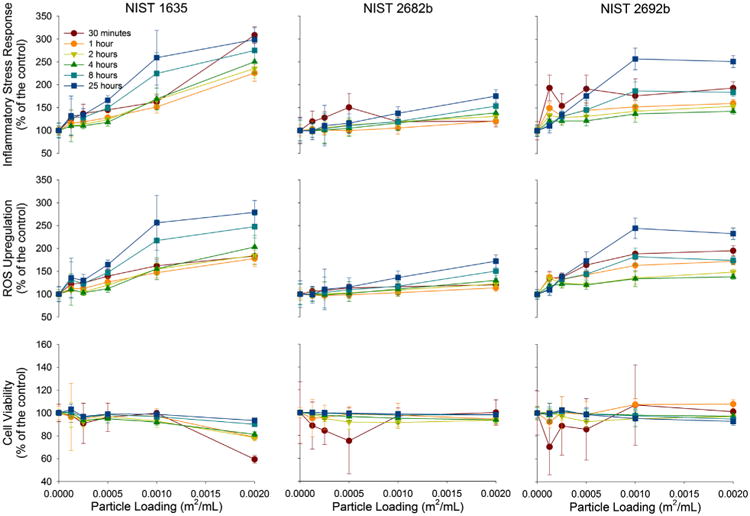
ROS upregulation, cell viability and ISR of A549 cells to relatively inert coal reference material numbers 1635 2682b and 2692b. The cellular response to coal reference material numbers 1635, 2682b and 2692b with increasing particle loading is presented with three different figures, all normalized to a control. ROS upregulation (middle), cell viability (bottom), and ISR – ROS upregulation divided by cell viability (top).
Fig. 3.
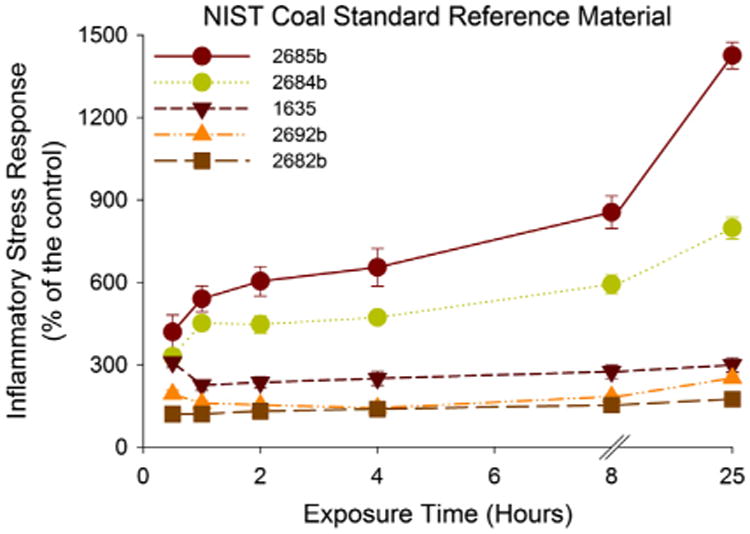
Evolution of ISR over time. The ISR over time of all the coal reference materials at the highest particle loading (0.002 m2 mL−1) is represented.
Coal reference material numbers 2684b and 2685b, containing the highest pyritic sulfur contents, generated the greatest ISR values (798% and 1426% of the control) (Fig. 2). Although number 2685b generated an ISR nearly a factor of two greater than 2684b, the temporal trends of these reference coals are similar. Generating similar ROS upregulation, the disparity in ISR is derived by the greater amount of cell death in the sample containing the most pyritic sulfur (67% versus 43% of the viable cells when compared to a control at 24 h).
Fig. 2.
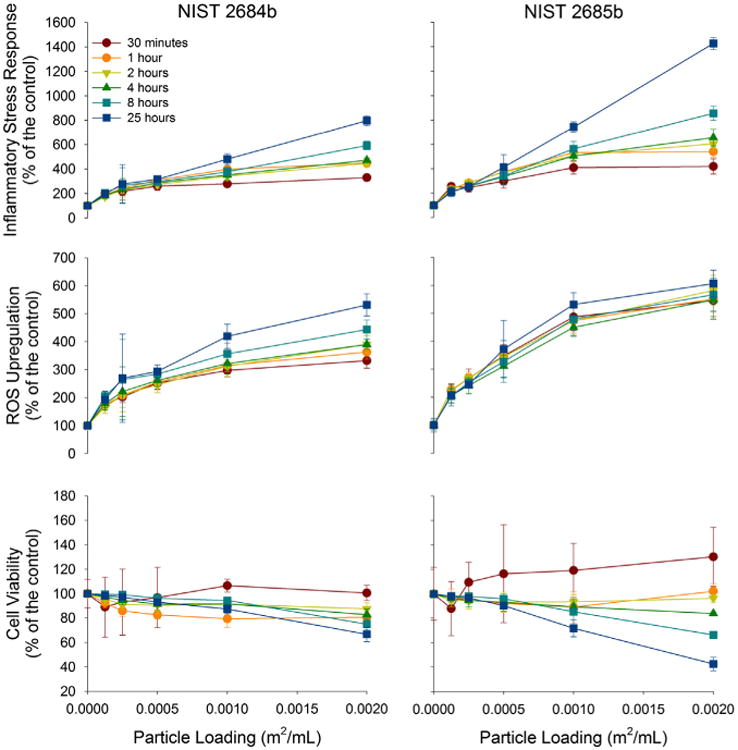
ROS upregulation, cell viability and ISR of A549 cells to relatively reactive coal reference material numbers 2684b and 2685b. The cellular response to coal reference material number 2684b and 2685b with increasing particle loading is presented with three different figures, all normalized to a control. ROS upregulation (middle), cell viability (bottom), and ISR – ROS upregulation divided by cell viability (top).
4. Discussion
The 4.6-fold ISR difference between the CSRM containing no pyrite and coal containing the highest concentration of pyrite supports the hypothesis that the reactivity of pyrite in the coal plays an important role in the pathogenesis of CWP. The much lower ISR for CSRM reference material number 1635 is somewhat lower than the stress generated by glass beads and anatase, which are considered relatively inert materials (Harrington et al., 2012b). Therefore, exposure to coal without pyrite generates the same level of response as exposure to inert particulate matter. This is consistent with the fact that there is a low prevalence of CWP among miners who are exposed to coal without pyrite (Huang et al., 2005).
CSRM number 2684b, containing the second highest concentration of pyrite, generated 2.6-fold the ISR of the sample 1635. CSRM number 2685b generated nearly twice the ISR as CSRM number 2684d. It is important to note we have normalized to total surface area and not the surface area of the pyrite within the standard reference material. Therefore, one would not necessarily expect a linear increase in ISR with increase pyritic sulfur contents (Finkelman, 1992). The amount of “available” iron, or total iron released in 1.5 mg L−1 RNA solution over a 7 h period, would also contribute to Fenton chemistry (Cohn et al., 2006a). The high available iron content of CSRM number 2684b (a factor of 17.3 greater than of number 2692b and 62% of number 2685b) likely plays a role in the increased ISR, which may be an indication of the accessibility and reactivity of the pyrite within the coal.
5. Conclusion
CWP is a protracted disease caused by chronic inflammation in the lungs induced by inhaled coal particles. This study builds upon and extends earlier acellular (Harrington et al., 2012a) (Cohn et al., 2006a) and epidemiological work (Huang et al., 2005) citing pyrite as the major contributing factor in CWP pathogenesis. Specifically, our data shows that epithelial cells challenged with coal containing modest amounts of pyrite (0.52 and 1.15 wt.%) undergo cell death, and the remaining surviving cells show an upregulation of ROS allowing for a dramatically high ISR (798% and 1426% compared to a control, respectively). These findings support the notion that pyrite is a key determinant the pathogenesis of CWP.
Highlights.
Pyrite's role in causing inflammatory stress in the A549 lung cell line is examined.
High pyrite content correlates with high available iron contents in coal.
The high pyritic sulfur coals generate the greatest inflammatory stress responses.
Coals with elevated pyrite contents are likely to induce a significant health burden.
Pyrite within coal may contribute to coal workers' pneumoconiosis pathogenesis.
Acknowledgments
This work was supported by the Minerals, Metals, Metalloids and Toxicity (3MT) program at Stony Brook University, which is funded by NSF-IGERT, and NIH R0142168 (S.E.T.).
Footnotes
Abbreviations: (ISR), Inflammatory stress response; (DCFH-DA), 2′,7′-dichlorofluoroscein-diacetate; (MTS), 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; (CSRM), coal standard reference material; (FBS), Fetal bovine serum; (HBSS), Hank's Buffered Salt Solution; (EDTA), Ethylenediaminetetraacetic acid; (CWP), Coal Workers' Pneumoconiosis; (ROS), Reactive oxygen species; (˙OH), Hydroxyl radical; (H2O2), Hydrogen peroxide; (NIST), National Institute of Standards and Technology.
Competing interests: The authors declare that they have no competing interests.
Authors' contributions: A.D.H. helped design the study, performed the experiments, and drafted the manuscript. S.E.T. and M.A.A.S. helped design the experiments, supervised the study, and edited the manuscript. All authors have read and approved the final manuscript.
Contributor Information
Andrea D. Harrington, Email: ndrea.Harrington@nyumc.org.
Stella E. Tsirka, Email: stella@pharm.stonybrook.edu.
Martin A.A. Schoonen, Email: martin.schoonen@stonybrook.edu.
References
- Biegler T, Rand DAJ, Woods R. Oxygen reduction on sulphide minerals: Part I. Kinetics and mechanism at rotated pyrite electrodes. J Electroanal Chem Interf Electrochem. 1975;60:151–162. [Google Scholar]
- Bleck B, Tse DB, Gordon T, Ahsan MR, Reibman J. Diesel exhaust particle-treated human bronchial epithelial cells upregulate Jagged-1 and OX40 ligand in myeloid dendritic cells via thymic stromal lymphopoietin. J Immunol. 2010;185:6636–6645. doi: 10.4049/jimmunol.1000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn CA, Laffers R, Simon SR, O'Riordan T, Schoonen MAA. Role of pyrite in formation of hydroxyl radicals in coal: possible implications for human health. Part Fibre Toxicol. 2006a;3:16. doi: 10.1186/1743-8977-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn CA, Mueller S, Wimmer E, Leifer N, Greenbaum S, Strongin DR, Schoonen MAA. Pyrite-induced hydroxyl radical formation and its effect on nucleic acids. Geochem Trans. 2006b;7:3–11. doi: 10.1186/1467-4866-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsetinow AR, Schoonen MAA, Strongin DR. Aqueous geochemical and surface science investigation of the effect of phosphate on pyrite oxidation. Environ Sci Technol. 2001;35:2252–2257. doi: 10.1021/es0016809. [DOI] [PubMed] [Google Scholar]
- Finkelman RB. Characteristics of acid-forming materials in coal: Proceedings American Society of Surface Mining Reclamation. Acid Forming Materials Symposium Montana Reclamation Research. 1992;9202:1–16. [Google Scholar]
- Fubini B. Surface reactivity in the pathogenic response to particulates. Environ Health Perspect. 1997;105(Suppl. 5):1013–1020. doi: 10.1289/ehp.97105s51013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fubini B, Hubbard A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Rad Biol Med. 2003;34:1507–1516. doi: 10.1016/s0891-5849(03)00149-7. [DOI] [PubMed] [Google Scholar]
- Harrington AD, Hylton S, Schoonen MAA. Pyrite-driven reactive oxygen species formation in simulated lung fluid: implications for coal workers' pneumoconiosis. Environ Geochem Health. 2012a;34:527–538. doi: 10.1007/s10653-011-9438-7. [DOI] [PubMed] [Google Scholar]
- Harrington AD, Tsirka SE, Schoonen MAA. Quantification of particle-induced inflammatory stress response: a novel approach for toxicity testing of earth materials. Geochemical Transactions. 2012b;13 doi: 10.1186/1467-4866-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Frenkel K, Klein CB, Costa M. Nickel Induces increased oxidants in intact cultured mammalian cells as detected by dichlorofluorescein fluorescence. Toxicol Appl Pharmacol. 1993;120:29–36. doi: 10.1006/taap.1993.1083. [DOI] [PubMed] [Google Scholar]
- Huang X, Li WH, Attfield MD, Nadas A, Frenkel K, Finkelman RB. Mapping and prediction of coal workers' pneumoconiosis with bioavailable iron content in the bituminous coals. Environ Health Persp. 2005;113:964–968. doi: 10.1289/ehp.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundholt BK, Scudder KM, Pagliaro L. A simple technique for reducing edge effect in cell-based assays. J Biomol Screening. 2003;8:566–570. doi: 10.1177/1087057103256465. [DOI] [PubMed] [Google Scholar]
- Martin TR, Frevert CW. Innate immunity in the lungs. Proc Am Thoracic Soc. 2005;2:403–411. doi: 10.1513/pats.200508-090JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses CO, Herman JS. Pyrite oxidation at circumneutral pH. Geochim Cosmochim Acta. 1991;55:471–482. [Google Scholar]
- Moses C, Nordstrom D, Herman J, Mills A. Aqueous pyrite oxidation by dissolved oxygen and by ferric iron. Geochim Cosmochim Acta. 1987;51:161–1572. [Google Scholar]
- NIOSH. Work-Related Lung Disease Surveillance Report 2002. Cincinnati, OH: National Occupational Safety and Health; 2003. [Google Scholar]
- Commerce, D.o., editor. NIST. Certificate of analysis – standard reference material 2682b. National Institute of Standards & Technology; Gaithersburg: 2007a. [Google Scholar]
- Commerce, D.o., editor. NIST. Certificate of analysis – standard reference material 2685b. National Institute of Standards & Technology; Gaithersburg: 2007b. [Google Scholar]
- Commerce, D.o., editor. NIST. Certificate of analysis – standard reference material 2692b. National Institute of Standards & Technology; Gaithersburg: 2007c. [Google Scholar]
- Commerce, D.o., editor. NIST. Certificate of analysis – standard reference material 1635. National Institute of Standards & Technology; Gaithersburg: 2008. [Google Scholar]
- Commerce, D.o., editor. NIST. Certificate of analysis – standard reference material 2684b. National Institute of Standards & Technology; Gaithersburg: 2010. [Google Scholar]
- Rada Bz, Leto TL. Characterization of hydrogen peroxide production by Duox in bronchial epithelial cells exposed to Pseudomonas aeruginosa. FEBS Lett. 2010;584:917–922. doi: 10.1016/j.febslet.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso KM, Becker U, Hochella MF. Atomically resolved electronic structure of pyrite 100 surfaces: an experimental and theoretical investigation with implications for reactivity. Am Miner. 1999a;84:1535–1548. [Google Scholar]
- Rosso KM, Becker U, Hochella MF. The interaction of pyrite 100 surfaces with O-2 and H2O: fundamental oxidation mechanisms. Am Miner. 1999b;84:13. [Google Scholar]
- Schoonen MAA, Cohn CA, Roemer E, Laffers R, Simon SR, O'Riordan T. Mineral-induced Formation of Reactive Oxygen Species. In: Sahai N, Schoonen MAA, editors. Medical Mineralogy and Geochemistry Mineralogical Society of America. 2006. pp. 179–221. [Google Scholar]
- Schoonen MAA, Harrington AD, Laffers RA, Strongin DR. Role of hydrogen peroxide and hydroxyl radical in pyrite oxidation by molecular oxygen. Geochim Cosmochim Acta. 2010;74:4971–4987. [Google Scholar]
- Spector A. Review: oxidative stress and disease. J Ocul Pharmacol Therap. 2000;16:193–201. doi: 10.1089/jop.2000.16.193. [DOI] [PubMed] [Google Scholar]
- USEIA U.S.E.I.A. International Energy Outlook. 2009 < http://www.eia.doe.gov/oiaf/ieo/highlights.html< DOE/EIA-0484.
- Vallyathan V, Shi XL, Castranova V. Reactive oxygen species: their relation to pneumoconiosis and carcinogenesis. Environ Health Persp. 1998;106:1151–1155. doi: 10.1289/ehp.98106s51151. [DOI] [PMC free article] [PubMed] [Google Scholar]


