Abstract
Purpose
To explore functional visual recovery after retinal reattachment surgery employing full-field electroretinography (ffERG).
Methods
In this case series, scotopic and photopic ffERGs were compared 2 days before, and 1, 3 and 6 months after successful scleral buckling for total rhegmatogenous retinal detachment (RRD). Main outcome measures were changes in ERG a-and b-wave amplitudes postoperatively.
Results
Twenty eyes of 20 patients including 14 male and 6 female subjects with mean age of 34.7±8.2 (range, 23 to 50) years were enrolled. Preoperatively, mean a-wave amplitude in the maximal combined response was 27.5±11.7 mV which was increased to 110.7±41.9 (P<0.001), 175.7±53.1 (p<0.001) and 174.6±51.4 (P<0.001) mV at 1, 3 and 6 months, respectively. Mean preoperative a-wave amplitude of the cone ERG response was 2.1±0.8 mV, which was increased to 2.2±0.9 (P=0.03), 5.1±1.7 (P<0.001) and 5.3±1.6 (P<0.001) mV at 1, 3 and 6 months, respectively. Mean preoperative b-wave amplitude in the maximal combined response was 97.6±28.9 mV which was increased to 179.2±44.9 (P<0.001), 264.2±56.3 (P<0.001) and 267.8±54.2 (P<0.001) mV at 1, 3 and 6 months, respectively. Mean preoperative b-wave amplitude of the cone ERG response was 2.9±0.9 mV which was increased to 3±0.9 (P=0.32), 9.9±1.9 (P<0.001) and 9.8±1.9 (P<0.001) mV at 1, 3 and 6 months, respectively.
Conclusion
After retinal reattachment surgery, photoreceptor and visual function show parallel improvement. The scotopic ERG response recovered faster than the photopic response. Incomplete recovery of ERG parameters indicates that photoreceptor cell damage in retinal detachment is not completely reversible.
Keywords: Retinal Detachment, Electroretinography, Visual Function, Retinal Sensitivity
INTRODUCTION
Retinal detachment is the separation of the retinal pigment epithelium (RPE) from the neural retina and a serious condition that can lead to blindness. Scleral buckling is a well-established surgical procedure for the treatment of rhegmatogenous retinal detachment (RRD); the anatomical success rate of this operation has been reported to exceed 90%.1-5
Although there are a number of reports expressing concern about functional visual recovery following retinal reattachment surgery, electrophysiologic function may not recover in parallel.5-7 Recovery of visual function in the reattached retina is accomplished through regeneration of photoreceptor outer segments and restoration of anatomical relations between the neurosensory retina and the RPE.8 After retinal reattachment, photoreceptor outer segments regenerate and the RPE attains good contact with the retina.9,10
Regeneration of photoreceptor outer segments and restoration of the anatomical relationship between the neurosensory retina and the RPE may explain recovery of retinal function after retinal reattachment.11-13 The fact that vision is at least partially restored in most humans after retinal reattachment implies that the adverse anatomical and physiological effects of retinal detachment can be halted or even reversed after successful reattachment.9-11 Recent clinical studies have demonstrated that cone photopigments show slow recovery after reattachment and visual acuity may continue to improve on a long term basis.11-13 Anatomical reattachment of the retina usually stabilizes three months after successful surgery.14-19 However, this anatomic reattachment is not always followed by full functional recovery and visual dysfunction may persist.12,16,20 The visual dysfunction depends on the duration of RRD, preoperative visual acuity, type and extent of retinal detachment, and macular involvement.14,16,19 Moreover, it has been suggested that RRD has greater morphologic impact on cones than rods. In other words, after retinal reattachment, the rod system recovers more quickly than the cone system.11,13
The fact that partial visual improvement and electrophysiological recovery have been observed in most patients with total RRD suggests irreversible damage to both types of photoreceptors even after successful retinal reattachment.21,22 ERG is a useful tool to objectively assess retinal function and can be used to analyze the function of different layers of the retina.23
In the present study, ERG alterations after retinal reattachment surgery along with functional changes in both rod and cone photoreceptors were evaluated in eyes with RRD undergoing scleral buckling.
Methods
Twenty eyes of 20 patients, including 14 male and 6 female subjects with mean age of 34.7±8.2 (range, 23 to 50) years with RRD undergoing scleral buckling for retinal reattachment were studied. Twenty healthy normal individuals were enrolled as the control group. The duration of retinal detachment, estimated from the onset of symptoms to the day of surgery, was less than seven days in all study participants. All patients had successful retinal reattachment following scleral buckling.
Patients with retinal redetachment within 6 months after surgery, hereditary retinal disorders, proliferative vitreoretinopathy (PVR) grade C or more, vascular retinal disorders, chorioretinal inflammation, and media haziness of any cause were excluded from the study.
ffERG readings were obtained according to the methods described by the International Society for Clinical Electrophysiology of Vision (ISCEV)24, using the Mono Elec2 system (Metrovision Inc. France).
Pupil dilatation was accomplished for patients and controls to a diameter of 8 mm with 1% tropicamide and 2.5% phenylephrine drops after topical anesthesia with 0.5% tetracaine. The ERG-jet contact lens electrode was used as the recording electrode and 0.5% methylcellulose gel was deposited into its concavity. The reference electrode was placed at the center of the forehead and the grounding electrode was attached to the ear lobe. Scotopic (rod response and maximal combined response) and photopic (cone response) ERGs were recorded after 20 minutes of dark and 10 minutes of light adaptation, respectively. Preoperative ERGs were obtained 2 days before surgery and postoperative studies were performed 1, 3 and 6 months after surgery. Amplitudes of a- and b-waves of the rod response, maximal combined response and cone response were recorded, analyzed and compared with those of the control group before and after surgery.
To evaluate changes during the study, mixed model adjusted for the multiple comparisons by Bonferroni method was used. This method was also applied to evaluate the proportions of change among different types of responses. To compare the results between a-and b-waves, the paired sample t-test was used. P-values less than 0.05 were considered as statistically significant. All analyses were performed using SPSS software (version 17.0, SPSS, Chicago, IL, USA).
Results
A total of 20 eyes of 20 patients including 14 (70%) male and 6 (30%) female subjects with mean age of 34.7±8.2 (range, 23 to 50) years were included in this study. Table 1 summarizes demographics and clinical data of the patients. The underlying condition that had led to scleral buckling surgery was total RRD in all patients. All retinas in affected eyes were successfully reattached and the anatomical reattachment remained unchanged during follow-up. A representative ERG wave tracing from a patient after surgery is compared to that from a normal healthy control in Figure 1.
Table 1.
Demographics and clinical data of the patients before and after surgery
| Sex | Age | Eye | b-wave maximal combined response |
b-wave rod response |
b-wave cone response |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Mon 1 | Mon 3 | Mon 6 | Pre | Mon 1 | Mon 3 | Mon 6 | Pre | Mon 1 | Mon 3 | Mon 6 | ||||
| 1 | M | 27 | OD | 93.2 | 175.1 | 286.4 | 287.0 | 43.5 | 112.2 | 240.0 | 238.0 | 2.4 | 3.1 | 10.2 | 10.2 |
|
| |||||||||||||||
| 2 | M | 32 | OD | 71.5 | 152.8 | 201.2 | 211.2 | 27.4 | 62.5 | 135.6 | 140.0 | 1.8 | 1.8 | 8.1 | 8.2 |
|
| |||||||||||||||
| 3 | F | 25 | OD | 86.3 | 161.2 | 235.2 | 225.9 | 92.2 | 184.1 | 325.1 | 314.6 | 2.1 | 2.3 | 8.6 | 8.0 |
|
| |||||||||||||||
| 4 | M | 50 | OS | 126.4 | 212.3 | 310.6 | 308.1 | 101.8 | 172.4 | 337.2 | 335.0 | 2.6 | 2.7 | 9.0 | 8.9 |
|
| |||||||||||||||
| 5 | M | 42 | OS | 98.0 | 198.5 | 325.8 | 315.4 | 98.7 | 156.7 | 294.6 | 334.0 | 2.8 | 3.0 | 8.1 | 8.0 |
|
| |||||||||||||||
| 6 | F | 37 | OD | 67.1 | 134.3 | 255.0 | 263.1 | 48.9 | 72.5 | 162.4 | 293.1 | 2.2 | 2.2 | 8.0 | 7.9 |
|
| |||||||||||||||
| 7 | F | 26 | OS | 72.4 | 141.2 | 242.6 | 249.0 | 65.3 | 110.2 | 211.0 | 168.0 | 1.8 | 1.9 | 7.4 | 7.4 |
|
| |||||||||||||||
| 8 | M | 33 | OD | 125.0 | 150.0 | 215.7 | 217.2 | 119.1 | 192.7 | 365.7 | 210.6 | 3.0 | 3.1 | 11.5 | 12.1 |
|
| |||||||||||||||
| 9 | M | 43 | OD | 121.0 | 211.5 | 312.8 | 301.6 | 113.0 | 185.7 | 267.5 | 366.0 | 2.6 | 2.8 | 12.6 | 12.6 |
|
| |||||||||||||||
| 10 | M | 29 | OD | 39.5 | 86.6 | 126.0 | 129.1 | 39.6 | 43.7 | 111.0 | 119 | 4.5 | 2.9 | 14.5 | 14.0 |
|
| |||||||||||||||
| 11 | M | 47 | OS | 144.3 | 271.2 | 316.6 | 311.0 | 112.7 | 201.7 | 344.0 | 135.0 | 2.5 | 3.1 | 10.3 | 11.0 |
|
| |||||||||||||||
| 12 | F | 40 | OD | 134.0 | 245.1 | 301.2 | 301.6 | 137.0 | 198.2 | 375.2 | 346.0 | 3.6 | 3.9 | 11.4 | 11.2 |
|
| |||||||||||||||
| 13 | M | 29 | OD | 110.7 | 192.1 | 265.5 | 270.0 | 101.7 | 192.7 | 366.5 | 369.4 | 3.4 | 3.9 | 10.3 | 9.4 |
|
| |||||||||||||||
| 14 | M | 24 | OD | 90.6 | 176.1 | 282.1 | 312.1 | 98.4 | 142.1 | 296.5 | 252.0 | 2.2 | 2.2 | 8.6 | 8.4 |
|
| |||||||||||||||
| 15 | M | 35 | OS | 143.1 | 242.7 | 365.0 | 362.0 | 106.3 | 192.6 | 334.7 | 313.2 | 4.9 | 5.0 | 11.7 | 11.5 |
|
| |||||||||||||||
| 16 | F | 31 | OS | 81.9 | 156.9 | 202.1 | 311.2 | 79.2 | 112.4 | 201.6 | 211.6 | 2.1 | 2.2 | 8.5 | 9.0 |
|
| |||||||||||||||
| 17 | M | 23 | OD | 58.2 | 125.2 | 232.6 | 234.0 | 86.4 | 122.1 | 210.4 | 210.0 | 2.3 | 2.3 | 8.6 | 8.6 |
|
| |||||||||||||||
| 18 | M | 46 | OD | 81.0 | 154.2 | 212.6 | 198.7 | 85.8 | 166.2 | 286.7 | 279.4 | 2.3 | 2.7 | 8.3 | 8.0 |
|
| |||||||||||||||
| 19 | F | 41 | OS | 108.1 | 201.0 | 285.6 | 246.0 | 92.0 | 175.0 | 282.0 | 272.8 | 4.2 | 4.3 | 11.6 | 11.6 |
|
| |||||||||||||||
| 20 | M | 34 | OD | 98.7 | 195.4 | 310.0 | 301.6 | 88.7 | 164.5 | 290.4 | 290.0 | 4.0 | 4.1 | 10.3 | 9.7 |
|
| |||||||||||||||
| P for change from baseline | <0.001 | <0.001 | <0.001 | 0.06 | <0.001 | <0.001 | 0.318 | <0.001 | <0.001 | ||||||
|
| |||||||||||||||
|
| |||||||||||||||
| Sex | Age | Eye | a-wave maximal combined response |
a-wave rod response |
a-wave cone response |
||||||||||
| Pre | Mon 1 | Mon 3 | Mon 6 | Pre | Mon 1 | Mon 3 | Mon 6 | Pre | Mon 1 | Mon 3 | Mon 6 | ||||
|
| |||||||||||||||
| 1 | M | 27 | OD | 29.4 | 128.2 | 213.7 | 221.4 | 4.2 | 7.1 | 14.2 | 13.9 | 1.4 | 1.4 | 4.7 | 4.3 |
|
| |||||||||||||||
| 2 | M | 32 | OD | 16.6 | 76.7 | 144.0 | 143.0 | 2.1 | 4.3 | 7.2 | 6.9 | 1.3 | 1.3 | 3.6 | 3.7 |
|
| |||||||||||||||
| 3 | F | 25 | OD | 28.2 | 119.3 | 198.2 | 201.3 | 3.2 | 6.1 | 18.1 | 17.1 | 2.3 | 2.3 | 6.4 | 5.9 |
|
| |||||||||||||||
| 4 | M | 50 | OS | 38.5 | 152.0 | 214.2 | 219.3 | 2.3 | 4.5 | 8.9 | 8.9 | 2.2 | 2.1 | 5.6 | 5.6 |
|
| |||||||||||||||
| 5 | M | 42 | OS | 35.2 | 141.0 | 201.7 | 198.7 | 3.4 | 5.9 | 9.0 | 9.1 | 2.0 | 2.0 | 4.3 | 4.1 |
|
| |||||||||||||||
| 6 | F | 37 | OD | 13.4 | 61.2 | 129.1 | 131.2 | 3.1 | 6.1 | 10.1 | 11.1 | 1.2 | 1.2 | 3.2 | 3.2 |
|
| |||||||||||||||
| 7 | F | 26 | OS | 13.9 | 58.9 | 124.6 | 112.6 | 2.1 | 4.1 | 16.3 | 15.8 | 1.7 | 1.7 | 3.2 | 5.2 |
|
| |||||||||||||||
| 8 | M | 33 | OD | 38.6 | 141.2 | 231.4 | 201.7 | 2.7 | 4.2 | 5.1 | 6.1 | 2.8 | 2.8 | 7.1 | 7.1 |
|
| |||||||||||||||
| 9 | M | 43 | OD | 28.5 | 119.4 | 189.7 | 191.0 | 2.1 | 3.9 | 11.5 | 10.9 | 2.7 | 2.8 | 8.1 | 8.3 |
|
| |||||||||||||||
| 10 | M | 29 | OD | 8.3 | 38.3 | 60.9 | 66.2 | 3.1 | 6.3 | 19.7 | 18.8 | 1.7 | 3.9 | 5.1 | 5.0 |
|
| |||||||||||||||
| 11 | M | 47 | OS | 41.8 | 156.1 | 262.4 | 254.0 | 4.3 | 7.5 | 13.8 | 12.9 | 1.8 | 2.1 | 4.2 | 4.9 |
|
| |||||||||||||||
| 12 | F | 40 | OD | 38.8 | 161.2 | 235.2 | 241.5 | 3.8 | 6.0 | 9.2 | 10.1 | 2.9 | 3.1 | 8.8 | 9.1 |
|
| |||||||||||||||
| 13 | M | 29 | OD | 46.6 | 181.3 | 210.3 | 216.4 | 2.1 | 4.1 | 7.4 | 7.3 | 2.4 | 2.4 | 3.8 | 4.1 |
|
| |||||||||||||||
| 14 | M | 24 | OD | 14.8 | 72.1 | 120.7 | 119.0 | 1.8 | 2.1 | 4.8 | 4.4 | 1.3 | 1.3 | 3.2 | 3.3 |
|
| |||||||||||||||
| 15 | M | 35 | OS | 39.2 | 151.6 | 237.8 | 229.7 | 3.2 | 6.0 | 8.4 | 7.9 | 3.8 | 3.8 | 6.2 | 6.1 |
|
| |||||||||||||||
| 16 | F | 31 | OS | 24.5 | 98.1 | 150.6 | 149.6 | 2.2 | 3.8 | 6.7 | 2.6 | 1.2 | 1.3 | 3.7 | 4.1 |
|
| |||||||||||||||
| 17 | M | 23 | OD | 13.9 | 58.1 | 128.1 | 125.0 | 2.9 | 3.0 | 5.4 | 6.1 | 1.3 | 1.1 | 5.1 | 5.0 |
|
| |||||||||||||||
| 18 | M | 46 | OD | 15.6 | 72.1 | 115.0 | 117.1 | 2.0 | 2.2 | 4.9 | 4.9 | 1.2 | 1.1 | 4.4 | 4.7 |
|
| |||||||||||||||
| 19 | F | 41 | OS | 27.2 | 95.0 | 145.2 | 150.6 | 3.2 | 5.8 | 9.3 | 8.9 | 3.2 | 3.1 | 5.3 | 5.0 |
|
| |||||||||||||||
| 20 | M | 34 | OD | 37.6 | 131.5 | 201.2 | 198.1 | 3.2 | 5.1 | 10.3 | 9.9 | 3.2 | 3.2 | 7.1 | 7.2 |
|
| |||||||||||||||
| P for change from baseline | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.268 | <0.001 | <0.001 | ||||||
F, female; M, male; OD; right eye. OS; left eye; Pre; before operation; Mon, month
Table 2.
Visual acuity (log MAR) and its change (crude and proportion) before, 1, 3 and 6 month after operation
| Time | Mean ± SD | Median (Range) |
|---|---|---|
| Baseline | ||
| VA | 2.22 ± 0.35 | 2.1 (1.8 to 2.6) |
|
| ||
| Month 1 | ||
| VA | 1.38 ± 0.21 | 1.3 (1.1 to 1.8) |
|
| ||
| Change | -0.8 ± 0.2 | -0.8 (-1.2 to -0.5) |
|
| ||
| 95% CI | 0.74 - 0.95 | |
|
| ||
| Change % | -38 ± 6 | -38 (-48 to -26) |
|
| ||
| P-Within* | <0.001 | |
|
| ||
| Month 3 | ||
| VA | 0.91 ± 0.25 | 0.95 (0.4 to 1.2) |
|
| ||
| Change | -1.3 ± 0.3 | -1.4 (-2 to -0.9) |
|
| ||
| 95% CI | -1.44to -1.19 | |
|
| ||
| Change % | -59 ± 9 | -57 (-78 to -50) |
|
| ||
| P-Within* | <0.001 | |
|
| ||
| Month 6 | ||
| VA | 0.88 ± 0.23 | 0.95 (0.4 to 1.2) |
|
| ||
| Change | -1.3 ± 0.3 | -1.4 (-2 to -0.9) |
|
| ||
| 95% CI | -1.47 to-1.21 | |
|
| ||
| Change % | -60 ± 9 | -59 (-78 to -48) |
|
| ||
| P-Within* | <0.001 | |
Based on mixed model adjusted for the multiple comparisons by Bonferroni method
SD, standard deviation; CI, confidence interval; VA, visual acuity
Figure 1.
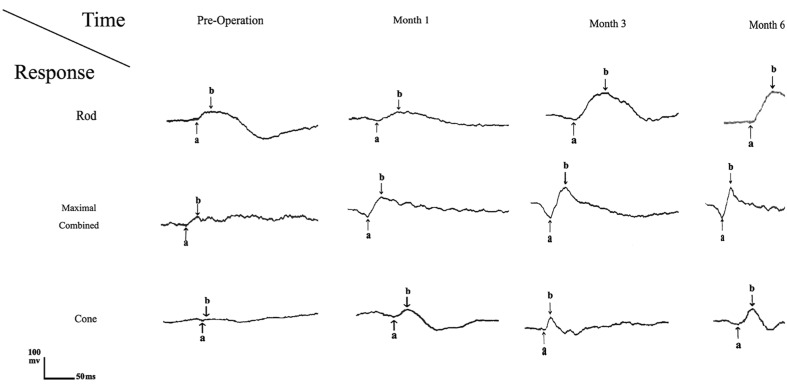
A representative case (No.10) demonstrates minimal response ERG; no significant electroretinographic amplitudes could be recorded especially under photopic conditions (cone response) preoperatively. ERG in the same patient at 1, 3 and 6 months, after retinal reattachment illustrates a significant increase in both a– and b-wave amplitudes especially under scotopic condition at month 3 postoperatively. However values were still lower than those recorded from normal subjects.
Preoperatively, mean a-wave amplitude in the maximal combined response was 27.5±11.7 increasing to 110.7±41.9 (P<0.001), 175.7±53.1 (P<0.001) and 174.6±51.4 (P<0.001) mV one, three and six months after retinal reattachment, respectively. Mean a-wave amplitude of the rod response ERG was 2.8±0.7, which increased to 4.9±1.5 (P<0.001), 10±4.4 (P<0.001) and 9.7±4.3 (P<000.1) mV in the same order. Mean a-wave amplitude of the cone response ERG before surgery was 2.1±0.8, which increased to 2.2±0.9 (P=0.027), 5.1±1.7 (P<0.001) and 5.3±1.6 (P<0.001) mV one, three and six months after surgery, respectively.
Mean b-wave amplitude in the maximal combined response was 97.6±28.9 increasing to 179.2±44.9 (P<0.001), 264.2±56.3 (P<0.001) and 267.8±54.2 (P<0.001) mV one, three and six months after retinal reattachment, respectively. Mean b-wave amplitude of the rod response ERG was 86.9±28.7 which increased in the same order to 148±48.5 (P=0.107), 271.9±78.3 (P<0.001) and 259.9±78.2 (P<0.001) mV at one, three and six months. Mean b-wave amplitude of the cone response ERG before surgery was 2.9±0.9 which increased to 3±0.9 (P=0.318), 9.9±1.9 (P<0.001) and 9.8±1.9 (P<0.001) mV at one, three and six months after surgery, respectively.
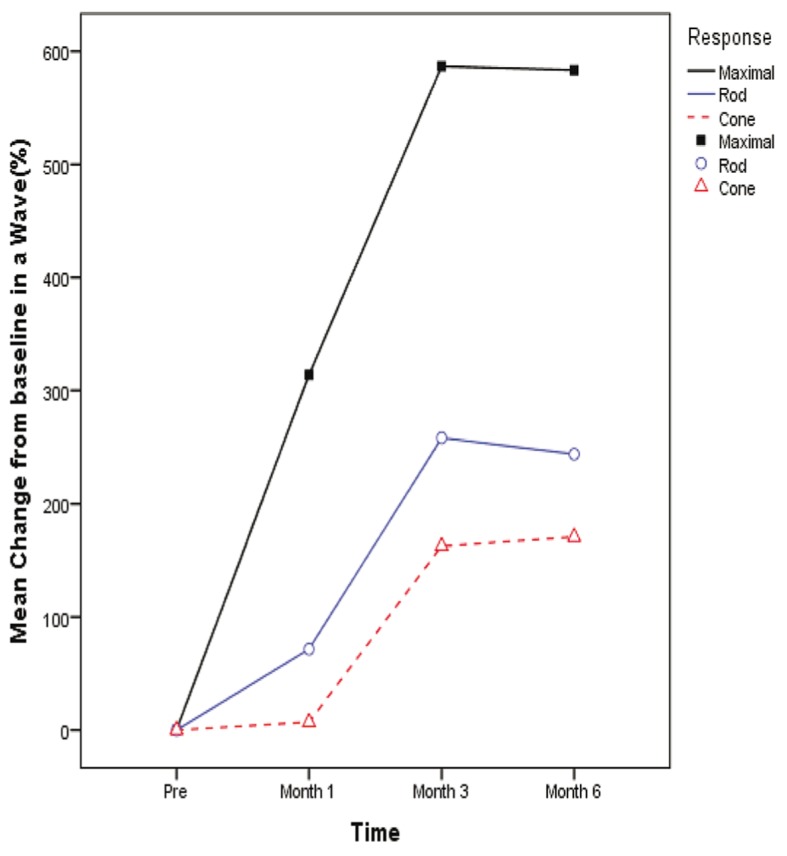
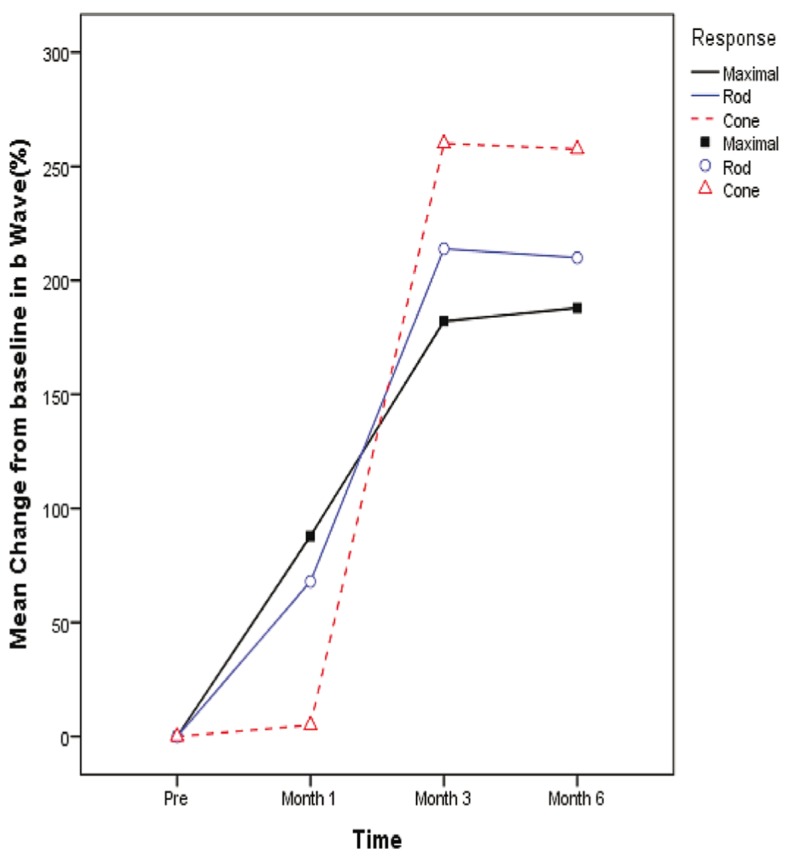
Comparison between a- and b-wave amplitudes before and after surgery in maximal combined, rod and cone response ERGs are detailed in tables 3, 4 and 5 respectively. All changes from baseline and month one were statistically significant compared to month three (P<0.001). Amplitude of a- and b-waves (µV) in maximal combined, rod and cone response ERGs in normal healthy control eyes are shown in table 6. Mean percentage of change from baseline to month 1, 3 and 6 in a- and b-wave amplitudes based on the type of ERG response are shown in figures 2 and 3.
Table 3.
a- and b- wave amplitudes (microvolts) of the maximal combined response ERG and their changes (percentage) before and 1, 3 and 6 months after surgery
| Time | a-wave |
b-wave |
P† | ||
|---|---|---|---|---|---|
| Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | ||
| Baseline | |||||
| Value | 27.5 ± 11.7 | 28.4 (8.3 to 46.6) | 97.6 ± 28.9 | 95.6 (39.5 to 144.3) | <0.001 |
|
| |||||
| Month 1 | |||||
| Value | 110.7 ± 41.9 | 119.4 (38.3 to 181.3) | 179.2 ± 44.9 | 175.6 (86.6 to 271.2) | <0.001 |
|
| |||||
| Change % | 314 ± 39 | 317 (249 to 387) | 88 ± 21 | 89 (20 to 119) | <0.001 |
|
| |||||
| P-Within* | <0.001 | <0.001 | |||
|
| |||||
| Month 3 | |||||
| Value | 175.7 ± 53.1 | 194 (60.9 to 262.4) | 264.2 ± 56.3 | 273.8 (126 to 365) | <0.001 |
|
| |||||
| Change % | 587 ± 144 | 547 (351 to 863) | 182 ± 55 | 168 (73 to 300) | <0.001 |
|
| |||||
| P-Within* | <0.001 | <0.001 | |||
|
| |||||
| Month 6 | |||||
| Value | 174.4 ± 51.9 | 194.6 (66.2 to 254) | 267.8 ± 54.2 | 278.5 (129.1 to 362) | <0.001 |
|
| |||||
| Change % | 583 ± 143 | 546 (364 to 879) | 188 ± 64 | 179 (74 to 302) | <0.001 |
|
| |||||
| P-Within* | <0.001 | <0.001 | |||
Based on mixed model adjusted for the multiple comparisons by Bonferroni method
Based on paired t-test
Table 4.
a- and b- wave amplitudes (microvolts) of the rod response ERG and their changes (percentage) before and 1, 3 and 6 months after surgery
| Time | a-wave |
b-wave |
P† | ||
|---|---|---|---|---|---|
| Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | ||
| Baseline | |||||
| Value | 2.8 ± 0.7 | 3 (1.8 to 4.3) | 86.9 ± 28.7 | 92.1 (27.4 to 137) | <0.001 |
|
| |||||
| Month 1 | |||||
| Value | 4.9 ± 1.5 | 4.8 (2.1 to 7.5) | 148 ± 48.5 | 165.4 (43.7 to 201.7) | <0.001 |
|
| |||||
| Change % | 72 ± 30 | 80 (3 to 105) | 68 ± 47 | 69 (-89 to 158) | 0.97 |
|
| |||||
| P-Within* | <0.001 | 0.107 | |||
|
| |||||
| Month 3 | |||||
| Value | 10 ± 4.4 | 9.1 (4.8 to 19.7) | 271.9 ± 78.3 | 288.5 (111 to 375.2) | <0.001 |
|
| |||||
| Change % | 258 ± 153 | 225 (86 to 667) | 214 ± 101 | 211 (-72 to 452) | 0.794 |
|
| |||||
| P-Within* | <0.001 | <0.001 | |||
|
| |||||
| Month 6 | |||||
| Value | 9.7 ± 4.3 | 9 (2.6 to 18.8) | 259.9 ± 78.2 | 276.1 (119 to 369.4) | <0.001 |
|
| |||||
| Change % | 244 ± 150 | 208 (21 to 645) | 210 ± 133 | 210 (-70 to 499) | 0.97 |
|
| |||||
| P-Within* | <0.001 | <0.001 | |||
Based on mixed model adjusted for the multiple comparisons by Bonferroni method
Based on paired t-test
Table 5.
a- and b-wave amplitudes (microvolts) of the cone response ERG and their changes (percentage) before and 1, 3 and 6 months after surgery
| Time | a-wave |
b-wave |
P† | ||
|---|---|---|---|---|---|
| Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | ||
| Baseline | |||||
| Value | 2.1 ± 0.8 | 1.9 (1.2 to 3.8) | 2.9 ± 0.9 | 2.6 (1.8 to 4.9) | <0.001 |
|
| |||||
| Month 1 | |||||
| Value | 2.2 ± 0.9 | 2.1 (1.1 to 3.9) | 3 ± 0.9 | 2.8 (1.8 to 5) | <0.001 |
|
| |||||
| Change % | 7 ± 29 | 0 (-13 to 129) | 5 ± 12 | 5 (-36 to 29) | 0.006 |
|
| |||||
| P-Within† | 0.268 | 0.318 | |||
|
| |||||
| Month 3 | |||||
| Value | 5.1 ± 1.7 | 4.9 (3.2 to 8.8) | 9.9 ± 1.9 | 9.6 (7.4 to 14.5) | <0.001 |
|
| |||||
| Change % | 163 ± 67 | 162 (56 to 302) | 260 ± 65 | 269 (141 to 377) | <0.001 |
|
| |||||
| P-Within† | <0.001 | <0.001 | |||
|
| |||||
| Month 6 | |||||
| Value | 5.3 ± 1.6 | 5 (3.2 to 9.1) | 9.8 ± 1.9 | 9.2 (7.4 to 14) | <0.001 |
|
| |||||
| Change % | 171 ± 66 | 166 (56 to 295) | 258 ± 71 | 265 (137 to 377) | <0.001 |
|
| |||||
| P-Within† | <0.001 | <0.001 | |||
Based on mixed model adjusted for the multiple comparisons by Bonferroni method
Based on paired t-test
Table 6.
ERG a- and b-wave amplitudes (microvolts) in normal subjects
| ID | Age | Sex | Eye | Maximal combined response (microvolts) |
Cone response (microvolts) |
Rod response (microvolts) |
|||
|---|---|---|---|---|---|---|---|---|---|
| a-wave | b-wave | a-wave | b-wave | a-wave | b-wave | ||||
| 1 | 22 | M | OD | 179.0 | 381.0 | 15.0 | 56.0 | 23.0 | 266.0 |
|
| |||||||||
| 2 | 18 | M | OS | 176.0 | 392.0 | 18.0 | 62.0 | 19.0 | 259.0 |
|
| |||||||||
| 3 | 29 | F | OD | 199.0 | 375.0 | 16.0 | 61.5 | 19.0 | 245.0 |
|
| |||||||||
| 4 | 40 | F | OD | 201.0 | 402.0 | 15.0 | 59.0 | 16.0 | 204.0 |
|
| |||||||||
| 5 | 31 | M | OS | 196.0 | 364.0 | 21.0 | 66.0 | 22.0 | 259.0 |
|
| |||||||||
| 6 | 19 | F | OS | 202.0 | 375.0 | 19.0 | 71.0 | 23.0 | 276.0 |
|
| |||||||||
| 7 | 23 | F | OD | 199.0 | 380.0 | 21.0 | 66.0 | 19.0 | 252.0 |
|
| |||||||||
| 8 | 31 | M | OD | 186.0 | 345.0 | 18.0 | 63.5 | 21.0 | 280.0 |
|
| |||||||||
| 9 | 29 | F | OD | 213.0 | 385.0 | 19.0 | 70.0 | 19.0 | 245.0 |
|
| |||||||||
| 10 | 17 | M | OS | 196.0 | 403.0 | 20.0 | 69.0 | 19.0 | 256.0 |
|
| |||||||||
| 11 | 46 | F | OD | 211.0 | 375.0 | 18.0 | 66.0 | 21.0 | 245.0 |
|
| |||||||||
| 12 | 61 | M | OS | 198.0 | 410.0 | 16.0 | 70.0 | 19.0 | 232.4 |
|
| |||||||||
| 13 | 44 | M | OS | 189.0 | 334.0 | 19.0 | 68.0 | 20.0 | 212.0 |
|
| |||||||||
| 14 | 19 | M | OD | 186.0 | 412.0 | 16.0 | 59.0 | 18.7 | 260.0 |
|
| |||||||||
| 15 | 32 | M | OS | 183.0 | 391.0 | 15.0 | 66.0 | 18.0 | 270.0 |
|
| |||||||||
| 16 | 59 | F | OD | 176.0 | 404.0 | 19.0 | 72.0 | 22.1 | 266.0 |
|
| |||||||||
| 17 | 40 | M | OS | 189.0 | 325.0 | 21.0 | 69.0 | 19.5 | 249.0 |
|
| |||||||||
| 18 | 18 | M | OD | 201.0 | 401.0 | 20.0 | 81.0 | 18.0 | 259.0 |
|
| |||||||||
| 19 | 47 | F | OS | 190.0 | 345.0 | 19.0 | 79.0 | 20.0 | 244.0 |
|
| |||||||||
| 20 | 50 | M | OS | 189.0 | 394.0 | 21.0 | 74.0 | 21.0 | 258.0 |
|
| |||||||||
| Mean | 33.8 | 193.0 | 379.7 | 18.3 | 67.4 | 19.9 | 251.9 | ||
|
| |||||||||
| SD | 13.9 | 10.5 | 26.0 | 2.1 | 6.4 | 1.8 | 19.4 | ||
M, male; F, female; OD, right eye; OS, left eye
We evaluated these parameters in 20 normal subjects with mean age ± SD of 33.8 ± 13.9 (median: 31, range: 17 to 61) which revealed the following results: the maximal combined response in a- and b- waves amplitude were 193±10.5 and 379.7±26.0, respectively. Also, cone response were 18.3±2.1 and 67.4±6.4 in a- and b- wave correspondingly. Rod response in a- and b- waves amplitude were 19.9±1.8 and 251.9 ±19.4 respectively.
Figure 2.
Mean percentage of change in a-wave amplitude from baseline by the type of response. Mixed model analysis (adjusted for multiple comparisons by the Bonferroni method) showed a significant difference in the mean proportion of change between different response types at month 1 (all pairwise comparisons, P<0.001), month 3 (P maximal vs rod <0.001, P maximal vs cone <0.001, P rod vs cone =0.076) and month 6 (P maximal vs rod <0.001, P maximal vs cone<0.001, P rod vs cone =0.180)
Figure 3.
Mean percentage of change in b- wave amplitude from baseline by the type of response. Mixed model analysis (adjusted for multiple comparisons by the Bonferroni method) showed a significant difference in the mean proportion of change between different response types at month 1 (P maximal vs rod =0.393, P maximal vs cone <0.001, P rod vs cone <0.001), month 3 (P maximal vs rod =0.732, P maximal vs cone =0.002, P rod vs cone =0.198) and month 6 (P maximal vs rod >0.99, P maximal vs cone=0.009, P rod vs cone =0.469)
Mean BCVA before surgery was 2.22±0.35 (range, 1.8 to 2.6) logMAR which was significantly improved to 1.38±0.21 (range, 1.1 to 1.8) logMAR, 0.91±0.25 (range 0.4 to 1.2) logMAR and 0.88±0.23 (range, 0.4 to 1.2) logMAR one, three and six months after surgery, respectively. This indicates a mean difference of 0.8±2 logMAR (95% confidence interval, -0.95 to -0.74, P<0.001), -1.3±0.3 logMAR (95% confidence interval, -1.44 to -1.19, P<0.001) and -1.3±0.3 logMAR (95% confidence interval, -1.47to -1.21, P<0.001) at 1, 3 and 6 months, respectively (Table 2).
Discussion
In the current study, both scotopic and photopic a- and b-wave amplitudes had been reduced to almost non-recordable levels in eyes with total RRD preoperatively. After successful anatomical reattachment, the scotopic ERG a- and b-wave amplitudes demonstrated higher values than the photopic response at 1, 3 and 6 months. Although, greater improvement in a- and b-wave amplitudes was seen at six months, there was no significant difference between the six and three month amplitudes. Moreover, visual acuity increased in parallel to improvement in ERG responses.
The results of the current study indicated that despite partial recovery in ERG wave amplitudes after retinal reattachment, these values still remained lower than normal eyes.23 In addition, there were differences in the recovery pattern of a- and b-wave amplitudes indicating dissimilarities in the functional recovery of different retinal cell types.
Few experimental studies have shown that one month after retinal reattachment surgery, photoreceptor ultrastructure recovers completely.12,21,25 Moreover, most of the outer segments regain their normal appearance within 2 weeks. However, there are often defects in the outer segment, especially in the outer segment of cone cells. In cases with detachments of short duration (less than 1 week) morphological recovery in the reattached retina is complete while with detachments of more than one month duration, recovery is usually incomplete. These observations are similar to some other published studies.26
Hayashi et al22 reported that reduction in ERG b-wave amplitude was significantly greater in short wavelength (s) cones than in long and medium wave length (L-M) cones postoperatively. They demonstrated such differences to result from dissimilarities in postoperative recovery among the three cone cell types. In the current study, rod photoreceptors demonstrated better recovery in ERG wave amplitudes than cone photoreceptors at all-time points. This indicates that the rod system repairs more rapidly and suggests that certain functional disorders may persist in the cone system.27,28 Similar to this observation, Schatz et al29, have reported in a group of patients with preoperative foveal detachment that rod system function was significantly improved while the single flash white light response and cone amplitudes did not improve to the same extent.
In contradiction, another study30 reported no obvious improvement in ERG parameters within the first 6 months post-operation. Our results showed that one month after surgery, both a- and b-wave amplitudes tend to rise, completing at three months with no further improvement up to 6 months. In eyes with retinal detachment, it is believed that there is a reduction in the number of photoreceptor synaptic terminals in the outer plexiform and in inner nuclear layers.31,32 In an experimental study, it has been demonstrated that after retinal detachment in animals the number of photoreceptors is reduced.31,32 However, demonstration of such findings is not possible in vivo.
The exact mechanism for delayed recovery of the cone system after retinal detachment is not clear. It is believed that the cone pathway is more vulnerable to hypoxia than the rod system.33 Regarding the minimum required duration of four weeks for photoreceptor recovery after retinal reattachment, we believe that during this period the photoreceptors should have made ultimate recovery and therefore ERG amplitudes can be considered as a reliable objective measure of improvement after retinal reattachment.23,34-36
Earlier improvement of ERG a-wave amplitudes following retinal reattachment is due to photoreceptor recovery which takes place sooner than improvement of inner retinal layers. Improvement of the b-wave, which occurred one to three months after reattachment, reflects recovery of inner retinal layers.
In summary, retinal detachment may cause severe reduction of ERG a- and b-wave amplitudes which usually recover shortly after reattachment surgery with maximum recovery at three months. However, this improvement may be partial and amplitudes may not reach normal pre-detachment levels. This study reemphasizes the need for reattachment surgery as soon as possible in cases with macula on detachments to prevent further damage to cone cells.
Acknowledgments
We would like to thank Mehdi Yaseri, PhD for his kind assistance with statistical analysis.
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.Kang IS, Kim IT. Clinical study on rhegmatogenous retinal detachment treated with sclera buckling. J Korean Ophthalmol Soc. 1991;32:232–239. [Google Scholar]
- 2.Wilkinson CP, Rice TA. Michel’s retinal detachment. 2nd. St Louis: Mosby; 1997. [Google Scholar]
- 3.American Academy of Ophthalmology. The repair of rhegmatogenous retinal detachments. Ophthalmology. 1990;97:1562–1572. doi: 10.1016/s0161-6420(90)32376-x. [DOI] [PubMed] [Google Scholar]
- 4.Isernhagen RD, Wilkinson CP. Recovery of visual acuity following the repair of pseudophakic retinal detachment. Trans Am ophthalmol Soc. 1988;86:291–306. [PMC free article] [PubMed] [Google Scholar]
- 5.Rachal WF, Burton TC. Changing concepts of failures after retinal detachment surgery. Arch Ophthalmol. 1979;97:480–483. doi: 10.1001/archopht.1979.01020010230008. [DOI] [PubMed] [Google Scholar]
- 6.Gundry MF, Davies EW. Recovery of visual acuity after retinal detachment surgery. Am J Ophthalmol. 1974;77:310–314. doi: 10.1016/0002-9394(74)90735-1. [DOI] [PubMed] [Google Scholar]
- 7.Friberg TR, Eller AW. Prediction of visual recovery after scleral buckling of macula-off retinal detachments. Am J ophthalmol. 1992;114:715–722. doi: 10.1016/s0002-9394(14)74050-4. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman PL, Alm A. Adler’s physiology of the eye. 10th. St Louis: Mosby; 2003. [Google Scholar]
- 9.Mowatt L, Shun-Shin GA, Arora S, Price N. Macula off retinal detachments. How long can they wait before it is too late? Eur J Ophthalmol. 2005;15:109–117. [PubMed] [Google Scholar]
- 10.Yang CH, Lin HY, Huang JS, Ho TC, Lin CP, Chen MS, et al. Visual outcome in primary macula-off rhegmatogenous retinal detachment treated with scleral buckling. J Formos Med Assoc. 2004;103:212–217. [PubMed] [Google Scholar]
- 11.Montrone L, Ziccardi L, Stifano G, Piccardi M, Molle F, Focosi F, et al. Regional assessment of cone system function following uncomplicated retinal detachment surgery. Doc Ophthalmol. 2005;110:103–110. doi: 10.1007/s10633-005-7554-3. [DOI] [PubMed] [Google Scholar]
- 12.Anderson DH, Stern WH, Fisher SK, Erickson PA, Borgula GA. Retinal detachment in the cat: the pigment epithelial-photoreceptor interface. Invest Ophthalmol Vis Sci. 1983;24:906–926. [PubMed] [Google Scholar]
- 13.Lewis GP, charteris DG, Sethi CS, Leitner WP, Linberg KA, Fisher SK. The ability of rapid retinal reattachment to stop or reverse the cellular and molecular events initiated by detachment. Invest Ophthalmol Vis Sci. 2002;43:2412–2420. [PubMed] [Google Scholar]
- 14.Kanski JJ. Retinal detachment. In: Kanski JJ, editor. Clinical ophthalmology: a systemic approach. 6th ed. London: Butterworth-Heinemann/Elsevier; 2007. [Google Scholar]
- 15.Liesegang TJ, Skuta GL, Cantor LB. Retina and Vitreous 2013-2014.Section 12. San Fransisco: American Academy of ophthalmology (AAO); 2004. Retinal detachment. [Google Scholar]
- 16.Rose A. Duration of rhegmatogenous retinal detachment predicts recovery of retinal sensitivity. Universa Medicina. 2009;28:133–138. [Google Scholar]
- 17.Polkinghorne PJ, Craig JP. Northern New Zealand rhegmatogenous retinal detachment study: epidemiology and risk factors. Clin Experiment Ophthalmol. 2004;32:159–163. doi: 10.1111/j.1442-9071.2004.00003.x. [DOI] [PubMed] [Google Scholar]
- 18.Gariano RF, Kim CH. Evaluation and management of suspected retinal detachment. Am Fam Physician. 2004;69:1691–1698. [PubMed] [Google Scholar]
- 19.Fisher SK, Lewis GP. Cellular effects of detachment and reattachment on the neural retina and the retinal pigment epithelium. In: Ryan SJ, editor. Retina. 4th. Philadelphia: Mosby; 2006. [Google Scholar]
- 20.Liu F, Meyer CH, Mennel S, Hoerle S, Kroll P. Visual recovery after scleral buckling surgery in macula-off rhegmatogenous retinal detachment. Ophthalmologica. 2006;220:174–180. doi: 10.1159/000091761. [DOI] [PubMed] [Google Scholar]
- 21.Guerin CJ, Lewis GP, Fisher SK, Anderson DH. Recovery of photoreceptor outer segment length and analysis of membrane assembly rates in regenerating primate photoreceptor outer segments. Invest Ophthalmol Vis Sci. 1993;34:175–183. [PubMed] [Google Scholar]
- 22.Hayashi M, Yamamoto S. Changes of cone electroretinograms to colour flash stimuli after successful retinal detachment surgery. Br J Ophthalmol. 2001;85:410–413. doi: 10.1136/bjo.85.4.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong Y, Wu X, Sun X, Zhang X, Zhu P. Electroretinogram changes after scleral buckling surgery of retinal detachment. Doc Ophthalmol. 2008;117:103–109. doi: 10.1007/s10633-007-9109-2. [DOI] [PubMed] [Google Scholar]
- 24.Marmor MF, Holder GE, Seeliger MW, Yamamoto S. Standard for clinical electroretinography (2004 update). Doc Ophthamol. 2004;108:107–114. doi: 10.1023/b:doop.0000036793.44912.45. [DOI] [PubMed] [Google Scholar]
- 25.Machemer R, Steinhorst UH. Retinal separation, retinotomy, and macular relocation: I. experimental studies in the rabbit eye. Graefes Arch Clin Exp Ophthalmol. 1993;231:629–634. doi: 10.1007/BF00921956. [DOI] [PubMed] [Google Scholar]
- 26.Anderson DH, Guerin CJ, Erickson PA, Stern WH, Fisher SK. Morphological recovery in the reattached retina. Invest Ophthalmol Vis Sci. 1986;27:168–183. [PubMed] [Google Scholar]
- 27.Yamamoto S, Hayashi M, Takeuchi S. Cone electroretinograms in response to color stimuli after successful retinal detachment surgery. Jpn J Ophthalmol. 1998;42:314–317. doi: 10.1016/s0021-5155(98)00014-8. [DOI] [PubMed] [Google Scholar]
- 28.Tani P, Robertson DM, Langworthy A. Prognosis for central vision and anatomic reattachment in rhegmatogenous retinal detachment with macula detached. Am J Ophthalmol. 1981;92:611–620. doi: 10.1016/s0002-9394(14)74651-3. [DOI] [PubMed] [Google Scholar]
- 29.Schatz P, Holm K, Andreasson S. Retinal function after scleral buckling for recent onset rhegmatogenous retinal detachment: assessment with electroretinography and optical coherence tomography. Retina. 2007;27:30–36. doi: 10.1097/01.iae.0000256659.71864.83. [DOI] [PubMed] [Google Scholar]
- 30.Imai K, Hayashi A, de Juan E. Method and evaluation of experimental retinal detachment. Nippon Ganka Gakkai Zasshi. 1998;102:161–166. [PubMed] [Google Scholar]
- 31.Lewis GP, Linberg KA, Fisher SK. Neurite outgrowth from bipolar and horizontal cells after experimental retinal detachment. Invest Ophthalmol Vis Sci. 1998;39:424–434. [PubMed] [Google Scholar]
- 32.Mervin K, Valter K, Maslim J, Lewis G, Fisher S, Stone J. Limiting photoreceptor death and deconstruction during experimental retinal detachment: the value of oxygen supplementation. Am J Ophthalmol. 1999;128:155–164. doi: 10.1016/s0002-9394(99)00104-x. [DOI] [PubMed] [Google Scholar]
- 33.Smith VC, Ernest JT, pokorny J. Effect of Hypoxia on FM 100-hue test performance. Mod Probl Ophthalmol. 1976;17:248–256. [PubMed] [Google Scholar]
- 34.Wu D, Gao R, Zhang G, Wu L. Comparison of pre-and post-operational multifocal electroretinograms of retinal detachment. Chin Med J (Engl) 2002;115:1560–1563. [PubMed] [Google Scholar]
- 35.Guerin CJ, Anderson DH, Fariss RN, Fisher SK. Retinal reattachment of the primate macula. Photoreceptor recovery after short- term detachment. Invest Ophthamol Vis Sci. 1989;30:1708–1725. [PubMed] [Google Scholar]
- 36.Sakai T, Calderone JB, Lewis GP, Linberg KA, Fisher SK, Jacobs GH. Cone photoreceptor recovery after experimental detachment and reattachment: an immunocytochemical, morphological, and electrophysiological study. Invest Ophthalmol Vis Sci. 2003;44:416–425. doi: 10.1167/iovs.02-0633. [DOI] [PubMed] [Google Scholar]