Abstract
Purpose
To describe the polymorphic expression of Stargardt disease in a large Tunisian family with clinical intra- and interfamilial variation of the condition.
Methods
Twelve subjects from two related families with autosomal recessive Stargardt disease were enrolled. A detailed clinical examination including visual acuity and visual field measurement, fundus photography, fluorescein angiography, electroretinography (ERG) and color vision testing was performed for all subjects.
Results
The youngest child from family A manifested typical Stargardt disease while her two brothers presented with Stargardt disease-fundus flavimaculatus (STGD-FFM) and her two sisters demonstrated a peculiar phenotype overlapping Stargardt disease and cone-rod dystrophy; their phenotypic manifestation corresponded well with ERG groups I, II and III, respectively. This uncommon occurrence of an age-related decline in ERG amplitude and worsening of fundus changes is suggestive of a grading pattern in Stargardt disease. Their two cousins in family B, displayed the STGD-FFM phenotype. Despite clinically similar STGD-FFM patterns in both families, age of onset and progression of the phenotype in family B differed from family A.
Conclusion
This is the first report on phenotypic variation of Stargardt disease in a large Tunisian family. Regarding phenotype and severity of visual symptoms, family A demonstrated Stargardt disease at various stages of progression. In addition, STGD-FFM appeared to be an independent clinical entity in family B. These findings imply that further parameters are required to classify Stargardt’s disease.
Keywords: Stargardt Disease, Fundus Flavimaculatus, Intrafamilial, Interfamilial, Progression, Classification, Prognosis
INTRODUCTION
Stargardt disease (STGD), first described in 1909, is a progressive macular dystrophy characterized initially by loss of vision with no detectable fundus changes, subsequent appearance of atrophic macular degeneration with flecks developing in the paramacular area and posterior pole, mild loss of color vision, normal peripheral visual fields and normal night vision. Symptoms typically appear in the first or second decades of life and the condition is the most frequent early-onset macular dystrophy leading to central vision loss.1,2 Years later, Franceschetti used the term fundus flavimaculatus (FFM) to describe the appearance of irregularly shaped yellow-white flecks within the retinal pigment epithelium (RPE).3
Attempts have been made to make a clear distinction between STGD and FFM using clinical criteria. Recently, molecular investigations substantiated that these entities are allelic disorders linked to the ABCA4 gene thus making the classification of STGD more complicated.4,5
The classification of STGD has been primarily based on fundus appearance; ranging from normal appearing fundus to atrophy of the macula with or without flecks, or flecks with or without atrophy of the macula. Further clinical findings broadened the terminology to include “macular form of tapetoretinal degeneration (TRD)”, “mixed TRD”, “centroperipheral tapetoretinal pigmentary dystrophy” (TRD), “diffuse or central TRD” and peripheral involvement resembling “retinitis pigmentosa inversa”.6
Later on, differences were identified in the severity of photoreceptor function loss on electroretinograms.7,8 However, it remains unclear whether STGD and FFM are actually different entities. While some authors acknowledge similar involvement patterns and severity within families,9-13 others have described the coexistence of different phenotypes in members of the same family.7,14,15
The present study reports the polymorphic expression of STGD in a large Tunisian family. A thorough clinical investigation was performed for all members of this large consanguineous family which represents the largest affected sibship reported so far. Our results demonstrate remarkable intrafamilial variation with cosegregation of STGD-FFM, typical STGD and advanced STGD resembling a cone-rod dystrophy (CRD) in family A. In addition, an interfamilial variation regarding age of onset and progression of the condition was also noted.
METHODS
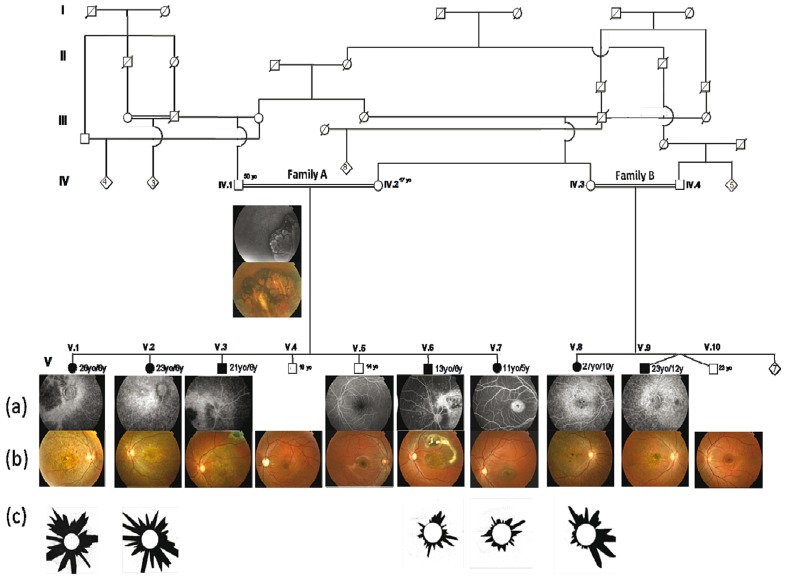
A large multiplex family from Southern Tunisia affected with autosomal recessive retinal dystrophy was ascertained through Hedi Rais Institute of Ophthalmology in Tunis, Tunisia. This pedigree was composed of two consanguinity loops (nuclear families A and B) including seven affected (3 male and 4 female) subjects and five healthy (4 male and 1 female) individuals (Figure 1).
Figure 1.
Phenotypic variation in the studied Tunisian family with autosomal recessive retinal dystrophy: (a) Fluorescein angiography images. (b) Fundus appearance. (c) The Farnsworth-Munsell 100 Hue test studies; this is a color vision test that highlights chromatic pathway dysfunctions. Age at the time of eye exam (yo) and at disease onset (y) appear next to the symbols representing each individual in the pedigree. The genealogy describes seven patients from two families (A and B) with macular degeneration starting in the first or second decades of life. In both families, the disease was confined to one generation and affected both males and females. Several loops of consanguinity are present and autosomal recessive inheritance seems likely. Family A includes healthy parents IV-1, IV-2 and their offspring. Family B includes healthy parents IV-3, IV-4 and their offspring. Individuals IV-1, V-4, V-5 and V-10 dizygotic twin are unaffected.
Informed consent was obtained from all participants who were examined between March 2005 and April 2008; demographic characteristics, age at onset, and personal and family history were recorded for all participants. Affected subjects ranged from 11 to 27 years of age. Age of onset was defined as the age at which decreased visual acuity was first noted.
All patients underwent a standard ophthalmological examination including determination of best corrected visual acuity (BCVA) using standard Snellen charts. Clinical examination was supplemented by fundus photography, color vision assessment using the Farnsworth-Munsell 100 hue color vision test (FM100, Munsell Color Company Inc., Baltimore, MD, USA) and analysis of dark adaptation. Goldmann kinetic perimetry (Carl Zeiss Meditec Inc., Dublin, CA, USA) using V-4e and I-4e targets; fluorescein angiography (FA) and indocyanine green angiography (ICGA) were also performed. Electrophysiological investigation was performed using Vision monitor Métrovision (Métrovision, Pérenchies, France) according to the International Society for Clinical Electrophysiology of Vision (ISCEV) protocol.16 Full-field electroretinography (ERG), including rod-specific response, bright white flash mixed response, 30-Hz flicker response, photopic single-flash ERG, and electro-oculography (EOG) were performed.
The medical files and retinal photographs of the participants were scrutinized in order to outline the clinical vignette. The diagnosis of STGD was based upon deterioration of visual acuity, dyschromatopsia and typical ophthalmoscopic appearance of the macular region (macular and peripheral atrophy together with distribution of flecks). Fundus flavimaculatus (STGD-FFM) was characterized by the presence of white-yellow flecks within the RPE involving the posterior pole or extending to the midperipheral retina, with or without overt atrophic macular lesions. However, if severe macular granularity and atrophy were noted in the presence of retinal flecks and altered photopic ERG, a diagnosis of STGD was made. A dark choroid on FA helped to differentiate cone dystrophy from STGD; the atrophic area and loss of function may extend to the peripheral retina characterizing CRD.
Results
Family A included 9 members among whom 5 were affected (Fig. 1). Photophobia and decreased visual acuity were the first visual symptoms appearing at the age of 5 or 6 years in all affected subjects of family A (Table 1). In this large sibship, three different retinal phenotypes were observed (Fig. 2).
Table 1.
Clinical data of Tunisian patients with Stargardt disease
| Studied Family | Code Sex | Age/Age-of- onset | Phenotype |
|||
|---|---|---|---|---|---|---|
| VA | Fundus and FA | Full field ERG | Diagnosis | |||
| Family A | V-1 | 26/6 | <20/400 | Diffuse macular,peripapillary and peripheral RPE atrophy; hyperfluorescent dots | Altered photopic and scotopic responses | Severe Stargardt or cone rod dystrophy “phenotype III” |
| F | ||||||
|
| ||||||
| V-2 | 23/6 | <20/400 | Diffuse macular,peripapillary and peripheral RPE atrophy; hyperfluorescent dots | Altered photopic and scotopic responses | Severe Stargardt or cone rod dystrophy “phenotype III” | |
| F | ||||||
|
| ||||||
| V-3 | 21/6 | 20/330 | Macular atrophy; white-yellow flecks; hyperfluorescent atrophic spots; silent choroid | Altered photopic responses | Stargardt fundus flavimaculatus “phenotype II” | |
| M | ||||||
|
| ||||||
| V6 | 13/6 | 20/400 | Central atrophy with white-yellow flecks; macular atrophy; hyperfluorescent atrophic spots; silent choroid | Altered photopic responses | Stargardt fundus flavimaculatus “phenotype II” | |
| M | ||||||
|
| ||||||
| V-7 | 11/5 | 20/200 | Bull’s eye maculopathy; temporal peripapillary atrophy; silent choroid; fibroglial scar | Slightly altered photopic responses | Stargardt maculopathy “phenotype I” | |
| F | ||||||
|
| ||||||
| Family B | V-8 | 27/10 | 20/500 | Central atrophy with white-yellow flecks; hyperfluorescent atrophic spots; silent choroid | Altered photopic responses | Stargardt fundus flavimaculatus |
| F | ||||||
|
| ||||||
| V-9 | 23/12 | 20/400 | Central atrophy with white-yellow flecks; macular atrophy hyperfluorescent atrophic spots; silent choroid | Altered photopic responses | Stargardt fundus flavimaculatus | |
| M | ||||||
VA, visual acuity; FA, fluorescein angiography; ERG, electroretinography
F, female; M, male; RPE, retinal pigment epithelium
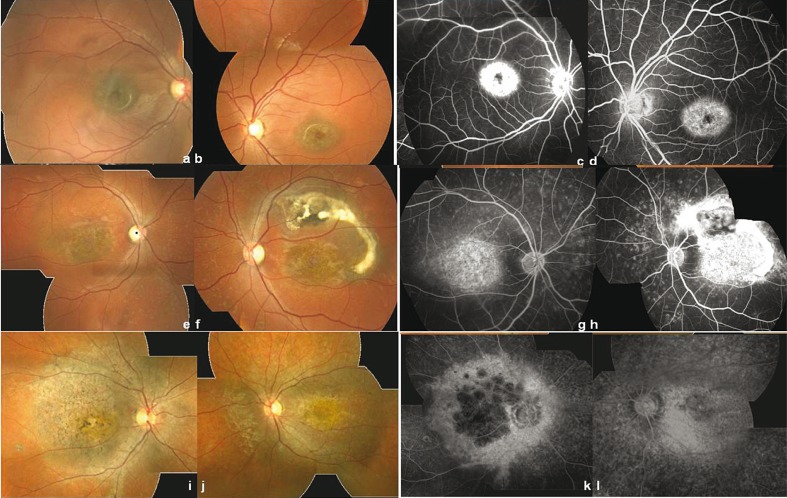
Figure 2.
Polymorphic expression of Stargardt disease in family A based on funduscopy and angiography. Typical Stargardt with bull’s eye maculopathy; fundus appearance (a, b) and angiograms (c, d) in phenotype I (right eye results: a, c; left eye results: b, d). Fluorescein angiography shows the typical appearance of reduced transmission of background fluorescence (dark choroid) in patient V-7. Stargardt disease associated with fundus flavimaculatus; fundus appearance (e, f) and angiograms (g, h) in phenotype II (right eye results: e, g; left eye results: f, h). Composite image of the right eye shows flecks throughout the posterior pole, atrophic macular changes and several flecks. Patient V-6 also displays a fibroglial scar in the left eye (f, h). Fluorescein angiography clearly reveals the dark choroid. Advanced stage of Stargardt disease or cone rod dystrophy; fundus appearance (i, j) and angiograms (k, l) in phenotype III (right eye results: i, k; left eye results: j, l ). Patient V-1 presents a large demarcated atrophic area in the macula with pigment clumping and migration extending to the peripheral retina illustrating an overlapping phenotype.
Phenotype I (11-year-old patient, V-7) includes mild salt and pepper changes in the macula, temporal peripapillary atrophy and normal retinal periphery without flecks (Figures 2a and 2b). FA revealed a silent choroid together with bull’s eye maculopathy (Figures 2c and 2d). The patient had a protan defect on FM100 (Fig. 1). The full-field photopic ERGs showed that the dysfunction was confined to the macula (Fig. 3). This phenotype corresponded to typical Stargardt maculopathy (Table 1).
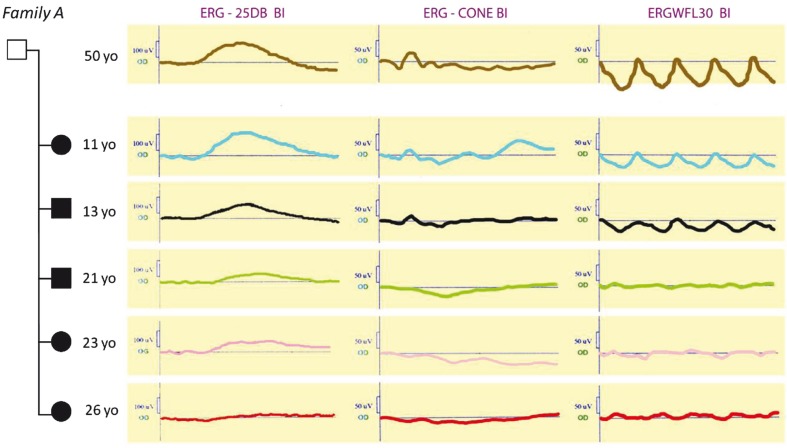
Figure 3.
Scotopic and photopic ERGs show progressive amplitude reduction related to age. Electrophysiological measurements were recorded under three conditions (ERG-25DB BI, ERG-CONE and ERGWFL30). Scotopic phase: response 25DB on blue light stimulus [rod b-wave amplitude (in microvolts]; photopic phase: response to cone and cone flicker stimulus. For patients in family A, first symptomatic visual impairment began at the age of 5-6 years. ERG showed hypovolted responses with predominant cone involvement and progressive worsening of these signs related to age. In the oldest patients, ERG recordings are consistent with macular and peripheral dysfunctions. yo, years old
Phenotype II (21-year-old patient V-3 and 13-year-old patient V-6) is characterized by a salt and pepper macular appearance, yellowish white flecks distributed in the posterior pole and midperiphery, and normal peripapillary area (Figures 2e and 2f). FA showed a silent choroid in both patients and a fibroglial scar in patient V-6 (Figures 2g and 2h). FM100 detected color discrimination loss along deutan and tritan axes (Fig. 1). Cone dysfunction was also reflected by altered photopic responses on ERG (Fig. 3). These clinical findings corresponded to STGD/FFM (Table 1).
Phenotype III (26-year-old patient, V-1 and 23-year-old patient, V-2) included a large demarcated area of RPE atrophy, pigment clumping and migration extending to the peripheral retina associated with peripapillary atrophy; the atrophy was worse in the right eye (Figures 2i and 2k) compared to the left eye (Figures 2j and 2l) in patient V-1. FA showed a larger central area of pronounced chorioretinal atrophy in both eyes. The borders between the confluent regions and the outer lesion were less distinct on FA images. The dyschromatopsia affected the three protan, deutan and tritan axes (Fig. 1). These two patients had severely reduced full-field cone ERG amplitudes (90% reduction or greater), reduced rod ERG amplitudes and markedly delayed cone implicit times (Fig. 3). This clinical presentation illustrated an uncommon Stargardt phenotype overlapping clinical features of an advanced stage of STGD and CRD (Table 1).
Family B included twelve individuals among whom two were affected (Fig. 1). Their initial complaints included blurred near vision in the first decade of life (10 or 12 years of age) (Table 1). Twenty-seven-year-old patient V-8 and 23-year-old patient V-9 showed central atrophy, diffuse yellowish white flecks with sparing of the peripapillary area on fundus examination. FA revealed a dark choroidal pattern. In addition, these patients had reduced full-field cone ERGs. These clinical findings characterized the STGD-FFM phenotype (Table 1). No other cases with similar visual complaints or clinical manifestations were reported among the remaining siblings.
DISCUSSION
In the current study, we report a large consanguineous Tunisian family demonstrating a variety of phenotypes including typical STGD, STGD-FFM, as well as forms of advanced Stargardt disease with diffuse atrophy resembling CRD. From a total of 7 affected subjects, 5 belonged to the same sibship, the largest number reported so far.
Classically, STGD patients begin to lose vision in the first two decades of life. The condition is often associated with an initially normal fundus and later with macular atrophy and yellowish deep retinal flecks.1
Patient V-7 from family A presented with “phenotype I” which is an example of typical STGD macular disease similar to what Stargardt described in 1909 (Table 2). This 11 -year-old patient presented with blurred central vision at the age of 5-6 years. Her visual acuity (VA) was 20/200. She had impaired color vision and fundus examination showed ‘‘bull’s eye'' macular atrophy while the peripheral retina was preserved.
Table 2.
History of classification of Stargardt’s Disease
| Description of Stargardt's Disease | Criteria for diagnosis | ||||
|---|---|---|---|---|---|
| Stargardt1 1909 | 1- A disease of probable autosomal recessive inheritance with age of onset in the 1st or 2nd decade. 2- Initial loss of vision before definite retinal changes are seen. 3- The eventual appearance of both an atrophic macular degeneration and prominent yellowish flecks in the macular and often posterior polar areas as well. The area of degeneration becomes larger with time and prominent flecks disappear. 4- Essentially normal peripheral visual fields and night vision throughout the patient's life. 5- A rather mild loss of color vision even with fairly severe visual loss. | ||||
|
| |||||
| Stargardt17 1913 |
Conditions designated as Stargardt's disease
|
||||
| Vitelliruptive macular degeneration | X-chromosome-linked juvenile retinoschisis | Cone degenerations | Fundus flavimaculatus | Age of onset (1st and 22nd decade) Absence of peripheral retinal changes | |
|
| |||||
| Franceschetti3 1962 | The same clinical picture reported by Stargardt (1909) is designated as Fundus flavimaculatus | Electroretinography dark adaptation | |||
|
| |||||
| Krill and Deutman9 1972 |
Stargardt's disease as a part of Fundus flavimaculatus
|
Eyeground changes Complete retinal function | |||
| Group I | Group II | Group III | |||
| Pure form without atrophy, central visual loss due to invasion of the fovea with one of the typical flecks | Atrophy, macular degeneration followed by flecks (frequent) or flecks preceding macular degeneration (rare). No loss of peripheral retinal function | (rare) Progressive deterioration of peripheral retinal function (4th-5th decade), Macular atrophy | |||
| Subgroup A (frequent) No diffuse cone abnormality (clinical picture reported by Stargardt 1909) | Subgroup B (rare) Diffuse severe cone disease on the ERG such as described in cone degenerations | ||||
|
| |||||
| Fishman7 1976 | Stage I | Stage II | Stage III | Stage IV | The extent of fundus fleck-like lesions and their degree of resorption The extent of choroidal atrophy |
| Central RPE and possibly choriocapillaris disease, often associated with a discrete ring of perimacular flecks | Macular atrophy with flecks often extending to the equator. Atrophy of choriocapallaris and RPE within the macula. | Extensive fleck resorption within the posterior pole and marked RPE atrophy. | Diffusely resorbed flecks, extensive choriocapillaris atrophy | ||
|
| |||||
| Gass18 1987 | Group I | Group II | Group III | Group IV | Macula appearance |
| Vermillion fundi and hidden choroidal fluorescence | Atrophic maculopathy with or without flecks | Atrophic maculopathy with late signs and symptoms of retinitis pigmentosa | Flecks not associated with macular atrophy | ||
|
| |||||
| Noble and Carr13 1979 | Group I | Group II | Group III | Group IV | Macula appearance |
| Macular degeneration without flecks | Macular degeneration with flecks | Macular degeneration with diffuse flecks | Diffuse flecks without macular degeneration | ||
|
| |||||
| Aaberg14 1986 | Stage I | Stage II | Stage III | Stage IV | The degree of damage to the retina |
| Purely central macular degeneration with or without perifoveal flecks | Central macular degeneration and pericentral flecks extending outside the posterior fundus | Centroperipheral retinal pigmentary degeneration with an intact peripheral visual field but pigment migration, depigmentation and normal retinal vessel size | Centroperipheral retinal pigmentary degeneration with peripheral visual field defects and "bone trabeculae" pigmentation with attenuated retinal vessels | ||
|
| |||||
| Lois et al8 2001 | Group I | Group II | Group III | Electrophysiological attributes | |
| Normal rod and cone-mediated ERGs | Relative loss of generalized cone function | Both abnormal rod and cone ERGs | |||
The term “group” does not allow the progression of a patient from one category to another while the term ”stage” is defined as a period or distinct phase in the course of a disease or any biologic process.
Meanwhile, VA of her older brothers (patients V-3 and V-6) was 20/400; macular changes and yellow flecks were more pronounced yet compatible with typical Stargardt. However, the retina in these two subjects displayed large atrophic areas and greyish discoloration with pigment migration and large pigment clumps in the posterior pole, midperiphery, and along the vessels. All of these characteristics more appropriately correspond to fundus flavimaculatus (STGD-FFM) or “phenotype II”, rather than STGD disease. These findings are suggestive of widespread retinal degeneration and are more drastic than what is usually observed in typical STGD disease. It is also noteworthy that STGD-FFM patients V-6 and V-3 (13 and 21 years of age respectively), showed more severe clinical presentation than their younger sister (V-7) affected with typical STGD disease.
Comparison of these two clinical pictures with “phenotype III” in older sisters V-2 and V-1, aged 23 and 26 respectively, suggests that there is a tendency toward progressive retinal degeneration within family A. The latter two subjects manifested deterioration of VA (<20/400) in addition to extensive atrophy of the RPE extending outside the macula. This peculiar phenotype included features of both an advanced STGD macular disease with diffuse atrophy and CRD. This phenotypic overlap and the intrafamilial variation in family A from typical STGD to advanced STGD with a diffuse atrophy are suggestive of progression of a single disease.
It has been understood that retinal function is more reliable than fundus appearance for characterization of this progression and its association with age 6-8,11,13,14,19,20 (Table 2). Recently, STGD has been subdivided into three groups based on ERG attributes: group I with normal rod and cone-mediated ERGs; group II with relative loss of generalized cone function; and group III with both abnormal rod and cone ERGs8 (Table 2). ERG findings in family A highlighted a decline in retinal function with age in cone, cone flicker and rod responses (Fig. 3). Interestingly, ERG findings in phenotypes I, II and III were compatible with Lois’s ERG groups I, II and III, respectively (Table 2). Therefore, our study represents the first instance of siblings displaying co-occurrence of three ERG groups I, II and III. Nevertheless, the apparent age-related progression from group I to III within the siblings, which seems to be unprecedented in the literature, raises questions about the definition of ERG groups as “distinct phenotypic subtypes that do not represent different stages in the progression course of the disease”.8
Different classifications for STGD have already been proposed but there is no clear consensus about the issue (Table 2); some subclassify the condition into separate groups and others support the concept of a single disease. At the beginning of the 20th century, differences were noted in the age of onset of the disease and fundus appearance of STGD patients.9 Later on, attention was given to the fleck component of the disorder and clinical investigation methods improved.3,7,21-23 This prompted many authors to review the proposed classification and seek more reliable criteria for clinical diagnosis of the condition, mainly by comparing funduscopy and electroretinography.8,11,14,20,25,26
Recently, it has been described that only STGD patients who are in the more severe ERG groups (II and III), reveal peripapillary sparing in their fundus.27 However, our study demonstrated peripapillary sparing in phenotypes I and III in family A (ERG groups I and III) and in their affected cousins whose phenotype likely corresponded to ERG group II. All of these findings suggest that sparing is not an accurate clinical marker for severity of STGD. Interestingly, this change appears to be independent of retinal function and fundus appearance which both worsened with age in family A. Consequently, this further supports the idea of disease progression.
The controversy surrounding disease course is reflected by the terminology used for classifying patients with STGD disease (Table 2) and by the large studied family. On one hand, family A displayed (1) an overlapping phenotype, (2) intrafamilial variation with macular defects that appeared at a same age (5-6 years old), and (3) an age-related decline in ERG amplitude suggesting an age-related increase in severity. Altogether, these findings strongly support the idea that the three phenotypes are variable expressions or stages of a single progressive disease.
On the other hand, family B showed one STGD-FFM phenotype in 27-year-old (V-8) and 23-year old (V-9) patients. However, the disease progression hypothesis implies that patients in family B should also manifest the same severe phenotype as their cousins V-1 (26 years old) and V-2 (23 years old). This discrepancy is likely due to various haplotype combinations (data not shown) and different ages of onset in family A (5-6 years old) and B (10-12 years old) that may influence the severity and prognosis of the condition. Indeed, age-of-onset is correlated with the amount of ABCR activity in photoreceptors.5,16,20 In addition, STGD-FFM appears at an older age, has slower progression and thus better prognosis.28 Therefore, age-of-onset remains an important criterion for diagnosis and classification of STGD.
Although patients V-3 and V-6 from family A, and patients V-8 and V-9 from family B expressed the same STGD-FFM phenotype on examination, the clinical presentation appeared at 10-12 years of age in family B. Therefore, STGD-FFM in family B should be considered a separate nosology from phenotype II in family A in which the disease appeared earlier in patients V-3 and V-6. These observations highlight the polymorphic expression of STGD disease.
It is currently not clear if STGD and STGD-FFM are different clinical entities or should be incorporated into one.9-13,19 Regarding the characteristic fleck components in STGD-FFM, it should be noted that perifoveal or diffuse flecks would eventually develop in some patients who were initially diagnosed with nothing but maculopathy and that the reverse occurrence has also been reported.12,13
Although genotype-phenotype models seemed to be the most accurate tool to differentiate STGD from STGD-FFM regarding the allelic combination of ABCR gene,8,29,30 it was later described that sporadic patients may progress from FFM/STGD to CRD, or to retinitis pigmentosa over many years of follow-up.31-35 However, most cases demonstrate unchanging clinical presentation. Likewise, progression or non-progression of the disease is required to resolve this issue. Only Fishman and Aaberg considered the progressive nature of this disease in their classifications by using the term “stage” instead of “group” considering the degree of retinal damage7,14 (Table 2).
In family A, the peculiar phenotype which corresponded to an advanced stage of STGD disease confounded with CRD; this overlap well demonstrates a bridge between two phenotypes in a single individual. In addition, different phenotypes in family A form a continuum that exemplify a grading pattern (STGD-FFM-CRD) from less severe (in younger cases) to more severe phenotypes (in older cases), which implies the tendency of STGD for progression.
In contrast, STGD progression was slower in family B in which the STGD-FFM phenotype was apparently similar to phenotype II in family A but was considered a separate nosology due to older age-of-onset. Therefore, the term “stage” appears to be more appropriate than “group” to describe the phase of morphologic changes, which is followed by either stability or progressive change.
It is noteworthy that FFM can have two independent traits: (1) a stage of progressive STGD expression in family A, designated as phenotype II and (2) a separate clinical entity in family B which differs from STGD in family A by age-of-onset and degree of progression. This difference could be due to different modifiers that act on ABCR alleles depending on age. Therefore, phenotypic variation dictates caution in classifying and in extrapolating prognosis from one affected family member to others within the same family.
This report demonstrated polymorphic expression of Stargardt disease within the same family in terms of morphology and severity of visual symptoms (intrafamilial variation), and differences between families in terms of age-of-onset and degree of disease progression (interfamilial variation). In order to classify STGD, all of these parameters should be carefully considered to properly differentiate STGD from STGD-FFM. These observations may contribute to better understanding of the disease spectrum and may also have implications for categorization and prognosis.
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.Stargardt K. Über familiäre, progressive degeneration in der makulagegend des auges. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1909;71:534–550. [Google Scholar]
- 2.Blacharski PA. Fundus flavimaculatus. In: Newsome DA, editor. Retinal Dystrophies and Degenerations. New York: Raven Press; 1988. [Google Scholar]
- 3.Franceschetti A. Über tapeto-retinale degenerationen im kindesalter: Dritter Fortbildungs-kurs der Deutschen Ophthalmologischen Gesellschaft, Hamburg 1962. In: Sautter H, editor. Entwicklung und Fortschrift in der Augenhielkunde. Stuttgart: Enke; 1963. [Google Scholar]
- 4.Gerber S, Rozet JM, Bonneau D, Souied E, Camuzat A, Dufier JL, et al. A gene for late-onset fundus flavimaculatus with macular dystrophy maps to chromosome 1p13. Am J Hum Genet. 1995;56:396–399. [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis RA, Shroyer NF, Singh N, Allikmets R, Hutchinson A, Li Y, et al. Genotype/Phenotype analysis of a photoreceptor-specific ATP-binding cassette transporter gene, ABCR, in Stargardt disease. Am J Hum Genet. 1999;64:422–434. doi: 10.1086/302251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klien BA, Krill AE. Fundus flavimaculatus. Clinical, functional and histopathologic observations. Am J Ophthalmol. 1967;64:3–23. [PubMed] [Google Scholar]
- 7.Fishman GA. Fundus flavimaculatus. A clinical classification. Arch Ophthalmol. 1976;94:2061–2067. doi: 10.1001/archopht.1976.03910040721003. [DOI] [PubMed] [Google Scholar]
- 8.Lois N, Holder GE, Bunce C, Fitzke FW, Bird AC. Phenotypic subtypes of Stargardt macular dystrophy-fundus flavimaculatus. Arch Ophthalmol. 2001;119:359–369. doi: 10.1001/archopht.119.3.359. [DOI] [PubMed] [Google Scholar]
- 9.Krill AE, Deutman A. The various categories of juvenile macular degeneration. Trans Am Ophthalmol Soc. 1972;70:220–245. [PMC free article] [PubMed] [Google Scholar]
- 10.Irvine R, Wergeland FL. Stargardt's hereditary progressive macular degeneration. Br J Ophthalmol. 1972;56:817–826. doi: 10.1136/bjo.56.11.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadden OB, Gass JDM. Fundus flavimaculatus and Stargardt's disease. Am J Ophthalmol. 1976;82:527–539. doi: 10.1016/0002-9394(76)90539-0. [DOI] [PubMed] [Google Scholar]
- 12.Krill AE, Archer DB. Incomplete rod-cone degenerations. In: Krill AE, Archer DB, editors. Krill's hereditary retinal and choroidal diseases: clinical characteristics. New York: Harper & Row; 1977. [Google Scholar]
- 13.Noble KG, Carr RE. Stargardt's disease and fundus flavimaculatus. Arch Ophthalmol. 1979;97:1281–1285. doi: 10.1001/archopht.1979.01020020023005. [DOI] [PubMed] [Google Scholar]
- 14.Aaberg TM. Stargardt’s disease and fundus flavimaculatus: evaluation of morphologic progression and intrafamilial co-existence. Trans Am Ophthalmol Soc. 1986;84:453–487. [PMC free article] [PubMed] [Google Scholar]
- 15.Klevering BJ, Maugeri A, Wagner A, Go SL, Vink C, Cremers FP, et al. Three families displaying the combination of Stargardt's disease with cone-rod dystrophy or retinitis pigmentosa. Ophthalmology. 2004;111:546–553. doi: 10.1016/j.ophtha.2003.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Marmor MF, Holder GE, Seeliger MW, Yamamoto S. Standard for clinical electroretinography. Doc Ophthalmol. 2004;108:107–114. doi: 10.1023/b:doop.0000036793.44912.45. [DOI] [PubMed] [Google Scholar]
- 17.Stargardt K. Familiare progressive degeneration in der makulagegend des auges. Zeit. für. Augenheilkd. 1913;30:95. [Google Scholar]
- 18.Gass JDM. Stargardt's disease (fundus flavimaculatus). In: Gass JDM, editor. Stereoscopic atlas of macular diseases: diagnosis and treatment. 3rd ed. St Louis: CV Mosby; 1987. [Google Scholar]
- 19.Franceschetti A, François J. Fundus flavimaculatus. Arch Ophtalmol Rev Gen Ophtalmol. 1965;25:505–530. [PubMed] [Google Scholar]
- 20.Armstrong JD, Meyer D, Xu S, Elfervig JL. Long-term follow-up of Stargardt's disease and fundus flavimaculatus. Ophthalmology. 1998;105:448–457. doi: 10.1016/S0161-6420(98)93026-3. [DOI] [PubMed] [Google Scholar]
- 21.Krill AE, Klien BA. Flecked retina syndrome. Arch Ophthalmol. 1965;74:496–508. doi: 10.1001/archopht.1965.00970040498011. [DOI] [PubMed] [Google Scholar]
- 22.Ernest JT, Krill AE. Fluorescein studies in fundus flavimaculatus and drusen. Am J Ophthalmol. 1966;62:1–6. doi: 10.1016/0002-9394(66)91668-0. [DOI] [PubMed] [Google Scholar]
- 23.Carr RE. Fundus flavimaculatus. Arch Ophthalmol. 1965;74:163–168. doi: 10.1001/archopht.1965.00970040165007. [DOI] [PubMed] [Google Scholar]
- 24.Krill AE, Newell FW, Chishti MI. Fluorescein studies in diseases affecting the pigment epithelium. Trans Am Ophthalmol Soc. 1968;66:269–317. [PMC free article] [PubMed] [Google Scholar]
- 25.Krill AE. The electroretinographic and electrooculographic findings in patients with macular lesions. Trans Am Acad Ophthalmol Otolaryngol. 1966;70:1063–1083. [PubMed] [Google Scholar]
- 26.Moloney JB, Mooney DJ, O'Connor MA. Retinal function in Stargardt's disease and fundus flavimaculatus. Am J Ophthalmol. 1983;96:57–65. doi: 10.1016/0002-9394(83)90455-5. [DOI] [PubMed] [Google Scholar]
- 27.Burke TR, Allikmets R, Smith RT, Gouras P, Tsang SH. Loss of peripapillary sparing in non-group I Stargardt disease. Exp Eye Res. 2010;91:592–600. doi: 10.1016/j.exer.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yatsenko AN, Shroyer NF, Lewis RA, Lupski JR. Late-onset Stargardt disease is associated with missense mutations that map outside known functional regions of ABCR (ABCA4). Hum Genet. 2001;108:346–355. doi: 10.1007/s004390100493. [DOI] [PubMed] [Google Scholar]
- 29.Rozet JM, Gerber S, Souied E, Perrault I, Châtelin S, Ghazi I, et al. Spectrum of ABCR gene mutations in autosomal recessive macular dystrophies. Eur J Hum Genet 1998;6:291-295. Erratum in: Eur J Hum Genet. 1999;7:102. doi: 10.1038/sj.ejhg.5200221. [DOI] [PubMed] [Google Scholar]
- 30.Maugeri A, van Driel MA, van de Pol DJ, Klevering BJ, van Haren FJ, Tijmes N, et al. The 2588G-->C mutation in the ABCR gene is a mild frequent founder mutation in the Western European population and allows the classification of ABCR mutations in patients with Stargardt disease. Am J Hum Genet. 1999;64:1024–1035. doi: 10.1086/302323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerth C, Andrassi-Darida M, Bock M, Preising MN, Weber BH, Lorenz B. Phenotypes of Stargardt macular dystrophy/fundus flavimaculatus patients with known ABCA4 mutations and evaluation of genotype-phenotype correlation. Graefes Arch Clin Exp Ophthalmol. 2002;240:628–638. doi: 10.1007/s00417-002-0502-y. [DOI] [PubMed] [Google Scholar]
- 32.Simonelli F, Testa F, Zernant J, Nesti A, Rossi S, Rinaldi E, et al. Association of a homozygous nonsense mutation in the ABCA4 (ABCR) gene with cone-rod dystrophy phenotype in an Italian family. Ophthalmic Res. 2004;36:82–88. doi: 10.1159/000076886. [DOI] [PubMed] [Google Scholar]
- 33.Klevering BJ, Deutman AF, Maugeri A, Cremers FP, Hoyng CB. The spectrum of retinal phenotypes caused by mutations in the ABCA4 gene. Graefes Arch Clin Exp Ophthalmol. 2005;243:90–100. doi: 10.1007/s00417-004-1079-4. [DOI] [PubMed] [Google Scholar]
- 34.Lorenz B, Preising MN. Age matters--thoughts on a grading system for ABCA4 mutations. Graefes Arch Clin Exp Ophthalmol. 2005;243:87–89. doi: 10.1007/s00417-004-1078-5. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg T, Klie F, Garred P, Schwartz M. N965S is a common ABCA4 variant in Stargardt-related retinopathies in the Danish population. Mol Vis. 2007;13:1962–1969. [PubMed] [Google Scholar]