Abstract
Graft versus host disease (GVHD) is a common complication of allogeneic stem cell transplantation (allo-SCT). Ocular GVHD develops in approximately 40-60% of patients following allo-SCT and its most common clinical manifestations include keratoconjunctivitis sicca and cicatricial conjunctivitis. Ocular GVHD may lead to severe ocular surface disease, which can significantly diminish quality of life and restrict daily activities. It is thus important to monitor the condition closely since with timely diagnosis, irreversible damage can be avoided. The current review will focus on updated information regarding ocular GVHD.
Keywords: Allogeneic Stem Cell Transplantation, Bone Marrow Transplantation, Ocular Graft Versus Host Disease, Dry Eye, Keratoconjunctivitis Sicca
Introduction
Hematopoietic stem cell transplantation (SCT), including bone marrow transplantation (BMT), peripheral blood stem cell transplantation and cord blood transplantation, is the routine treatment and a potential cure for life-threatening hematologic malignancies, aplastic anemia, severe combined immunodeficiency, and certain metabolic diseases such as mucopolysaccharidoses and lysosomal storage disorders.1 Graft versus host disease (GVHD) remains one of the major complications of allogenic (allo-) SCT; the condition occurs in 25-70% of patients and is responsible for non-relapse mortality and morbidity in patients undergoing allo-SCT.2
According to the National Institutes of Health (NIH), GVHD can be classified into two broad categories.3 Acute GVHD (aGVHD) is an immediate multi-organ inflammatory syndrome primarily affecting the skin, liver and digestive tract. Chronic GVHD (cGVHD), which previously referred to instances developing 100 days after transplantation, according to the new NIH classification has no time limit; it involves multiple systems such as the musculoskeletal and hematologic systems, as well as various organs including the skin, gut, lungs, and eyes. Clinical features are very complex; there are manifestations of mixed autoimmune/collagen vascular diseases and the hallmarks are comprised of fibrosis, stenosis, and atrophy of tissues in the skin, lung, and mucous membranes such as in the mouth, vagina, and eyes.4
Ocular complications develop in a substantial percentage of patients after allo-SCT as part of acute or chronic GVHD. Ocular GVHD has the potential to lead to severe ocular problems, impair quality of life, and restrict daily activities, and thus, warrants close ophthalmic monitoring in patients undergoing allo-SCT.5 The current article will focus on updated information regarding ocular GVHD.
Pathophysiology
The complex interaction between donor T-cells and host tissues in aGVHD has been described as a three-step process that includes 1) damage to recipient tissue by the pre-transplant conditioning regimen, 2) donor T-cell activation caused by recipient antigen presentation followed by clonal expansion, and 3) cell death induced by activated T-cells, cytokines such as tumor necrosis factor-alpha (TNF-α), and other innate immune cells. In particular, the inflammatory process in aGVHD is thought to involve type 1 T-helper cells, interleukin (IL)-2, interferon-γ (IFN-γ), and IL-1.6 In ocular aGVHD, such T-cell mediated processes are mainly detected in conjunctival and lacrimal gland tissues. In cases with pseudomembranous conjunctivitis, donor-derived mononuclear T lymphocytes, and fibrinoid material with cellular debris and inflammatory cells have been observed.7
The pathophysiology of cGVHD is less understood. The hallmark is IFN-γ expression, and in light of distinctive similarities, especially with collagen vascular disorders, it is often considered an autoimmune disease. There is excessive fibrosis, collagen deposition, antibody production, and suppression of the acute inflammatory response.8 In animal models of cGVHD, type 2 T-helper cells produce cytokines such as IL-4, IL-10, transforming growth factor-β1 and IFN-γ in the absence of IL-2.9 Contrary to aGVHD, donor antigen-presenting cells play a role in the pathogenesis of the chronic form of the disease.10
The eye is a target organ for GVHD, and the ocular surface shows major changes even in the absence of dry eye.11 Ocular involvement in cGVHD appears as inflammatory destruction of the conjunctiva and lacrimal glands with fibrosis, decreased goblet cell density, and a resultant decrease in tear production.12 Tear physiology is found to be severely impaired in most aspects, and compared to Sjögren’s syndrome and meibomian gland dysfunction, tear turnover rate is lowest, evaporation and osmolarity are highest, and the lipid layer appears the most unstable.13
Late ocular complications following BMT include retinal lesions and cataracts as well. The retinal microvasculopathy seen with GVHD seems to reflect a generalized process, and similar to cataract formation, is attributed to other factors such as the use of steroids, irradiation, and systemic hypertension.14
Prevalence and Risk Factors
Ocular GVHD develops in 40-60% of patients after allo-SCT, and 60-90% of patients with acute or chronic GVHD.10 Although signs and symptoms such as photophobia, hyperemia, hemorrhagic conjunctivitis, pseudomembrane formation, lagophthalmos, and corneal ulceration may occur as early as 50 days during the course of aGVHD, ocular GVHD is mainly associated with, and more severe in, cGVHD and occurs in 40-60% of such cases.5 While ocular symptoms may be the first manifestation of systemic GVHD, the presence of skin and/or mouth involvement puts patients at a higher risk for ocular GVHD.15 A higher risk was also detected in patients with allo-SCT from related donors compared to those receiving transplants from matched unrelated donors, probably due to conditioning with anti-thymocyte globulins in the latter group. The choice of stem cell source has shown no clear association with the development of ocular GVHD.5
Manifestations
Ocular tissues affected by acute and chronic forms of GVHD include the eyelid and periorbital skin, conjunctiva, cornea, lens, lacrimal system, sclera, uvea, and retina. While certain complications such as dry eye are very common and are used for diagnosis, scoring, and prognosis assessment of GVHD, some occur less frequently. Nonetheless, many ocular symptoms can be the first and only presentation of ocular GVHD, and some have the potential to progress to severe ocular conditions such as corneal ulceration, melting, and perforation which may, on occasion, lead to evisceration.12 Thus, close monitoring and timely diagnosis are necessary to avoid such drastic complications that can permanently impair vision and quality of life.
Ocular Surface Manifestations
Ocular surface and corneal complications of GVHD can be the direct result of conjunctival goblet cell involvement, or an indirect outcome of lacrimal gland stasis caused by immunosuppression or total body irradiation.12 Major findings in the conjunctiva and cornea include keratinization, epithelial thinning, and squamous metaplasia.5
Dry Eye
Dry eye syndrome (DES) or keratoconjunctivitis sicca is the most frequent complication of GVHD, and is reported to occur in 40 to 76% of patients.4,5,12,15 It may be the initial presentation and sole complication of GVHD and may occur in the absence of other systemic complications.10 While the main cause of DES is cGVHD, other reasons such as irradiation, chemotherapy, immuno-suppressive therapy, and infection can contribute as well. Other risk factors, especially for severe dry eye, include meibomian gland disease and female to male BMT.16 Dry eye can develop any time from a few weeks up to 100 months after transplantation, and the median time is usually around 6 months.12
Subjective symptoms of dry eye are the hallmark of this disease. Patients most commonly experience dry eye and foreign body sensation followed by ocular fatigue, discharge, and dull sensations.17 In the majority of patients, symptoms progress to severe dry eye resembling Sjögren syndrome. Other symptoms include burning, stinging, itching, soreness and heaviness of the eyelids, and photophobia.4,5,12,15Table 1 contains DES symptom classification used for cGVHD scoring.
Table 1.
Grading ocular symptoms in graft versus host disease (GVHD)
| GVHD score | Ocular symptom | |
|---|---|---|
| Dry eye syndrome | 0 | No dry eye symptoms |
|
| ||
| 1 | Dry eye symptoms not affecting activities of daily living (eye drops ≤3 per day) or asymptomatic signs of keratoconjunctivitis sicca | |
|
| ||
| 2 | Dry eye symptoms partially affecting activities of daily living (eye drops >3 per day or punctal plugs) without vision impairment | |
|
| ||
| 3 | Dry eye symptoms, significantly affecting activities of daily living (special eyewear to relieve pain) or unable to work because of ocular symptoms or loss of vision caused by keratoconjunctivitis sicca | |
|
| ||
| Conjunctivitis | 0 | None |
|
| ||
| 1 | Conjunctival hyperemia | |
|
| ||
| 2 | Conjunctival hyperemia with chemotic response and serosanguinous exudate | |
|
| ||
| 3 | Pseudomembranous conjunctivitis | |
|
| ||
| 4 | Pseudomembranous conjunctivitis with corneal epithelial sloughing and subsequent conjunctival scar and symblepharon formation | |
|
| ||
| Cataract | 1 | Occasional subcapsular opacities and vacuoles in the central region of the lens |
|
| ||
| 2 | Small clusters of subcapsular opacities remaining discrete | |
|
| ||
| 3 | Multiple clusters of subcapsular opacities that have mostly coalesced | |
Conjunctival Disease
Conjunctival involvement in GVHD can be seen in about 10% of cases and is often indicative of severe systemic involvement.18 Acute GVHD that involves significant conjunctival inflammation and sloughing typically leaves behind sequelae such as conjunctival scarring and symblepharon. These cicatricial changes can further progress during the course of chronic GVHD. Cases of conjunctival chemosis with multiple central serous chorioretinopathy lesions occurring as early as day 16 and scleritis with choroidal detachment on day 40 have also been described.18 The severity of symptoms at this stage does not necessarily correlate with the clinical presentation.
Cataract
Cataract formation is a common late complication of allo-SCT (Table 1). It is mainly attributed to irradiation and steroid therapy, and is the most common cause of visual acuity loss among patients.12,15,18 Patients receiving total body irradiation are at higher risk of developing cataracts than recipients of fractionated total body irradiation (83% vs. 21% at 6 years); it tends to develop much earlier in the former group as well. Nonetheless, most surviving patients will eventually require cataract surgery.12,15,18
Other Findings
Other ocular manifestations of GVHD include cutaneous complications such as eyelid dermatitis, lagophthalmos and ectropion, poliosis, madarosis, and vitiligo.12,15 Uveitis can occur in up to 8% of cases with cGVHD, and it is important to distinguish infectious etiologies or neoplastic masquerade syndrome from noninfectious uveitis.12 Neuro-ophthalmologic complications such as disc edema are likely secondary to the toxic effects of chemotherapeutic agents such as cyclosporine A and/or coexisting medical conditions, and are usually reversible.19 The main vitreoretinal complication seen in association with GVHD is retinal microvasculopathy that may occur in 10% of cases. Findings include optic disc edema, cotton-wool spots in the fundus, intraretinal and vitreous hemorrhage, and lipid deposits.19 Posterior segment complications also include infections such as infectious retinitis from cytomegalovirus (CMV), herpes simplex virus, or varicella zoster virus, central serous chorioretinopathy and posterior scleritis.10,12,15
Diagnosis and Grading
The diverse range of ocular complications of GVHD calls for close monitoring, assessment of symptoms, and comprehensive ophthalmic examinations including visual acuity testing, slit lamp examination, dry eye workup, tonometry, and fundoscopy. In addition to manifestations and test results, the diagnosis of ocular GVHD can be made through conjunctival biopsies.
The clinical diagnosis of the most common ocular complication, i.e. DES, is not always straightforward. A combination of history taking and medical examination must be used because some patients may have significant symptoms with few findings, whereas others have significant clinical findings with only mild symptoms. After obtaining a thorough history, a careful examination is important to make the diagnosis of dry eye and determine the most likely etiology. There are a number of diagnostic tests for dry eye, which can be divided into four general categories including tear film stability, ocular surface health, tear film composition, and tear film flow.
Prophylaxis and Treatment
At present, aGVHD prophylaxis is mainly attempted by immunosuppression with drugs such as methotrexate, cyclosporine and anti-lymphocyte antibodies. Some studies have reported that combination therapy is more successful.20,21 Immunosuppressive drugs and steroids are used to prevent cGVHD, nonetheless, results are not satisfactory. As for ocular GVHD, cyclosporine eye drops appear to have a prophylactic effect.12,20 As a preventive measure against cataract, eye shielding during total body irradiation can delay its development and decrease its severity.22
Treatment of GVHD mainly constitutes immunosuppressive therapy, and there are guidelines for monitoring and treatment of organ-specific symptoms and complications.23 In ocular GVHD, most treatments are aimed at relieving DES by supporting the tear film, controlling inflammation, and maintaining mucosal integrity. To date, there have been no controlled trials for ocular GVHD treatment options; these have mostly provided unsatisfactory results and include a variety of topical, systemic, and surgical approaches, as well as eyewear and environmental strategies that are administered with respect to the severity of the condition. Treatment recommendations according to severity are summarized in Table 2.24 As with any other organ, the first level should include patient education and counseling. At this level, environmental management includes use of humidifiers and lower room temperatures. At higher levels, while priority is given to topical options, systemic therapy may be needed when the eye is resistant or when other organs are involved.
Table 2.
Treatment recommendations for dry eye based on severity; should treatment outcome at any given level be unsatisfactory, suggested measures of the next level are added
| Level | Recommended measure |
|---|---|
| 1 | Education and counseling Environmental management Elimination of offending systemic medications Preserved tear substitutes, allergy eye drops |
| 2 | Unpreserved tears, gels, ointments Steroids Cyclosporine A Secretagogues Nutritional supplements |
| 3 | Tetracyclines Autologous serum tears Punctal plugs (after control of inflammation) |
| 4 | Topical vitamin A Contact lenses Acetylcysteine Moisture goggles Surgery |
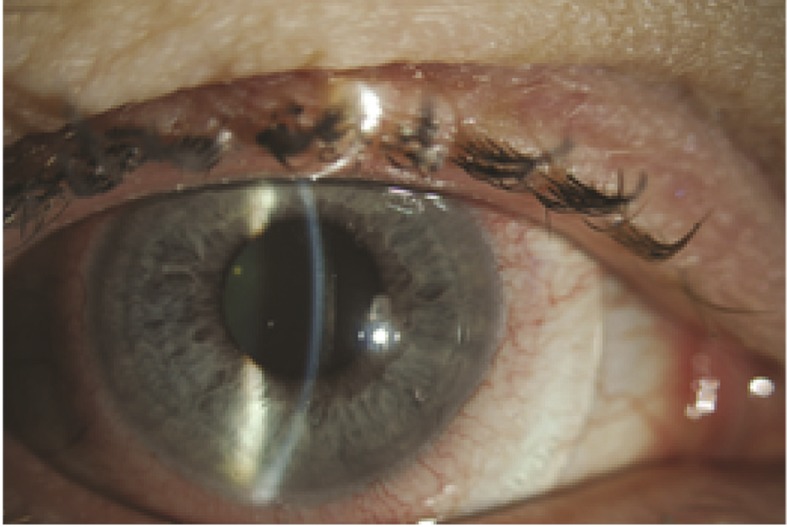
Tear function can be improved by controlling lubrication, evaporation, and drainage. The most common approach is instilling artificial tears, which are available in different brands; their efficacy may be comparable and patients should use the ones they tolerate best. Artificial tears decrease symptoms and improve vision by coating the ocular surface and minimizing punctate keratopathy.25 Autologous serum eye drops are especially beneficial for patients with DES due to GVHD and are now recommended to be used earlier in the course of the disease. Wearing eye protection such as moisture chamber goggles can help decrease evaporation. One of the most exciting new treatments that is useful for recalcitrant cases are contact lenses. Special contact lenses including soft and hard scleral lenses have been shown to be safe and effective in moderate to severe cases, and may help reduce patient dependency on lubricants, minimize symptoms, and improve quality of life.12,26 Prosthetic Replacement of the Ocular Surface Ecosystem (PROSE) is one of the exciting new treatments (Fig. 1). It has been developed by the Boston Foundation for Sight (Needham, MA, USA) to restore vision, support healing, reduce symptoms, and improve quality of life for patients experiencing complex corneal diseases, including irregular astigmatism and ocular surface disease. PROSE uses Food and Drug Administration (FDA) approved custom designed and fabricated prosthetic devices to replace or support impaired ocular surface system functions that protect and enable vision. The fluid-ventilated gas-permeable prosthetic devices clear the cornea and immerse the entire ocular surface in a reservoir of artificial tears. Device design features an optic portion linked to a customized bearing haptic portion, which is designed to align with the sclera. Oxygenation of the fluid-filled reservoir is principally maintained by oxygen transmission through the prosthetic material, but there is some oxygen supplied through tear-fluid exchange under the haptic region. PROSE treatment replaces the functions of the ocular surface system by creating a smooth optical surface over an irregular, damaged, or diseased cornea. It also provides an expanded artificial tear reservoir that yields constant lubrication, while maintaining necessary oxygen supply. Successful treatment re-establishes a healthy and stable ocular surface environment that supports healing, reduces symptoms, and also improves visual function. The lens also acts to mask surface corneal irregularities and prevents damage by protecting and shielding the cornea and conjunctiva against the environment and eyelids.27,28 In a study, PROSE significantly mitigated symptoms and improved quality of life in patients with severe dry eye from cGVHD.26
Figure 1.
A Prosthetic Replacement of the Ocular Surface Ecosystem (PROSE) lens fitted over the eye of a patient with severe dry eye due to chronic graft versus host disease.
The first line treatment in order to control ocular surface inflammation is topical steroids, as they exert non-specific inhibitory effects on the inflammatory response and can improve DES signs and symptoms. However, although some preparations such as loteprednol etabonate are relatively safer, they are more or less associated with an increased risk of infection, glaucoma, and cataract;29 therefore, they are recommended for pulse therapy with close monitoring rather than long-term treatment. The immune response is commonly controlled with cyclosporine eye drops which inhibit T-cell activation, down-regulate pro-inflammatory cytokines, increase the number and density of conjunctival goblet cells, and minimize signs and symptoms of DES with minimal side effects.30,31 Cases unresponsive to a twice daily regimen of cyclosporine 0.05% may benefit from more frequent administration of the medication.32 Topical vitamin A (retinyl palmitate) and topical retinoic acid are also helpful; the former has been found to be comparable to cyclosporine A 0.05% eye drops in the management of DES.33
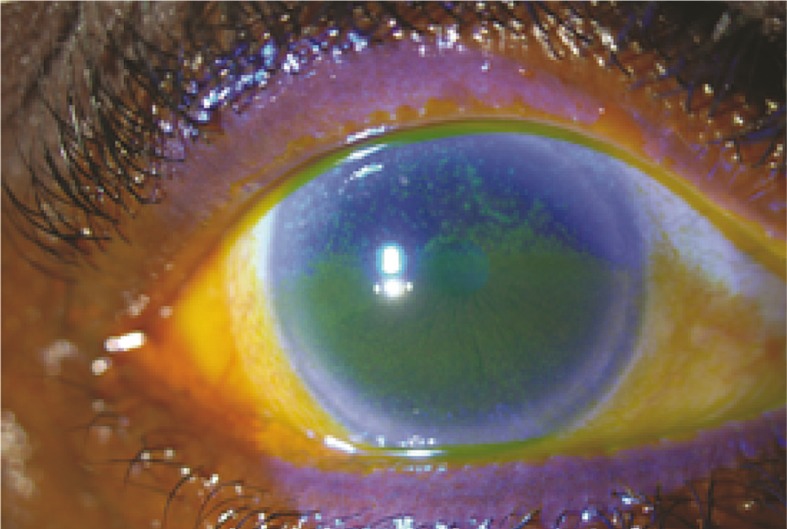
Another exciting new treatment for management of ocular surface disease in patients with GVHD is autologous serum drops. Treatment with autologous plasma rich in platelet-derived growth factor has shown an 80% success rate in refractory cases of cGVHD-related DES.34 It is generally advised to administer autologous tear drops to these patients with even milder symptoms of dry eye (Fig. 2). If topical therapy proves insufficient for controlling ocular surface inflammation, systemic immunosuppression should be started or increased in consultation with the hematologist/oncologist.35-39
Figure 2.
Severe corneal and conjunctival staining in a patient with chronic ocular graft versus host disease; this patient was started on autologous serum tears resulting in significant improvement of signs and symptoms.
Surgical management of DES includes punctal occlusion which is recommended for all patients early in the course once the ocular surface inflammation has been controlled. Partial tarsorrhaphy would be considered as a last resort option for patients with recurrent epithelial breakdown; however specialty contact lenses would be preferred over tarsorrhaphy. If severe complications such as corneal perforation ensue, multilayer amniotic membrane transplantation and keratoplasty may be required (along with tarsorrhaphy).12
Other manifestations of ocular GVHD should be treated accordingly. Conjunctival inflammation can be relieved using topical steroids such as prednisolone acetate 1%. Surgical approaches in this regard include superficial debridement of filamentary keratitis and removal of pseudomembranes. Complications such as uveitis should respond to topical and systemic medications administered to control GVHD, and posterior scleritis has been successfully treated with systemic corticosteroids and acetazolamide.40 The treatment for cataract is surgical, and when performed in GVHD patients, special attention should be given to maintaining normal tear physiology.12 It should be noted that most of these patients will typically have worsening of DES immediately after cataract surgery. Therefore, the dry eye should be fully controlled prior to cataract surgery.
Prognosis
GVHD following allo-SCT often has poor prognosis; ten-year, non-relapse survival rates range from 4 to 91% depending on severity. With cGVHD, the 2-year overall survival for 298 patients with mild, moderate and severe disease was 97%, 86%, and 62% respectively.41 The long-term impact of ocular GVHD on quality of life has not yet been systematically studied. Balaram et al followed 114 patients for more than 1 year after SCT and concluded that late-onset ocular surface disease can occur, and thus the need for long-term monitoring should not be neglected.42 Data on the long-term clinical course and visual outcomes of patients with ocular GVHD are required in order to determine prognostic indicators, develop appropriate measures and therapeutic guidelines, and also improve outcomes.
In conclusion, ocular GVHD is a common manifestation of hematopoietic allo-SCT which can result in significant morbidity and decreased quality of life, severe ocular surface disease and in some cases significant corneal complications. Treatment may require multiple strategies including topical and oral medications, surgical approach, environmental control and systemic immunosuppression. New therapies including autologous serum tears and scleral lenses have provided treatment options for management of ocular GVHD. More data on the impact of ocular GVHD, in addition to development of therapeutic and preventive measures are needed.
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.Malatack JJ, Consolini DM, Bayever E. The status of hematopoietic stem cell transplantation in lysosomal storage disease. Pediatr Neurol. 2003;29:391–403. doi: 10.1016/j.pediatrneurol.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Dean RM, Bishop MR. Graft-versus-host disease: emerging concepts in prevention and therapy. Curr Hematol Rep. 2003;2:287–294. [PubMed] [Google Scholar]
- 3.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabbara KF, Al-Ghamdi A, Al-Mohareb F, Ayas M, Chaudhri N, Al-Sharif F, et al. Ocular findings after allogeneic hematopoietic stem cell transplantation. Ophthalmology. 2009;116:1624–1629. doi: 10.1016/j.ophtha.2009.04.054. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol. 2006;43:3–10. doi: 10.1053/j.seminhematol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Saito T, Shinagawa K, Takenaka K, Matsuo K, Yoshino T, Kiura K, et al. Ocular manifestation of acute graft-versus-host disease after allogeneic peripheral blood stem cell transplantation. Int J Hematol. 2002;75:332–334. doi: 10.1007/BF02982052. [DOI] [PubMed] [Google Scholar]
- 8.Gilman AL, Serody J. Diagnosis and treatment of chronic graft-versus-host disease. Semin Hematol. 2006;43:70–80. doi: 10.1053/j.seminhematol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 9.McCormick LL, Zhang Y, Tootell E, Gilliam AC. Anti-TGF-beta treatment prevents skin and lung fibrosis in murine sclerodermatous graft-versus-host disease: a model for human scleroderma. J Immunol. 1999;163:5693–5699. [PubMed] [Google Scholar]
- 10.Kim SK. Ocular graft vs. host disease. Ocul Surf. 2005;3:S177–179. doi: 10.1016/s1542-0124(12)70250-1. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Ogawa Y, Dogru M, Tatematsu Y, Uchino M, Kamoi M, et al. Baseline profiles of ocular surface and tear dynamics after allogeneic hematopoietic stem cell transplantation in patients with or without chronic GVHD-related dry eye. Bone Marrow Transplant. 2010;45:1077–1083. doi: 10.1038/bmt.2009.312. [DOI] [PubMed] [Google Scholar]
- 12.Hessen M, Akpek EK. Ocular graft-versus-host disease. Curr Opin Allergy Clin Immunol. 2012;12:540–547. doi: 10.1097/ACI.0b013e328357b4b9. [DOI] [PubMed] [Google Scholar]
- 13.Khanal S, Tomlinson A. Tear physiology in dry eye associated with chronic GVHD. Bone Marrow Transplant. 2012;47:115–119. doi: 10.1038/bmt.2011.36. [DOI] [PubMed] [Google Scholar]
- 14.Moon SJ, Mieler WF. Retinal complications of bone marrow and solid organ transplantation. Curr Opin Ophthalmol. 2003;14:433–442. doi: 10.1097/00055735-200312000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Kim SK. Update on ocular graft versus host disease. Curr Opin Ophthalmol. 2006;17:344–348. doi: 10.1097/01.icu.0000233952.09595.d8. [DOI] [PubMed] [Google Scholar]
- 16.Kamoi M, Ogawa Y, Uchino M, Tatematsu Y, Mori T, Okamoto S, et al. Donor-recipient gender difference affects severity of dry eye after hematopoietic stem cell transplantation. Eye (Lond) 2011;25:860–865. doi: 10.1038/eye.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogawa Y, Okamoto S, Wakui M, Watanabe R, Yamada M, Yoshino M, et al. Dry eye after haematopoietic stem cell transplantation. Br J Ophthalmol. 1999;83:1125–1130. doi: 10.1136/bjo.83.10.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allan EJ, Flowers ME, Lin MP, Bensinger RE, Martin PJ, Wu MC. Visual acuity and anterior segment findings in chronic graft-versus-host disease. Cornea. 2011;30:1392–1397. doi: 10.1097/ICO.0b013e31820ce6d0. [DOI] [PubMed] [Google Scholar]
- 19.Coskuncan NM, Jabs DA, Dunn JP, Haller JA, Green WR, Vogelsang GB, et al. The eye in bone marrow transplantation. VI. Retinal complications. Arch Ophthalmol. 1994;112:372–379. doi: 10.1001/archopht.1994.01090150102031. [DOI] [PubMed] [Google Scholar]
- 20.Barrett AJ, Le Blanc K. Prophylaxis of acute GVHD: manipulate the graft or the environment? Best Pract Res Clin Haematol. 2008;21:165–176. doi: 10.1016/j.beha.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ringden O, Horowitz MM, Sondel P, Gale RP, Biggs JC, Champlin RE, et al. Methotrexate, cyclosporine, or both to prevent graft-versus-host disease after HLA-identical sibling bone marrow transplants for early leukemia? Blood. 1993;81:1094–1101. [PubMed] [Google Scholar]
- 22.van Kempen-Harteveld ML, van Weel-Sipman MH, Emmens C, Noordijk EM, van der Tweel I, Revesz T, et al. Eye shielding during total body irradiation for bone marrow transplantation in children transplanted for a hematological disorder: risks and benefits. Bone Marrow Transplant. 2003;31:1151–1156. doi: 10.1038/sj.bmt.1704076. [DOI] [PubMed] [Google Scholar]
- 23.Couriel D, Carpenter PA, Cutler C, Bolanos-Meade J, Treister NS, Gea-Banacloche J, et al. Ancillary therapy and supportive care of chronic graft-versus-host disease: national institutes of health consensus development project on criteria for clinical trials in chronic Graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2006;12:375–396. doi: 10.1016/j.bbmt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Behrens A, Doyle JJ, Stern L, Chuck RS, McDonnell PJ, Azar DT, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006;25:900–907. doi: 10.1097/01.ico.0000214802.40313.fa. [DOI] [PubMed] [Google Scholar]
- 25.Doughty MJ, Glavin S. Efficacy of different dry eye treatments with artificial tears or ocular lubricants: a systematic review. Ophthalmic Physiol Opt. 2009;29:573–583. doi: 10.1111/j.1475-1313.2009.00683.x. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs DS, Rosenthal P. Boston scleral lens prosthetic device for treatment of severe dry eye in chronic graft-versus-host disease. Cornea. 2007;26:1195–1199. doi: 10.1097/ICO.0b013e318155743d. [DOI] [PubMed] [Google Scholar]
- 27.Romero-Rangel T, Stavrou P, Cotter J, Rosenthal P, Baltatzis S, Foster CS. Gas-permeable scleral contact lens therapy in ocular surface disease. Am J Ophthalmol. 2000;130:25–32. doi: 10.1016/s0002-9394(00)00378-0. [DOI] [PubMed] [Google Scholar]
- 28.Rosenthal P, Croteau A. Fluid-ventilated, gas-permeable scleral contact lens is an effective option for managing severe ocular surface disease and many corneal disorders that would otherwise require penetrating keratoplasty. Eye Contact Lens. 2005;31:130–134. doi: 10.1097/01.icl.0000152492.98553.8d. [DOI] [PubMed] [Google Scholar]
- 29.Pflugfelder SC, Maskin SL, Anderson B, Chodosh J, Holland EJ, De Paiva CS, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol. 2004;138:444–457. doi: 10.1016/j.ajo.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 30.Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002;120:330–337. doi: 10.1001/archopht.120.3.330. [DOI] [PubMed] [Google Scholar]
- 31.Pflugfelder SC, Solomon A, Stern ME. The diagnosis and management of dry eye: a twenty-five-year review. Cornea. 2000;19:644–649. doi: 10.1097/00003226-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Ogawa Y, Dogru M, Kawai M, Tatematsu Y, Uchino M, et al. Ocular surface and tear functions after topical cyclosporine treatment in dry eye patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2008;41:293–302. doi: 10.1038/sj.bmt.1705900. [DOI] [PubMed] [Google Scholar]
- 33.Kim EC, Choi JS, Joo CK. A comparison of vitamin A and cyclosporine a 0.05% eye drops for treatment of dry eye syndrome. Am J Ophthalmol. 2009;147:206–213. doi: 10.1016/j.ajo.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Pezzotta S, Del Fante C, Scudeller L, Cervio M, Antoniazzi ER, Perotti C. Autologous platelet lysate for treatment of refractory ocular GVHD. Bone Marrow Transplant. 2012;47:1558–1563. doi: 10.1038/bmt.2012.64. [DOI] [PubMed] [Google Scholar]
- 35.Tam PM, Young AL, Cheng LL, Lam PT. Topical 0.03% tacrolimus ointment in the management of ocular surface inflammation in chronic GVHD. Bone Marrow Transplant. 2010;45:957–958. doi: 10.1038/bmt.2009.249. [DOI] [PubMed] [Google Scholar]
- 36.Yoon KC, Jeong IY, Im SK, Park YG, Kim HJ, Choi J. Therapeutic effect of umbilical cord serum eyedrops for the treatment of dry eye associated with graft-versus-host disease. Bone Marrow Transplant. 2007;39:231–235. doi: 10.1038/sj.bmt.1705566. [DOI] [PubMed] [Google Scholar]
- 37.Ogawa Y, Okamoto S, Kuwana M, Mori T, Watanabe R, Nakajima T, et al. Successful treatment of dry eye in two patients with chronic graft-versus-host disease with systemic administration of FK506 and corticosteroids. Cornea. 2001;20:430–434. doi: 10.1097/00003226-200105000-00020. [DOI] [PubMed] [Google Scholar]
- 38.Rocha EM, Pelegrino FS, de Paiva CS, Vigorito AC, de Souza CA. GVHD dry eyes treated with autologous serum tears. Bone Marrow Transplant. 2000;25:1101–1103. doi: 10.1038/sj.bmt.1702334. [DOI] [PubMed] [Google Scholar]
- 39.Murphy PT, Sivakumaran M, Fahy G, Hutchinson RM. Successful use of topical retinoic acid in severe dry eye due to chronic graft-versus-host disease. Bone Marrow Transplant. 1996;18:641–642. [PubMed] [Google Scholar]
- 40.Hettinga YM, Verdonck LF, Fijnheer R, Rijkers GT, Rothova A. Anterior uveitis: a manifestation of graft-versus-host disease. Ophthalmology. 2007;114:794–797. doi: 10.1016/j.ophtha.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 41.Arai S, Jagasia M, Storer B, Chai X, Pidala J, Cutler C, et al. Global and organ-specific chronic graft-versus-host disease severity according to the 2005 NIH Consensus Criteria. Blood. 2011;118:4242–4249. doi: 10.1182/blood-2011-03-344390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balaram M, Dana MR. Phacoemulsification in patients after allogeneic bone marrow transplantation. Ophthalmology. 2001;108:1682–1687. doi: 10.1016/s0161-6420(01)00675-3. [DOI] [PubMed] [Google Scholar]