Abstract
Rationale:
Closure of the ductus arteriosus (DA) is essential for the transition from fetal to neonatal patterns of circulation. Initial PO2-dependent vasoconstriction causes functional DA closure within minutes. Within days a fibrogenic, proliferative mechanism causes anatomical closure. Though modulated by endothelial-derived vasodilators and constrictors, O2-sensing is intrinsic to ductal smooth muscle cells (DASMC) and oxygen-induced DA constriction persists in the absence of endothelium, endothelin and cyclooxygenase mediators. O2 increases mitochondrial-derived H2O2 (mitoROS), which constricts DASMC by raising intracellular calcium and activating rho kinase. However, the mechanism by which oxygen changes mitochondrial function is unknown.
Objective:
Determine whether mitochondrial fission is crucial for O2-induced DA constriction and closure.
Methods and Results:
Using DA harvested from 30 term infants during correction of congenital heart disease, as well as DA from term rabbits, we demonstrate that mitochondrial fission is crucial for O2-induced constriction and closure. O2 rapidly (<5 minutes) causes mitochondrial fission by a cyclin-dependent kinase-mediated phosphorylation of dynamin-related protein 1 (Drp1) at serine 616. Fission triggers a metabolic shift in the DASMC that activates pyruvate dehydrogenase and increases mitochondrial H2O2 production. Subsequently fission increases complex I activity. Mitochondrial-targeted catalase overexpression eliminates PO2-induced increases in mitoROS and cytosolic calcium. The small-molecule Drp1 inhibitor, Mdivi-1, and siDRP1 yield concordant results, inhibiting O2-induced constriction (without altering the response to phenylephrine or KCl) and preventing O2-induced increases in oxidative metabolism, cytosolic calcium and DASMC proliferation. Prolonged Drp1 inhibition reduces DA closure in a tissue culture model.
Conclusions:
Mitochondrial fission is an obligatory, early step in mammalian O2-sensing and offers a promising target for modulating DA patency.
Keywords: Oxygen sensing, persistent patent ductus arteriosus, mitochondrial division inhibitor-1 (Mdivi-1), mitochondrial-targeted photoactivatable green fluorescent protein, mitochondrial fission
INTRODUCTION
Closure of the ductus arteriosus (DA) at birth diverts blood from the right ventricle through the newly expanded lungs, an essential step in the transition from the fetal to neonatal circulation. Functional DA closure due to vasoconstriction begins within minutes of birth and is required for subsequent anatomical closure1, 2. Anatomical closure occurs within days by a process that involves breakdown of the internal elastic lamina, proliferation of cells in the media3 and fibrosis. Persistent patency of the DA (PDA) is prevalent in low birth-weight, preterm infants4, resulting in pulmonary congestion and failure to thrive. Prostaglandin H synthase inhibitors such as ibuprofen5 can close the DA in ~70% of infants; however, morbidities (e.g. oliguria) are not uncommon6. Failure of medical therapy often leads to invasive closure7. Conversely, maintenance of DA patency, by prostaglandin E infusion8, is sometimes required as a bridge to corrective congenital heart surgery. This too can cause toxicity (notably apnea and morbidity related to central venous access).
Oxygen (O2) is the primary stimulus for DA constriction. O2-induced DA vasoconstriction is reinforced by endothelin9 and inhibited by vasodilator prostanoids10 and nitric oxide11. Coceani et al proposed that DA constriction to O2 results from the loss of the vasodilator prostaglandin E2 combined with an increase in levels/activity of the potent vasoconstrictor, endothelin-1 12. While endothelin can constrict the DA and endothelin levels gradually increase with oxygenation, endothelin is not essential for DA closure/constriction in animal models13, 14. Moreover, simultaneous inhibition of endothelin receptors and endothelin converting enzyme (at doses that inhibit constriction to exogenous endothelin and reduce endothelin levels) does not inhibit acute constriction of the human DA to oxygen15. Our group has proposed an alternative mechanism for the O2-induced DA16 in which the core mechanism of O2-induced constriction reflects changes in the function of a mitochondrial-based redox sensor that triggers a cascade of reinforcing constrictor mechanisms that are intrinsic to the DA smooth muscle cell (DASMC)15, 17-19. Within 5 minutes of increasing PO2, mitochondria-derived reactive oxygen species (mitoROS), notably H2O2, increase and act as diffusible signaling molecules that by inhibiting O2-sensitive potassium channels, lead to DASMC depolarization, activation of the L-type calcium channel, calcium influx and initiation of vasoconstriction. Subsequently, mitoROS activate rho kinase, which sustains the O2-constriction20-22. Some calcium also enters the DASMC via store-operated channels or transient receptor potential channels22. MitoROS originate from the electron transport chain (ETC) complexes I or III and inhibition of these complexes (by rotenone or antimycin A) selectively inhibits DA constriction to O2, without altering responses to other vasoconstrictors, such as phenylephrine or KCl20.
Anatomical closure of the DA follows, and may depend on, functional closure. There are several theories for the basis of the DA closure, including that hypoxic cell death occurs in cells in the wall of the full-term ductus due to reduced blood flow in vasa vasorum of the thick walled DA 23. This mechanism may not be active in all species and was found not to be crucial in mice24.
The precise mechanism by which O2 increases mitoROS and the relevance of mitochondrial O2-sensing to functional and anatomical DA closure were the subject of this study. We observed that physiologic increases in PO2 rapidly fragment the DASMC’s mitochondrial network, suggesting that structural changes in mitochondria are a key early step in O2-sensing. O2 induces fission by stimulating phosphorylation of the guanosine triphosphatase (GTPase), dynamin related protein 1 (Drp1), at serine 616. Fission activates pyruvate dehydrogenase (PDH), increases oxygen consumption rate (OCR) and initiates redox based O2-sensing. Drp1 inhibition selectively blocks oxygen-mediated DA constriction and, if sustained, prevents DA closure in a tissue culture model. Thus, mitochondrial fission is an obligatory early step in O2-sensing that is crucial to DA constriction and closure.
METHODS
Human ductus
DAs were obtained from infants with various forms of congenital heart disease at the time of surgical correction either at the University of Nebraska Children’s Hospital or the University of Chicago under protocols approved by the local IRB of both institutions. Demographics are provided in the supplemental table I. None of the patents had a persistently patent DA, defined as continued patency in infants older than 3 months25. The 2 oldest patients in this cohort had associated congenital heart disease (tetralogy of Fallot and coarctation of the aorta).
Animal studies
The University of Chicago Animal Care Committee approved all protocols.
Statistics
Values are stated as mean ± SEM. When applicable, normality was confirmed with a Kolmogorov-Smirnov test. Inter-group differences were assessed by Student’s t-tests (unpaired or paired) or ANOVA (simple or repeated-measures), as appropriate. Post hoc analysis was performed using Tukey’s test. A p<0.05 was considered statistically significant.
Term human DA ring physiology
The DA from 28 infants were immediately placed in sterile, iced, hypoxic M231 culture media and shipped overnight by FedEx to the University of Chicago. DA from two infants originated at the University of Chicago. Of 30 infants, 25 (83%) had been on prostaglandin prior to removal of DA at surgery. The DA was cut into rings and suspended in hypoxic Earle's Balanced Salt Solution (PO2~40mmHg) at an experimentally determined optimal resting tension of 1g, as previously described15. 10μm meclofenamate and 100μm L-NAME were present throughout all experiments. Mdivi-1 (20μM) was given 30 minutes before normoxic challenges (PO2~120mmHg).
Term rabbit DA ring physiology
Pregnant New Zealand White rabbits at 30-31 days of gestation (term =31days) were anesthetized with ketamine 75 mg and xylazine 20 mg, intramuscularly and 50 mg pentobarbital, intravenously. The pups were delivered by cesarean section and a midline sternotomy was performed, before initiation of respiration. The heart, lungs, and great vessels were excised en bloc and placed in deoxygenated Earle’s solution. The DA was carefully dissected free from adventitia under a dissecting microscope and severed distal to the takeoff of the left pulmonary artery and proximal to the insertion into the descending thoracic aorta. DA physiology was studied as previously described17, 18, 26. 10μm meclofenamate and 100μm l-NAME were present throughout all experiments.
DASMC culture (human and rabbit)
Human DASMCs from 14 infants (10 Male, 4 Female) were used in this part of the study. 4 term fetal pups from 4 different pregnant New Zealand White rabbits were used to isolate and culture rabbit DASMCs. The identity of DASMCs was confirmed morphologically and by immunohistochemical demonstration of the presence of SM α-actin and the absence of von Willebrand’s factor. A primary culture of DASMC was established and then the cells were harvested with trypsin, frozen in Freezing Media (10% DMSO, 20% FBS and 70% M231) and stored in Liquid Nitrogen for later use. DASMC were used within the first 5 passages in culture. DASMC were scrupulously maintained in hypoxia (PO2 40 mmHg, pH 7.35-7.45, PCO2 30-40mmHg) using an environmentally controlled, Tri-Gas CO2 incubator (Thermo Fisher Scientific), until the protocol called for exposure to normoxia.
DA tissue culture (human and rabbit)
The DA rings was cultured in M231 media with 5% smooth muscle growth media in normoxic conditions (PO2 120 mmHg, pH 7.35-7.45, PCO2 30-40 mmHg). The DAs were allowed to float in the culture medium. Once Mdivi-1 or vehicle (DMSO) was added to the media it was left in place for the duration of the experiment.
Exposure of hypoxic human DASMC to acute normoxia
The experiment was performed in a chamber flushed with nitrogen. Inside the hypoxic chamber, two small glass containers (500 ml) were filled with Earle’s solution (37°C). They were bubbled either with a hypoxic (0% O2, 5% CO2; PO2=28±2 mmHg) or normoxic (20% O2, 5% CO2; PO2=134±2 mmHg) gas mixture. DASMCs were immersed in the hypoxic solution for 30 minutes and then either maintained in hypoxia for 5 and 20 minutes longer or quickly switched to the normoxia solution for 5 or 20 minutes. At the end of the experiment, cells were flash frozen in liquid nitrogen and later lysed at 4°C under hypoxic conditions using the PhosphoSafe™ Extraction Reagent (Calbiochem) and a Phosphatase Inhibitor Cocktail (Calbiochem, 1:100).
Imaging Mitochondrial Network Fragmentation and Connectivity
Mitochondrial Fragmentation Counts (MFC)
Mitochondrial network fragmentation was assessed as previously described27, 28. The MFC quantifies discrete mitochondrial particles and greater fission results in higher MFC values. To calculate MFC, cells were loaded with tetramethylrhodamine (TMRM) (50 nM, 20 min at 37C, Molecular Probes, Eugene, OR) and imaged with the Zeiss 510 META confocal laser scanning microscope using an alpha-Plan Apo 100x/1.46NA objective with 3x digital zoom (excitation at 561 nm, emission recorded above 575 nm). Acquired images were background subtracted, filtered (median), thresholded and binarized to identify mitochondrial segments using ImageJ (NIH, Bethesda, MD). Continuous mitochondrial structures were counted with a particle counting subroutine and the number was normalized to the total mitochondrial area (in pixels) to obtain the MFC for each imaged cell (n>25 randomly selected cells/group).
Mitochondrial Networking Factor (MNF)
To quantify functional networking of the mitochondria, cells were doubly transfected with mito-PA-GFP29 and mito-Ds-Red30 using Fugene HD transfection reagent (Roche, Indianapolis, IN), as previously described27, 28. Imaging experiments were performed 24-96 hours following the transfection of the cells using a Zeiss 510 META confocal laser scanning microscope equipped with an environmental chamber, to ensure that experiments were conducted at 37C and at physiologic pH, PCO2 and either normoxic or hypoxic PO2. A 100X alpha Plan-Apochromat (1.46NA) objective and 2X zoom were used to image single cells. Sequential images of fluorescent mitochondria were collected every 17.6 seconds. Mito-Ds-Red was excited at 561 nm and the emission was recorded using 565 nm dichroic and 575 nm long pass filters. After acquiring 3 control images, localized photoactivation of mito-PA-GFP was achieved by 10 passes of the 2-photon laser (at 700 nm) within a 23 □m2 activation area. Mito-PA-GFP was excited at 488 nm and the images were acquired at 500-550 nm. Mito-Ds-Red permits visualization of the entire mitochondrial network, while the mito-PA-GFP enables real-time imaging of GFP diffusion from the discrete photoactivation region through the mitochondrial reticulum. MNF quantifies the spread of mito-PA-GFP after photoactivation and thus is proportional to the extent of network fusion, resulting in low values in states of fission27, 28.
Transmission Electron Microscopy
Two human ductus were used for this experiment. Each ductus was cut into 2 to 4 rings and treated with either hypoxia (20 minutes) or normoxia (20 minutes). These DA were then immediately fixed and processed for electron microscopy. After osmium tetroxide treatment, samples were stained with 1% uranyl acetate and embedded in SPURR (Electron Microscopy Sciences, Hatfield, PA). Images were collected using a scanning transmission electron microscope at 300 KV (Tecnai F30; FEI, Hillsboro, Oregon) with a high-performance Gatan CCD camera (Pleasanton, CA).
O2-consumption and metabolic measurements
Simultaneous measurements of oxygen consumption rate (OCR) and extracellular acidification rate (ECAR, a measure of lactate production) were performed on a XF24 extracellular flux analyzer (Seahorse Bioscience, North Billerica, MA). DASMC were plated on a XF24 cell culture microplate. Measurements were made the next day, after equilibration in XF assay medium supplemented with 4.5g/l glucose. To compare metabolic function in hypoxia versus normoxia, the entire XF24 extracellular flux analyzer was sealed in a plastic container which was flushed with either nitrogen (hypoxia) or room air (normoxia). The oxygen level within the chamber was monitored by an oxygen sensor and by the XF24’s PO2 sensor. OCR was measured at baseline and upon sequential challenge with 1 μM oligomycin (to inhibit ATP synthase), 2 μM FCCP (to uncouple the mitochondria and demonstrate maximal OCR) and 5 μM antimycin A (to inhibit the mitochondrial component of respiration).
qRT-PCR and immunofluorescence
These technqiues were performed as previously described27.
Immunoblotting and immunofluorescence
Immunoblotting was performed on 30 μg of protein from human DASMC. The primary antibodies used were anti-Drp1 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-phospho Ser616 Drp1, anti-phospho Ser637 Drp1 (Cell Signaling Technology, Boston, MA), anti-Actin (Millipore, Billerica, MA) and anti-translocase of outer mitochondrial membrane 20 (TOM20, Santa Cruz Biotechnology, Santa Cruz, CA). The mitochondria-enriched fraction was isolated using a mitochondria/cytosol fractionation kit (Abcam, Cambridge, MA).
Measurement of cytosolic calcium concentrations ([Ca2+]cyt)
DASMC were plated on coverslips and loaded with Fura-2 AM (3 μmol/L for 30 minutes at 37°C, Invitrogen, Carlsbad, CA). The ratio of 510 nm fluorescence signals elicited at 380 and 340nm stimulation was calculated to quantify [Ca2+]cyt, as previously described27. Compartmental calcium stores were assessed by measuring changes in [Ca2+]cyt when the sarcoplasmic reticulum pool was released by cyclopiazonic acid (CPA), or the mitochondrial pool was released by carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP).
Small interfering RNA (siRNA)
To achieve specific molecular knockdown of Drp1 or the putative Drp1phosphorylating kinase, PKCδ , we used siRNA, targeting Drp127 or PKCδ (IDT, San Jose, CA). Human DASMC were grown to ~80% confluence and then transfected using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA) and 10 nM of a validated Silencer Select siRNAs or a scrambled siRNA control (IDT, San Jose, CA). After 6 hours, normal culture medium was applied and gene knockdown was assessed using qRT-PCR (48 hours) and immunoblot (72 hours).
PDH enzyme activity
PDH enzyme activity assay was performed on freshly isolated DA proteins using a PDH Enzyme Activity Dipstick Assay Kit following the manufacturer’s instruction (MitoSciences-Abcam, ab109882, Eugene Oregon). This technique uses anti-PDH antibody immobilized on a capture line section of the dipstick. The native (active) PDH is immunocaptured and activity is measured by coupling PDH-dependent production of NADH to the reduction of nitroblue tetrazolium. PDH activity results in an insoluble blue precipitate at the capture line, the intensity of which is proportional to PDH activity.
ETC complex I activity
Protein isolated from DASMC analyzed using the Mitoscience Complex I Enzyme Activity Dipstick Assay Kit (ab109720). Complex I from the DASMC is captured in its native (active) form on the dipstick which is then immersed in a Complex I activity buffer solution containing substrate (NADH) and an electron recipient (nitroblue tetrazolium). The immunocaptured Complex I oxidizes NADH and the electron transfer reduces nitroblue tetrazolium forming a blue precipitate at the capture line that is proportional to complex activity.
Mitochondrial targeted increases in catalase activity
Human DASMC in culture (50% confluence) were infected with serotype 5 adenovirus carrying mitochondrial-targeted catalase (Ad-mitoCAT, 100 MOI) or an empty virus (100 MOI). Catalase activity was quantified after 3-5 days using a colorimetric activity assay, following the manufacturer’s instructions (Biovision, Milpitas, California).
DASMC proliferation and apoptosis assays
Proliferation was quantified using the EdU kit according the manufacturer's instructions (Invitrogen), as previously described27. Apoptosis was measured using an FITC annexin V apoptosis detection kit, as previously described (BD Pharmingen, San Diego, CA)27.
RESULTS
Mitochondrial fission is required for O2-constriction (Fig 1 and 2)
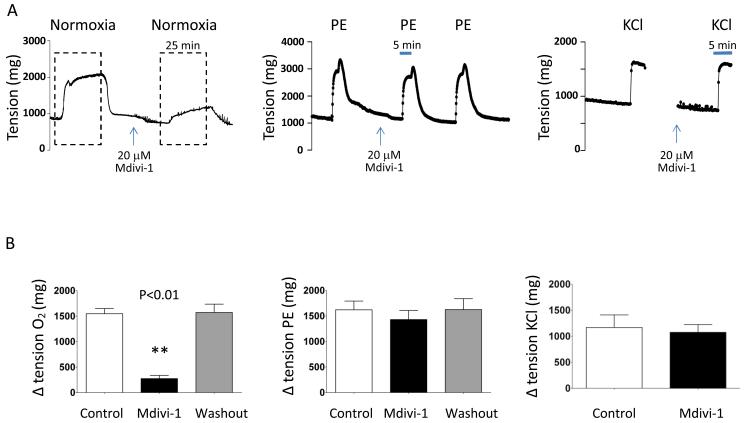
Figure 1. The Drp1 inhibitor Mdivi-1 selectively inhibits oxygen-induced constriction in rabbit ductus.
A-B: Representative images showing that Mdivi-1 prevents and rapidly reverses O2 constriction in rabbit ductus. Mean (±SEM) in panel B show that Mdivi-1 inhibits O2-constriction without altering phenylephrine- or KCl-mediated constriction, measured under hypoxic condition (** p<0.01 compared to control, n=8, 4, 4).
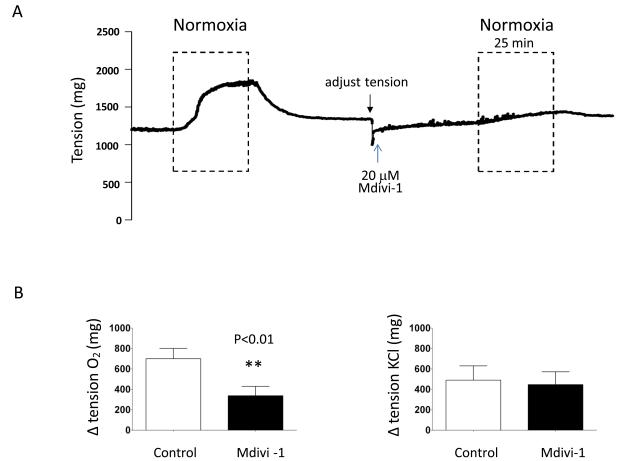
Figure 2. The Drp1 inhibitor Mdivi-1 selectively inhibits oxygen-induced constriction in human.
A-B: Representative image and mean data show that Mdivi-1 inhibits O2-constriction without altering KCl-constriction. n=6.
The small molecule inhibitor of Drp1, mitochondrial division inhibitor (Mdivi-1)31, prevents and reverses O2-induced constriction in rabbit and human DA. Mdivi-1 does this selectively, without altering constriction to agents that act by depolarizing the DASMC (KCl) or releasing intracellular calcium (phenylephrine). This suggests that Mdivi-1 interferes with a mitochondrial oxygen-sensor, rather than a downstream constrictor mechanism.
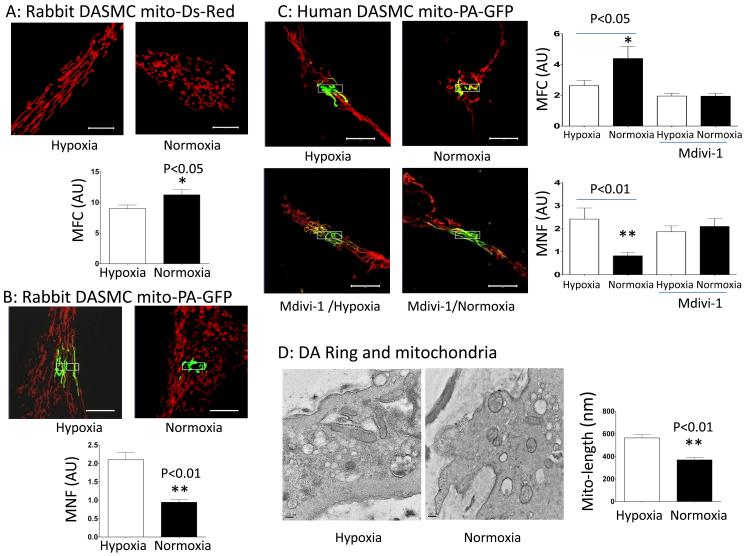
Oxygenation rapidly causes mitochondrial fission in DASMC
Exposure of hypoxic DASMC to O2 at tensions similar to those experienced at birth (increasing PO2 from 40 to 120 mmHg), fragments the DASMC’s mitochondrial network. This was quantified as an increase in the MFC and a reduction in MNF that occurred within 10 minutes of increasing PO2 (Fig 3A-C). Mdivi-1, prevents this O2-induced fission (Fig 3C), offering pharmacologic evidence that it is mediated by Drp1. To ensure that fission was not unique to DASMC in culture, we divided surgically resected human DAs into two pieces, one maintained in hypoxia (40 mmHg) and the other exposed to a PO2 of 120 mmHg for 15 minutes. Transmission electron microscopy (TEM) confirms that O2 causes mitochondrial fragmentation in DASMC within the media of freshly isolated DA rings (Fig 3D).
Figure 3. Increased PO2 causes mitochondrial fission in rabbit and human DASMC.
A: Serial observations of a rabbit DASMC transfected with mitochondrial targeted Ds-Red shows that 10-15 minutes of increased PO2 (from 40 to 120 mmHg) fragments the mitochondrial network, thus increasing the MFC. Scale bar=10 μM. n=18-19 cells. B: There is increased fission after exposure of rabbit DASMC to increased PO2. Representative images and mean ± SEM show impaired spread of mito-PA-GFP in oxygenated DASMC, evident as reduced MNF (n=18-19). C: The mitochondrial network in human DASMC fragments rapidly with brief (20 minutes) increases in PO2, evident as a increasing the MFC and reduction in MNF. This is corrected by exposure to Mdivi-1 (20 μM for 20 minutes). ** p<0.01 compared to hypoxic control (MFC n=19-20; MNF n=7-15). D: Transmission electron microscopy data confirming that in DASMC within freshly isolated human DA rings, mitochondrial length is shorter after 20 min exposure to normoxia vs. hypoxia (n=62 and 56 mitochondria, respectively).
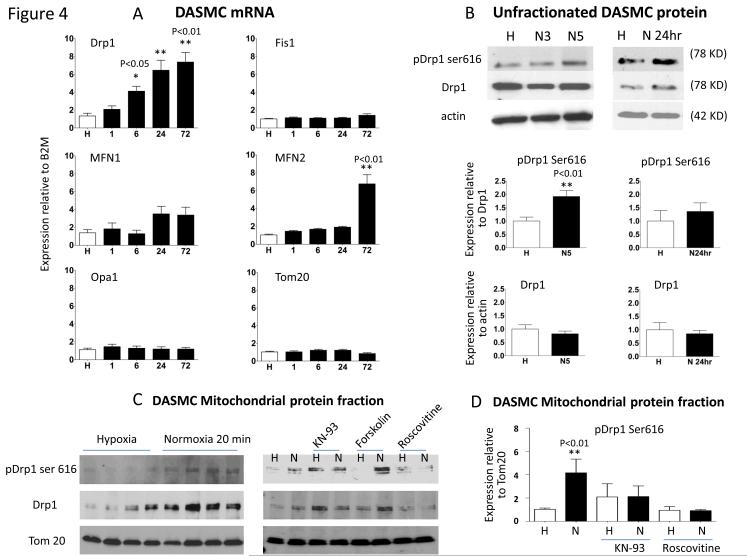
Expression profiling of fission and fusion mediators (Fig 4)
Figure 4. Increased PO2 leads to rapid phosphorylation and activation of Drp1 in human DASMC.
A: Incubation at PO2 120mmHg preferentially increases the expression of Drp1 mRNA in human DASMC. N=8 to 10. B: Immunoblot of whole cell lysate shows that brief O2-exposure causes phosphorylation (activation) of Drp1 at serine 616 within 3-5 minutes (n=12).C-D: Immunoblot of the mitochondrial fraction shows that brief O2-exposure (20 minutes) causes phosphorylation of Drp1 at serine 616, and causes Drp1 translocation to the mitochondria (n=4). Phosphorylation of Drp1 is prevented by inhibitors of its regulatory kinases (KN93- calcium/calmodulin-dependent protein kinase and roscovitine- cyclin-dependent kinase, n= 3 to 5). Forskolin, an activator of protein kinase A which can phosphorylate Drp1 at serine 637, had no effect.
Mitochondrial fragmentation could reflect impaired fusion, enhanced fission or both. We constructed an mRNA expression profile for mediators of fusion (mitofusin-1, mitofusin-2 and optic atrophy protein) and fission (Drp1 and fission protein 1) at time points from 0-72 hours after exposure to normoxia (Fig 4A). Drp1 expression began to increase within 1-hour of O2 exposure and by 24-hours Drp1 was the only fission or fusion mediator whose expression had changed (Fig 4A). After 72 hours of O2, Drp1 expression increased ~8-fold and mitofusin-2 expression also increased.
Changes in Drp1 phosphorylation (Fig 4)
DA constriction begins within 5 minutes of an increase in PO2, which is too rapid to reflect transcriptional regulation of Drp1. Consequently we assessed whether O2 changes Drp1 phosphorylation at serine 616, a post-translational modification known to cause Drp1 to translocate to the mitochondria and form multimers that circumferentially constrict the mitochondrion and initiate fission 32. As expected, brief O2-exposure (<10 minutes) did not change total Drp1 expression; however, it increased expression of activated DRP1 (Drp1-p-Ser-616, Fig 4C) in the mitochondrial protein fraction. O2 also promoted DRP-1 activation by decreasing expression of the inhibitory phosphorylation form, p-Ser-637 (supplemental Fig IA).
Identification of kinases that phosphorylate Drp1 (Fig 4C)
Next we examined the identity of the kinase(s) that activate/phosphorylate Drp1. Several kinases can cause Drp1 phosphorylation, including cyclin-dependent kinases 32, calcium/calmodulin-dependent protein kinase 33 and protein kinase Cδ 34, which has been reported to phosphorylate Drp1 at serine-616 in neurons 35, To identify the kinase(s) that mediate O2-induced increase in Drp1 p-Ser-616, DASMC were exposed to oxygen in the presence of roscovitine (Fig 4C), an inhibitor of cyclin-dependent kinase-1, -2 and -5,36 KN93, a calcium/calmodulin-dependent protein kinase inhibitor33 or siPKCδ. Roscovitine and KN93 reduced Drp1 p-Ser-616 (Fig 4D); whereas, siPKCδ had no effect (supplemental Fig I). All siRNA species were confirmed to achieve >80% knockdown of their targets. Thus cyclin-dependent kinase and calcium/calmodulin-dependent protein kinase (linked to cell proliferation and [Ca2+]cyt, respectively) contribute to O2-induced activation of Drp1 in DASMC.
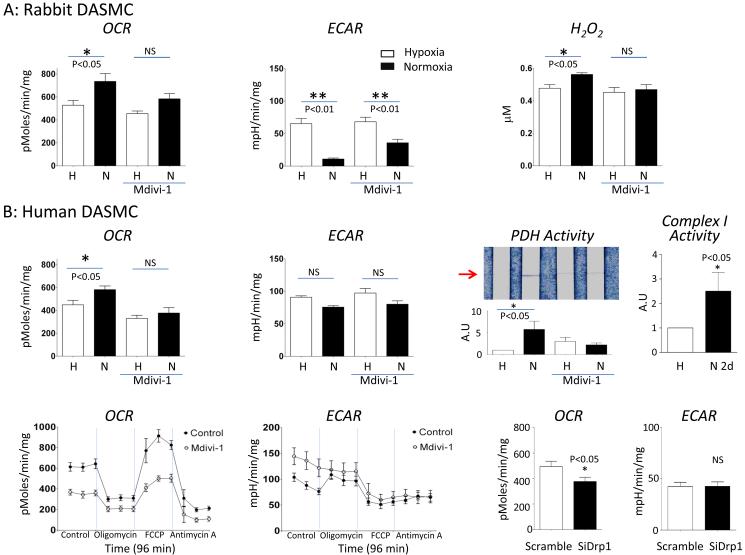
Fission increases O2-consumption and oxidative metabolism (Fig 5)
Figure 5. Normoxia increases oxidative metabolism and H2O2 production in rabbit and human DASMC.
A: Rabbit DASMC: Normoxia increases OCR (left, n=17), decreases ECAR (middle, n=17) and H2O2 (right, n=6). Mdivi-1 (20 μM) inhibits these changes. B: Human DASMC.
Top row: Normoxia (20 minutes) increases OCR (left, n=11) and PDH activity (third panel, n=9). Mdivi-1 (20 μM) inhibits the changes. Long-term normoxia (2 day) increases ETC complex 1 activity (right, n=10).
Bottom row: Mdivi-1 (20 μM) inhibits the basal and FCCP induced maximal OCR and increases basal ECAR in human DASMC during normoxia condition (n=10). Oligomycin, used to show coupling of respiration to ATP synthesis, had similar effects with or without Mdivi-1. Antimycin is an inhibitor of ETC complex 3 and demonstrates that the OCR in DASMC is almost exclusively based on mitochondrial respiration. Antimycin’s effects were unaltered by Mdivi-1. Like Mdivi-1, SiDrp1 inhibits the basal OCR in human DASMC during normoxia condition while scrambled siRNA had no effect (n=11).
We next explored how this Drp1-mediated fission elicits vasoconstriction. Because increases in mitochondrial respiration are thought to mediate O2-dependent increases in mitoROS in the DA, we investigated the effects of fission on mitochondrial metabolism. To do this we measured OCR and glycolysis (ECAR) in primary cultures of DASMC. To modulate PO2 the Seahorse analyzer itself was housed in a hypoxic chamber (Fig 5). DASMC maintained in hypoxia were glycolytic, with low OCR and high ECAR (Fig 5A-B). Elevating PO2 increased OCR and reduced ECAR. This metabolic change resulted from rapid, PO2-dependent activation of PDH, the key regulator of mitochondrial glucose oxidation (Fig 5B). The obligatory role for fission in this oxidative metabolic shift is demonstrated by the finding that Drp1 inhibition (whether achieved by Mdivi-1 or siDrp1) inhibits O2-induced increases in both OCR and PDH activity. As would be predicted, Mdivi-1 inhibits the rise in H2O2 production in response to increases in PO2 (Fig 5A). The finding that PO2-induced PDH activation is inhibited by Mdivi-1 demonstrates that fission is required for the burst of oxidative metabolism that serves as the redox signal of “normoxia” in the DA. This metabolic shift increases mitoROS, presumably by increasing electron flux through the ETC. Consistent with this, more sustained incubation in O2 (2 days) also increases ETC mega-Complex I activity (Fig 5B).
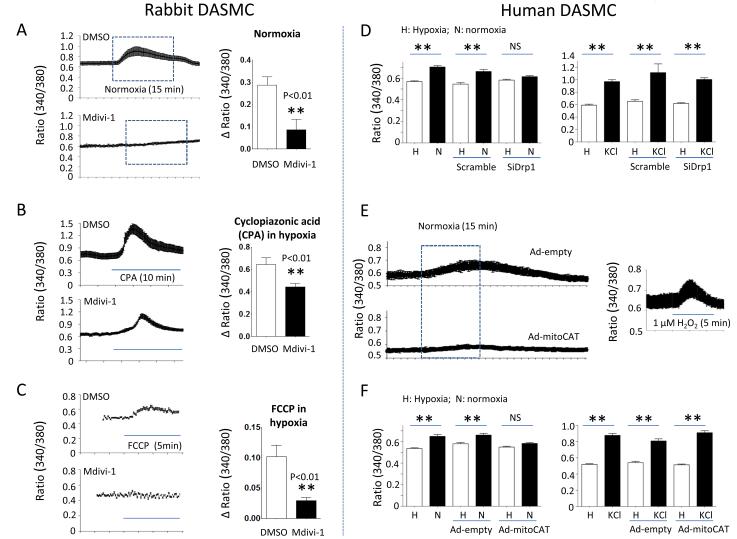
Inhibition of Drp1 lowers cytosolic calcium (Fig 6)
Figure 6. Drp1 inhibition impairs oxygen-induced increases in cytosolic calcium DASMC.
A-C: Mdivi-1 lowers calcium in all subcellular pools in DASMC and prevents oxygen-induced elevations in cytosolic calcium. A; n=44-46; B: n=8-13; C; n=38-57 cells. D: SiDrp1 inhibits increases in calcium caused by oxygen (n=90-109 cells) without altering the response to KCl (n=15-30 cells) in human DASMC. E-F: Representative images and mean data showing that elimination of mitochondrial H2O2 using Ad-mitoCAT prevents O2-induced elevation of cytosolic calcium (n=46 to 51) without altering the response to KCl (n=19 to 37 cells) in human DASMC. E right panel: t-butyl hydroperoxide (1 μm) increases cytosolic calcium (n=17 cells).
Elevation of [Ca2+]cyt is the accepted final common pathway for activation of the contractile apparatus in DASMC. Consequently, we examined the effects of Drp1 inhibition on O2-induced elevation of [Ca2+]cyt. Mdivi-1 impaired O2-induced increases in cytosolic calcium in rabbit DASMC (Fig 6A). Mdivi-1 reduced the calcium released from the sarcoplasmic reticulum and mitochondria by CPA and FCCP, respectively (Fig 6B and 6C). Mdivi-1 is thought to be a selective Drp1 inhibitor31; however, to confirm the putative mechanism, we used a highly specific molecular strategy (siDrp1) and observed concordant results. Like Mdivi-1, siDrp1 prevented O2-induced increases in [Ca2+]cyt without altering the response to KCl (Fig 6D). The trigger for the rise in calcium in DASMC is thought to be increasing H2O2. Supporting this, exogenous H2O2 increases [Ca2+]cyt in DASMC (Fig 6E).
Consistent with the signaling role of H2O2 in DASMC, prior studies had shown that augmenting intracellular catalase attenuated O2-sensing in DASMC20, 26, we established the compartmental specificity of the signaling H2O2 pool using Ad-mitoCAT. Mitochondria-specific overexpression of catalase inhibited O2-induced elevation of cytosolic calcium (Fig 6E-F and supplemental Fig III). Thus, fission triggers O2-sensitive H2O2 generation by the DASMC’s mitochondria.
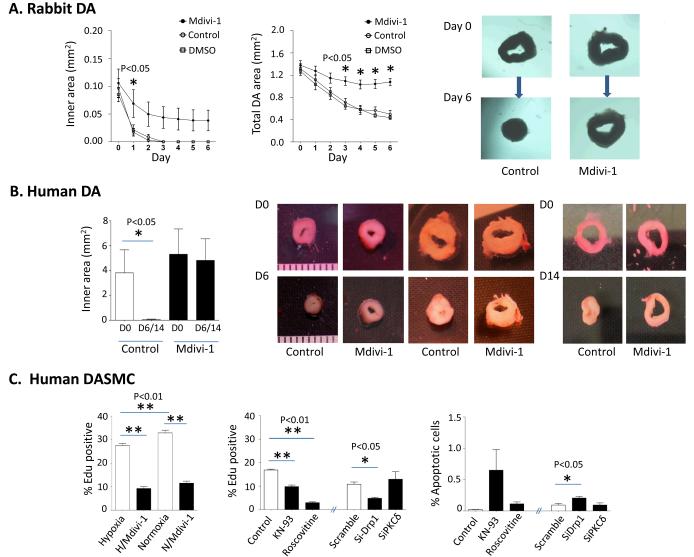
Prolonged Drp1 inhibition prevents DASMC proliferation and DA closure (Fig 7 & 8)
Figure 7. Prolonged Drp1 inhibition prevents DASMC proliferation and prevents DA closure.
A-B: In a tissue culture model, occlusion of the lumen occurs in response to incubation in oxygen after 3 days in rabbits (n=10 to 13 DA rings) and 6-14 days in humans (n=7 DA rings). Incubation with Mdivi-1 prevents DA closure in this model. C: Mdivi-1 prevents the proliferation of human DASMC, measured using EdU flow cytometry (n=6 to 9). Roscovitine, KN93 and siDrp1 inhibit the proliferation of human DASMC during normoxia, whereas siPKCδ has no effect (middle panel, n=4 cultures of DASMC). SiDrp1 induces apoptosis in human DASMC during normoxia condition (right panel, n=4 cultures of DASMC).
We next assessed whether prolonged inhibition of fission would prevent the anatomical closure of the DA. In a tissue culture model, we demonstrated that a PO2 of 140 mmHg caused DA occlusion after 3 days (rabbit) and 6-14 days (human) (Fig 7 A-B). Mdivi-1, but not vehicle, prevented closure of the DA. Because DA closure involves cellular proliferation and because prior experience shows Mdivi-1 can prevent SMC proliferation27, we assessed the antiproliferative effects of Mdivi-1 and siDrp1. Both interventions caused a dose-dependent decrease in DASMC proliferation (Fig 7C). Likewise inhibition of the kinases that activate Drp1, cyclin-dependent kinase (roscovitine) and calcium/calmodulin-dependent protein kinase (KN93) reduced DASMC proliferation (Fig 7C). In contrast, siRNA-induced inhibition of PKCδ had no effect (Fig 7C). Inhibiting proliferation with roscovitine or siDrp1 also increased apoptosis in cultured human DASMC (Fig 7C).
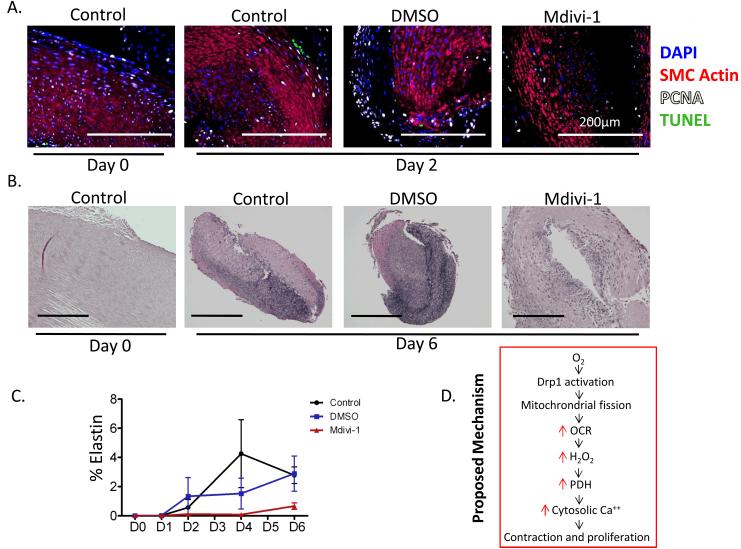
The antiproliferative effects of Mdivi-1 also occurred in DA rings in tissue culture, evident as a reduction in the number of PCNA-positive DASMC in rings cultured in the presence of Mdivi-1 (Fig 8A). Mdivi-1 significantly prevented fibrotic remodeling of the DA, which normally occurs with closure (Fig 8B-C). These findings, together with the time dependent increase in Drp1 expression suggest that fission is important to DA closure, as well as to the rapid initiation of O2-induced constriction.
Figure 8.
Mdivi-1 significantly prevented fibrotic remodeling of the rabbit DA. 8A: Time course of channels in apoptosis (TUNEL) and proliferation (PCNA) in rabbit DAs maintained in tissue culture with or without mdivi-1 (n=5 DA rings/group). 8B-C: Immunostaining shows that Mdivi-1 inhibits elastin production in vivo, impairing an important step in DA closure. The dramatic O2-induced reduction in DA lumen and overall DA size over time is also attenuated by Mdivi-1 (n=5-8 DA rings at D6). 8D: Proposed mechanism.
DISCUSSION
This study identified a critical role for Drp1-mediated mitochondrial fission in acute constriction of the DA to O2 and also strongly suggests this mechanism participates in the DA’s subsequent anatomical closure. Mitochondrial fission increases oxidative metabolism in DASMC. The resulting changes in redox signaling (particularly increases in mitochondrial-derived H2O2) elevate [Ca2+]cyt and initiate vasoconstriction. Drp1 activation is rapid, occurring with onset within 5 minutes, and reflects posttranslational modification. With normoxia cyclin-dependent kinase and calcium/calmodulin-dependent protein kinase increase Drp1 phosphorylation, increasing the ratio of p-Ser-616/p-Ser-637 and consequently favoring fission. These kinases link mitochondrial morphology to the regulation of cell proliferation (cyclin-dependent kinase) and cytosolic calcium (calcium/calmodulin-dependent protein kinase). The concordant findings in rabbits and humans speak to the conservation of this mechanism. The basis for the fission-induced redox signal is demonstrated to lie in an alteration in mitochondrial metabolism. Fission in DASMC rapidly activates PDH (within 10 minutes) and subsequently increases activity of Complex 1 in the mitochondrial electron transport chain (Fig 5B). The demonstration of the importance of Drp1-mediated fission to DA closure in DAs from human infants strengthens the translational relevance of this discovery (Supplemental Table I). The physiological normality of the term DAs was evident in their robust and reversible O2 constrictor responses (Figure 2A-B). None of these patients had persistent DA; rather the DA had been maintained patent by infusion of prostaglandin E1 where medically necessary.
The DA is a prototypic member of the specialized oxygen homeostatic system, which also includes the type 1 cell in the carotid body, the neuroepithelial body, and resistance pulmonary artery SMC16. Prior studies have showed that increasing PO2 caused a proportionate increase in the generation of H2O2 by DASMC16. H2O2 is produced by dismutation of the superoxide that results from the increased ETC activity that accompanies oxidative metabolism 20, 26, 37, 38. H2O2 serves as a diffusible oxidant and second messenger, signaling the PO2 to voltage-gated ion channels (e.g. Kv1.5 and the L-type calcium channel) 20, 26 and enzymes (e.g. rho kinase)16. Prior studies in mammals (including humans) and birds have demonstrated that the superoxide in DA originates from ETC Complexes I and III37, 39. However, it was assumed that oxygen increased mitoROS by changing mitochondrial function, rather than structure. It is now evident that the metabolic and redox changes reflect earlier dynamic changes in morphology.
Multiple lines of evidence indicate that fission in the DASMC results from the increased activity and expression of a single GTPase, Drp1. Drp1 exists as a cytosolic homo-tetramer; however, when activated, it is recruited to the mitochondria. Drp1’s activity is determined by GTP hydrolysis, which is regulated by its C-terminal GTPase effector domain. Upon activation Drp1 assembles in the outer mitochondrial membrane creating a multimeric collar that mediates mitochondrial division40,41. Mdivi-1 inhibits Drp1 multimerization31 and in DASMC this prevents oxygen-induced fission (Fig 3A). Fission results in more numerous, smaller mitochondria, which we demonstrate in DASMC (increased MFC) and in human DA rings (decreased mitochondrial size on TEM, Fig 3D).
Mdivi-1 is the most selective and effective of the antifission molecules identified from a screen of 23,000 candidates31, 42. While Mdivi-1 can inhibit cytochrome-c release in cell-free assays, where mitochondrial division does not occur42, the concordant results with Mdivi-1 and siDrp1 in this study provide mechanistic specificity. As an additional method of ensuring mechanistic certainty, we carefully quantified fission using two complementary techniques, MFC, a particle counting technique, and MNF, which measures the rapidity of diffusion of mito-PA-GFP. These assays yielded concordant results; physiological increases in PO2 fragmented the mitochondrial network and impaired the diffusion of mitochondrial matrix proteins. Finally, we replicated key findings with Mdivi-1 on metabolism, calcium levels and proliferation/apoptosis using a specific molecular approach-siDrp1 (Fig 5B, 6D and 7C).
The physiologic importance of fission is evident from the observation that Mdivi-1 selectively prevents constriction of the DA to O2, without altering the response to vasoconstrictor stimuli, such as KCl or phenylephrine (Fig 1). Prior to Mdivi-1, the only agents known to selectivity inhibit O2-induced DA constriction were redox agents that mimic hypoxia (e.g. the reducing agent, dithiothreitol26, 38) or ETC Complex I and III inhibitors, rotenone and antimycin A20, which in DASMC reduce mitoROS production. The commonality amongst these inhibitors of DA constriction is that each creates a more reduced redox environment in the DASMC. The central role for the oxidant stimulus, mitochondrial-derived H2O2, is confirmed by the demonstration that mitochondrial-targeted catalase prevents oxygen-induced elevation of [Ca2+]cyt, an accepted surrogate for DA constriction (Fig 6E and F).
To account for O2-sensing, fission must occur rapidly before the onset of vasoconstriction. Indeed, Drp1 activation occurs within 3-5-minutes of elevating PO2 (prior to elevation of tone). This rapidity reflects O2-induced, post-translational activation of Drp1 mediated by an increased ratio of active/inhibitory phospho forms (i.e. an increased p-Drp1-Ser 616/637 ratio) (Fig 4 & supplemental Fig I). Activated Drp1 rapidly translocates to the mitochondria (Fig 4C and D). Phosphorylation allows fission to occur in minutes, critical to the very rapid fission we observed. In this regard we identify two relevant kinases that link fission to mitosis (cyclin-dependent kinase) and elevation of intracellular calcium (calcium/calmodulin-dependent protein kinase). The importance of an increased ratio of phosphor-Drp1 Serine-616/637 is supported by the observation that inhibitors of Drp1 phosphorylation reduce O2-induced DASMC proliferation, while enhancing apoptosis (Fig 7C), similar to observations in lung cancer cells28. However, in longer-term exposure, there is also a selective upregulation of Drp1 expression, which may be crucial for anatomical closure, as discussed subsequently.
Inhibition of fission (by Mdivi-1 or siDrp1) largely eliminates the PO2-dependent rise in [Ca2+]cyt without altering the response to KCl (Fig 6A and D), consistent with the O2-specific effects of Drp1 inhibitors on DA constriction. Mdivi-1 appears to affect [Ca2+]cyt primarily by inhibition of mitochondrial H2O2 generation (Fig 6E-F). However, fission may alter [Ca2+]cyt by changing the connection between the mitochondria and organelles, such as the sarcoplasmic reticulum43-45. Supporting this, Mdivi-1 does alter compartmentalization of calcium, reducing calcium release from the sarcoplasmic reticulum and mitochondria during hypoxia (Fig 6 B-C). The changes in calcium may also be linked to metabolism. Lowering mitochondrial calcium, which is known to inhibit PDH46, 47, might contribute to Mdivi-1’s ability to inhibit oxidative metabolism (Fig 6C). A recent study investigating glucose-induced activation of mitochondrial fission and metabolism in the hypothalamus found that siDrp1 decreased mitoROS production and impaired substrate-driven respiration48, consistent with our findings (Fig 6D).
The consequence of fission that triggers vasoconstriction is a rapid increase in oxidative metabolism, evident as a increased OCR (with unchanged ECAR) in DASMC (Fig 5). That this shift results from fission is demonstrated by the observation that pharmacologic (Mdivi-1) or molecular Drp1 inhibition (with siDrp1) prevents the normoxic metabolic shift (Fig 5). We show that O2-induced PDH activation is inhibited by Mdivi-1 (Fig 5B).
There are several mechanisms by which inhibiting fission might inhibit respiration. The role of fission as a stimulant for oxidative metabolism has recently been identified in a heritable form of Parkinsonism that is caused by a mutation of a mitochondrial Ser/Thr kinase, PTEN-induced kinase 1 (PINK1)49. The PINK1 mutation impairs mitochondrial fission and depresses mitochondrial respiration in neurons by decreasing the expression and activity of ETC complex I and IV. Drosophila lacking PINK1 also display impaired mitochondrial fission and metabolism, both of which are rescued by Drp1 overexpression49. In these flies, reduced ETC complex activity reflects defective ETC complex assembly and is rescued by increasing mitochondrial fission. Consistent with this, fission in DASMC increases the activity of ETC Complex I (Fig 4B). We interpret this as indicating that fission changes the architecture of the mitochondria in a manner, which favors assembly and activation of Complex I, a major source of the ROS that signal PO2.
We acknowledge that the role of PINK1/Parkin in familial Parkinsonism is thought to lie in mitochondrial quality control and may not be directly relevant to the more dynamic role of fission in increasing ETC activity in the DA.
There are likely important temporal considerations in metabolic effects of fission. . Oxygen’s effects initial effects are on Drp1 phosphorylation (evident in <5 minutes) and this is sufficient to activate PDH activity (evident in <20 minutes). These early changes are critical for initial redox signaling. Inhibiting mitochondrial H2O2 production prevents the rise in cytosolic calcium that triggers vasoconstriction (Fig 5E-F). We show that fission is an important early upstream mediator of calcium signaling through its effects on mitochondrial metabolism (OCR) and redox signaling (H2O2 production). Our prior observations the normoxic redox signaling by H2O2 derives from the mitochondria is supported by the observation that oxygen-induced elevation of cytosolic calcium is prevented by eliminating mitochondrial-derived hydrogen peroxide, using Ad-Mito-Catalase (Fig 6E-F). Fission increases mitochondrial respiration and mitochondrial hydrogen peroxide generation. Mitochondria-derived hydrogen peroxide serves as a redox signaling molecule that triggers calcium entry and organellar calcium release thereby elevating cytosolic calcium and promoting calcium sensitization in DASMC17, 21, 22, 50-54. Inhibiting fission lowers calcium in all compartments (Fig 6A-C) by changing mitochondrial redox signaling (Fig 6E). Consequently blocking fission (using Mdivi-1) not only prevents constriction but also, inhibits PDH activity, a calcium-sensitive mitochondrial enzyme. Likewise, Mdivi-1 and siDRP1, which both inhibit respiration, abrogate O2dependent increases in cytosolic calcium (Fig 6 A and 6D). Thus there is a positive feedback between mitochondrial respiration, H2O2 generation and calcium levels, such that fission-induced increases in respiration increase the magnitude of redox-induced calcium levels.
In normal, term human infants, most DAs close by vasoconstriction within hours-days and by 2 months only 4.5% of DAs are patent (and most of these are clinically silent)55. However, in premature infants, immaturity of the mitochondrial O2-sensing system and the downstream ion channel pathways18, 20 is associated with a 21% incidence of persistent DA patency56. The demonstration that DA patency is maintained by inhibiting Drp1 is remarkable. Prolonged O2-exposure selectively increased Drp1 expression without altering expression of the other mediators of fission (e.g. Fis1, Fig 4A) or fusion mediators (e.g. mitofusin-1and -2 or optic atrophy protein-1, Fig 4A). Conversely, inhibiting Drp1 in tissue culture preserved DA patency (Fig 7B), at least in part by inhibiting oxygen-induced DASMC proliferation (Fig 7C) and preventing DA fibrosis (Fig 8B-C).
At least part of the effect of inhibiting Drp1 may relate to inhibition of proliferation. Prior work in hyperproliferative, pulmonary hypertensive pulmonary artery SMCs and lung cancer cells indicates that cyclin B- cyclin-dependent kinase 1, a key regulator of mitotic entry coordinates the cell cycle with mitotic mitochondrial division, ensuring equal distribution of mitochondria to daughter cells27, 28.
Because of the clinical efficacy of cyclooxygenase inhibitors (such as indomethacin) in achieving DA closure it might be questioned why other strategies are needed. Beyond the fundamental scientific importance of understanding the molecular basis for oxygen sensing, there is a practical imperative. First, cyclooxygenase inhibitors fail to close the ductus in 30% of neonates57, This lack of efficacy is even more common in extremely low gestational age infants. Second, indomethacin has adverse effects, including significant reductions in renal function (oliguria and reduced creatinine clearance), and reductions in mesenteric and cerebral blood flow58. The combination of indomethacin and steroids also increases the incidence and morbidity of necrotizing enterocolitis59.
LIMITATIONS
While Mdivi-1’s effects were largely mimicked by siDRP1, and while Mdivi-1 had no effect on phenylephrine or KCL constriction or calcium homeostasis, we cannot exclude the possibility that Mdivi-1 has unknown properties, such as acting as an antioxidant”
Here we show that DASMC proliferation is also reduced by inhibiting kinases that activate Drp1. Pharmacological strategies to activate Drp1 could be developed as a means to close the DA, whilst inhibition of Drp1, by congeners of Mdivi-1, could be used to maintain DA patency in infants awaiting congenital heart surgery.
Supplementary Material
Novelty and Significance.
What Is Known?
Closure of the ductus arteriosus (DA) is essential for the transition from fetal to neonatal circulation.
PO2-dependent vasoconstriction causes functional DA closure within minutes.
−PO2-dependent vasoconstriction occurs by a mechanism that is intrinsic to the DA smooth muscle cells and reflects the regulation of oxygen-sensitive potassium and calcium channels and rho kinase, by a mitochondrial redox sensor.
Within days at elevated PO2 a fibrogenic, proliferative mechanism causes anatomical closure.
What New Information Does This Article Contribute?
Mitochondrial fission is crucial for O2-induced constriction and closure of human and rabbit ducti.
O2 rapidly (<5 minutes) causes mitochondrial fission by post-translational modification and activation of dynamin-related protein 1.
Fission triggers an oxidative metabolic shift in the DA that increases production of the redox signaling molecule, hydrogen peroxide.
Dynamin-related protein 1 can be targeted to modulate ductal patency.
Closure of the ductus arteriosus (DA) is essential for the transition from the fetal to neonatal circulation. Functional closure, due to O2-induced vasoconstriction, begins within minutes of birth and is required for subsequent anatomical closure. Within minutes of increasing PO2 there is an increase in mitochondria-derived reactive oxygen species (mitoROS). The mitoROS inhibit O2-sensitive potassium channels and activate calcium channels and rho kinase, initiating vasoconstriction. The precise mechanism by which O2 increased mitoROS and the relevance to anatomical DA closure are unknown. We observed that physiologic increases in PO2 fragment the DASMC’s mitochondrial network, suggesting that structural changes in mitochondria are a key early step in O2-sensing. O2 induces fission by phosphorylating dynamin related protein 1 (Drp1), at serine 616. Fission activates pyruvate dehydrogenase, increases oxygen consumption and mitoROS and initiates redox-based O2-sensing. Drp1 inhibition selectively blocks oxygen-mediated DA constriction and, if sustained, prevents DA closure. These findings suggest that mitochondrial fission is an obligatory early step in O2-sensing that is crucial to DA constriction and closure. Drp1 and its regulatory kinases are appealing therapeutic targets for modulating DA patency. The translational relevance of these finding is enhanced by the proof of this concept in ducti from 30 human patients with congenital heart diseases.
ACKNOWLEDGMENTS
Thanks the following colleagues for providing us with the tools to study mitochondrial networking: Dr. Richard Youle (NIH, Bethesda, MD) for the mito-PA-GFP plasmid, Dr. Michael Frohman (Stony Brook University, Stony Brook, NY) for the mito-DsRed and mito-GFP plasmids, Drs. Frederick E. (Rick) Domann, Jr. and Shawn Flanagan (University of Iowa, Iowa City, IA, USA) for the adenovirus containing the cDNA of human mitochondrial targeted catalase.
SOURCES OF FUNDING
Supported in part by the Harold Hines Jr. Chair in Medicine and NIH-RO1-HL071115 (S.A), 1RC1HL099462-01 (S.A) and the American Heart Association (AHA).
Non-standard Abbreviations
- DA
ductus arteriosus
- DASMC
ductus arteriosus smooth muscle cell
- Drp1
dynamin-related protein 1
- Drp1 p-Ser-616
dynamin-related protein 1 (phosphorylated at serine 616)
- ECAR
extracellular acidification rate
- H2O2
hydrogen peroxide
- Mdivi-1
mitochondrial division inhibitor 1
- MFC
mitochondrial fragmentation count
- mitoCAT
mitochondrial-targeted catalase
- mitoGFP
mitochondrial-targeted green fluorescent protein
- mito-PA-GFP
mitochondrial-targeted photoactivatable green fluorescent protein
- mito-DsRED
mitochondrial targeted red fluorescent protein
- mitoROS
mitochondrial-derived reactive oxygen species
- MNF
mitochondrial Networking Factor
- OCR
oxygen consumption rate
- PDH
pyruvate dehydrogenase
Footnotes
DISCLOSURES
None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Seidner SR, Chen YQ, Oprysko PR, Mauray F, Tse MM, Lin E, Koch C, Clyman RI. Combined prostaglandin and nitric oxide inhibition produces anatomic remodeling and closure of the ductus arteriosus in the premature newborn baboon. Pediatr Res. 2001;50:365–373. doi: 10.1203/00006450-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Kajino H, Chen YQ, Seidner SR, Waleh N, Mauray F, Roman C, Chemtob S, Koch CJ, Clyman RI. Factors that increase the contractile tone of the ductus arteriosus also regulate its anatomic remodeling. Am J Physiol Regul Integr Comp Physiol. 2001;281:R291–301. doi: 10.1152/ajpregu.2001.281.1.R291. [DOI] [PubMed] [Google Scholar]
- 3.Ho SY, Anderson RH. Anatomical closure of the ductus arteriosus: A study in 35 specimens. J Anat. 1979;128:829–836. [PMC free article] [PubMed] [Google Scholar]
- 4.del moral T, Gonzalez-Quintero VH, Claure N, Vanbuskirk S, Bancalari E. Antenatal exposure to magnesium sulfate and the incidence of patent ductus arteriosus in extremely low birth weight infants. J Perinatol. 2007;27:154–157. doi: 10.1038/sj.jp.7211663. [DOI] [PubMed] [Google Scholar]
- 5.Casalaz D. Ibuprofen versus indomethacin for closure of patent ductus arteriosus. N Engl J Med. 2001;344:457–458. doi: 10.1056/NEJM200102083440612. [DOI] [PubMed] [Google Scholar]
- 6.Van Overmeire B, Smets K, Lecoutere D, Van de Broek H, Weyler J, Degroote K, Langhendries JP. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl J Med. 2000;343:674–681. doi: 10.1056/NEJM200009073431001. [DOI] [PubMed] [Google Scholar]
- 7.Hosking MC, Benson LN, Musewe N, Dyck JD, Freedom RM. Transcatheter occlusion of the persistently patent ductus arteriosus. Forty-month follow-up and prevalence of residual shunting. Circulation. 1991;84:2313–2317. doi: 10.1161/01.cir.84.6.2313. [DOI] [PubMed] [Google Scholar]
- 8.Hatem J, Sade RM, Upshur JK, Hohn AR. Maintaining patency of the ductus-arteriosus for palliation of cyanotic congenital cardiac malformations. The use of prostaglandin e1 and formaldehyde infiltration of the ductal wall. Ann Surg. 1980;192:124–128. doi: 10.1097/00000658-198007000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coceani F, Liu Y, Seidlitz E, Kelsey L, Kuwaki T, Ackerley C, Yanagisawa M. Endothelin a receptor is necessary for o(2) constriction but not closure of ductus arteriosus. Am J Physiol. 1999;277:H1521–1531. doi: 10.1152/ajpheart.1999.277.4.H1521. [DOI] [PubMed] [Google Scholar]
- 10.Olley PM, Coceani F. Prostaglandins and the ductus arteriosus. Annu Rev Med. 1981;32:375–385. doi: 10.1146/annurev.me.32.020181.002111. [DOI] [PubMed] [Google Scholar]
- 11.Sodini D, Baragatti B, Barogi S, Laubach VE, Coceani F. Indomethacin promotes nitric oxide function in the ductus arteriosus in the mouse. Br J Pharmacol. 2008;153:1631–1640. doi: 10.1038/bjp.2008.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coceani F, Baragatti B. Mechanisms for ductus arteriosus closure. Semin Perinatol. 2012;36:92–97. doi: 10.1053/j.semperi.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Fineman JR, Takahashi Y, Roman C, Clyman RI. Endothelin-receptor blockade does not alter closure of the ductus arteriosus. Am J Physiol. 1998;275:H1620–1626. doi: 10.1152/ajpheart.1998.275.5.H1620. [DOI] [PubMed] [Google Scholar]
- 14.Winters JW, Wong J, Van Dyke D, Johengen M, Heymann MA, Fineman JR. Endothelin receptor blockade does not alter the increase in pulmonary blood flow due to oxygen ventilation in fetal lambs. Pediatr Res. 1996;40:152–157. doi: 10.1203/00006450-199607000-00026. [DOI] [PubMed] [Google Scholar]
- 15.Michelakis E, Rebeyka I, Bateson J, Olley P, Puttagunta L, Archer S. Voltage-gated potassium channels in human ductus arteriosus. Lancet. 2000;356:134–137. doi: 10.1016/S0140-6736(00)02452-1. [DOI] [PubMed] [Google Scholar]
- 16.Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL. Acute oxygen-sensing mechanisms. N Engl J Med. 2005;353:2042–2055. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thebaud B, Michelakis ED, Wu XC, Moudgil R, Kuzyk M, Dyck JR, Harry G, Hashimoto K, Haromy A, Rebeyka I, Archer SL. Oxygen-sensitive kv channel gene transfer confers oxygen responsiveness to preterm rabbit and remodeled human ductus arteriosus: Implications for infants with patent ductus arteriosus. Circulation. 2004;110:1372–1379. doi: 10.1161/01.CIR.0000141292.28616.65. [DOI] [PubMed] [Google Scholar]
- 18.Thebaud B, Wu XC, Kajimoto H, Bonnet S, Hashimoto K, Michelakis ED, Archer SL. Developmental absence of the o2 sensitivity of l-type calcium channels in preterm ductus arteriosus smooth muscle cells impairs o2 constriction contributing to patent ductus arteriosus. Pediatr Res. 2008;63:176–181. doi: 10.1203/PDR.0b013e31815ed059. [DOI] [PubMed] [Google Scholar]
- 19.Tristani-Firouzi M, Reeve HL, Tolarova S, Weir EK, Archer SL. Oxygen-induced constriction of rabbit ductus arteriosus occurs via inhibition of a 4-aminopyridine-, voltage-sensitive potassium channel. J Clin Invest. 1996;98:1959–1965. doi: 10.1172/JCI118999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michelakis ED, Rebeyka I, Wu X, Nsair A, Thebaud B, Hashimoto K, Dyck JR, Haromy A, Harry G, Barr A, Archer SL. O2 sensing in the human ductus arteriosus: Regulation of voltage-gated k+ channels in smooth muscle cells by a mitochondrial redox sensor. Circ Res. 2002;91:478–486. doi: 10.1161/01.res.0000035057.63303.d1. [DOI] [PubMed] [Google Scholar]
- 21.Kajimoto H, Hashimoto K, Bonnet SN, Haromy A, Harry G, Moudgil R, Nakanishi T, Rebeyka I, Thebaud B, Michelakis ED, Archer SL. Oxygen activates the rho/rho-kinase pathway and induces rhob and rock-1 expression in human and rabbit ductus arteriosus by increasing mitochondria-derived reactive oxygen species: A newly recognized mechanism for sustaining ductal constriction. Circulation. 2007;115:1777–1788. doi: 10.1161/CIRCULATIONAHA.106.649566. [DOI] [PubMed] [Google Scholar]
- 22.Hong Z, Hong F, Olschewski A, Cabrera JA, Varghese A, Nelson DP, Weir EK. Role of store-operated calcium channels and calcium sensitization in normoxic contraction of the ductus arteriosus. Circulation. 2006;114:1372–1379. doi: 10.1161/CIRCULATIONAHA.106.641126. [DOI] [PubMed] [Google Scholar]
- 23.Kajino H, Goldbarg S, Roman C, Liu BM, Mauray F, Chen YQ, Takahashi Y, Koch CJ, Clyman RI. Vasa vasorum hypoperfusion is responsible for medial hypoxia and anatomic remodeling in the newborn lamb ductus arteriosus. Pediatr Res. 2002;51:228–235. doi: 10.1203/00006450-200202000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Richard C, Gao J, LaFleur B, Christman BW, Anderson J, Brown N, Reese J. Patency of the preterm fetal ductus arteriosus is regulated by endothelial nitric oxide synthase and is independent of vasa vasorum in the mouse. Am J Physiol Regul Integr Comp Physiol. 2004;287:R652–660. doi: 10.1152/ajpregu.00049.2004. [DOI] [PubMed] [Google Scholar]
- 25.Forsey JT, Elmasry OA, Martin RP. Patent arterial duct. Orphanet J Rare Dis. 2009;4:17. doi: 10.1186/1750-1172-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reeve HL, Tolarova S, Nelson DP, Archer S, Weir EK. Redox control of oxygen sensing in the rabbit ductus arteriosus. J Physiol. 2001;533:253–261. doi: 10.1111/j.1469-7793.2001.0253b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu X, Fang YH, Thenappan T, Piao L, Zhang HJ, Pogoriler J, Chen Y, Morrow E, Weir EK, Rehman J, Archer SL. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ Res. 2012;110:1484–1497. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rehman J, Zhang HJ, Toth PT, Zhang Y, Marsboom G, Hong Z, Salgia R, Husain AN, Wietholt C, Archer SL. Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer. Faseb J. 2012;26:2175–2186. doi: 10.1096/fj.11-196543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ. Spatial and temporal association of bax with mitochondrial fission sites, drp1, and mfn2 during apoptosis. J Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi SY, Huang P, Jenkins GM, Chan DC, Schiller J, Frohman MA. A common lipid links mfn-mediated mitochondrial fusion and snare-regulated exocytosis. Nat Cell Biol. 2006;8:1255–1262. doi: 10.1038/ncb1487. [DOI] [PubMed] [Google Scholar]
- 31.Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, Nunnari J. Chemical inhibition of the mitochondrial division dynamin reveals its role in bax/bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related gtpase drp1 participates in mitochondrial fission. J Biol Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- 33.Han XJ, Lu YF, Li SA, Kaitsuka T, Sato Y, Tomizawa K, Nairn AC, Takei K, Matsui H, Matsushita M. Cam kinase i alpha-induced phosphorylation of drp1 regulates mitochondrial morphology. J Cell Biol. 2008;182:573–585. doi: 10.1083/jcb.200802164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qi X, Disatnik MH, Shen N, Sobel RA, Mochly-Rosen D. Aberrant mitochondrial fission in neurons induced by protein kinase c{delta} under oxidative stress conditions in vivo. Mol Biol Cell. 2011;22:256–265. doi: 10.1091/mbc.E10-06-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu J, Huang Y, Chen G, Chen Z, Tweardy DJ, Dong S. Aberrant chromatin remodeling by retinoic acid receptor alpha fusion proteins assessed at the single-cell level. Mol Biol Cell. 2007;18:3941–3951. doi: 10.1091/mbc.E07-03-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, Inagaki M, Delcros JG, Moulinoux JP. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 37.Archer SL, Wu XC, Thebaud B, Moudgil R, Hashimoto K, Michelakis ED. O2 sensing in the human ductus arteriosus: Redox-sensitive k+ channels are regulated by mitochondria-derived hydrogen peroxide. Biol Chem. 2004;385:205–216. doi: 10.1515/BC.2004.014. [DOI] [PubMed] [Google Scholar]
- 38.Olschewski A, Hong Z, Peterson DA, Nelson DP, Porter VA, Weir EK. Opposite effects of redox status on membrane potential, cytosolic calcium, and tone in pulmonary arteries and ductus arteriosus. Am J Physiol Lung Cell Mol Physiol. 2004;286:L15–22. doi: 10.1152/ajplung.00372.2002. [DOI] [PubMed] [Google Scholar]
- 39.Dzialowski EM, Greyner H. Maturation of the contractile response of the emu ductus arteriosus. J Comp Physiol B. 2008;178:401–412. doi: 10.1007/s00360-007-0232-x. [DOI] [PubMed] [Google Scholar]
- 40.Zhu PP, Patterson A, Stadler J, Seeburg DP, Sheng M, Blackstone C. Intra- and intermolecular domain interactions of the c-terminal gtpase effector domain of the multimeric dynamin-like gtpase drp1. J Biol Chem. 2004;279:35967–35974. doi: 10.1074/jbc.M404105200. [DOI] [PubMed] [Google Scholar]
- 41.Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators fis1, drp1, and opa1 in apoptosis. Mol Biol Cell. 2004;15:5001–5011. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lackner LL, Nunnari J. Small molecule inhibitors of mitochondrial division: Tools that translate basic biological research into medicine. Chem Biol. 2010;17:578–583. doi: 10.1016/j.chembiol.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pizzo P, Pozzan T. Mitochondria-endoplasmic reticulum choreography: Structure and signaling dynamics. Trends Cell Biol. 2007;17:511–517. doi: 10.1016/j.tcb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 44.Ruiz-Meana M, Fernandez-Sanz C, Garcia-Dorado D. The sr-mitochondria interaction: A new player in cardiac pathophysiology. Cardiovasc Res. 2010;88:30–39. doi: 10.1093/cvr/cvq225. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y, Csordas G, Jowdy C, Schneider TG, Csordas N, Wang W, Liu Y, Kohlhaas M, Meiser M, Bergem S, Nerbonne JM, Dorn GW, 2nd, Maack C. Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle ca2+ crosstalk. Circ Res. 2012;111:863–875. doi: 10.1161/CIRCRESAHA.112.266585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terrand J, Papageorgiou I, Rosenblatt-Velin N, Lerch R. Calcium-mediated activation of pyruvate dehydrogenase in severely injured postischemic myocardium. Am J Physiol Heart Circ Physiol. 2001;281:H722–730. doi: 10.1152/ajpheart.2001.281.2.H722. [DOI] [PubMed] [Google Scholar]
- 47.Huang HM, Toral-Barza L, Sheu KF, Gibson GE. The role of cytosolic free calcium in the regulation of pyruvate dehydrogenase in synaptosomes. Neurochem Res. 1994;19:89–95. doi: 10.1007/BF00966734. [DOI] [PubMed] [Google Scholar]
- 48.Carneiro L, Allard C, Guissard C, Fioramonti X, Tourrel-Cuzin C, Bailbe D, Barreau C, Offer G, Nedelec E, Salin B, Rigoulet M, Belenguer P, Penicaud L, Leloup C. Importance of mitochondrial dynamin related protein 1 (drp1) in hypothalamic glucose sensitivity in rats. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2011.4254. [DOI] [PubMed] [Google Scholar]
- 49.Liu W, Acin-Perez R, Geghman KD, Manfredi G, Lu B, Li C. Pink1 regulates the oxidative phosphorylation machinery via mitochondrial fission. Proc Natl Acad Sci U S A. 2011;108:12920–12924. doi: 10.1073/pnas.1107332108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Archer SL, Wu XC, Thebaud B, Moudgil R, Hashimoto K, Michelakis ED. O2 sensing in the human ductus arteriosus: Redox-sensitive k+ channels are regulated by mitochondria-derived hydrogen peroxide. Biol Chem. 2004;385:205–216. doi: 10.1515/BC.2004.014. [DOI] [PubMed] [Google Scholar]
- 51.Clyman RI, Waleh N, Kajino H, Roman C, Mauray F. Calcium-dependent and calcium-sensitizing pathways in the mature and immature ductus arteriosus. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1650–1656. doi: 10.1152/ajpregu.00300.2007. [DOI] [PubMed] [Google Scholar]
- 52.Michelakis ED, Rebeyka I, Wu X, Nsair A, Thebaud B, Hashimoto K, Dyck JR, Haromy A, Harry G, Barr A, Archer SL. O2 sensing in the human ductus arteriosus: Regulation of voltage-gated k+ channels in smooth muscle cells by a mitochondrial redox sensor. Circ Res. 2002;91:478–486. doi: 10.1161/01.res.0000035057.63303.d1. [DOI] [PubMed] [Google Scholar]
- 53.Nakanishi T, Gu H, Hagiwara N, Momma K. Mechanisms of oxygen-induced contraction of ductus arteriosus isolated from the fetal rabbit. Circ Res. 1993;72:1218–1228. doi: 10.1161/01.res.72.6.1218. [DOI] [PubMed] [Google Scholar]
- 54.Tristani-Firouzi M, Reeve HL, Tolarova S, Weir EK, Archer SL. Oxygen-induced constriction of rabbit ductus arteriosus occurs via inhibition of a 4-aminopyridine-, voltage-sensitive potassium channel. J Clin Invest. 1996;98:1959–1965. doi: 10.1172/JCI118999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Connuck D, Sun JP, Super DM, Kirchner HL, Fradley LG, Harcar-Sevcik RA, Salvator A, Singer L, Mehta SK. Incidence of patent ductus arteriosus and patent foramen ovale in normal infants. Am J Cardiol. 2002;89:244–247. doi: 10.1016/s0002-9149(01)02214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siassi B, Blanco C, Cabal LA, Coran AG. Incidence and clinical features of patent ductus arteriosus in low-birthweight infants: A prospective analysis of 150 consecutively born infants. Pediatrics. 1976;57:347–351. [PubMed] [Google Scholar]
- 57.Van Overmeire B, Smets K, Lecoutere D, Van de Broek H, Weyler J, Degroote K, Langhendries JP. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl J Med. 2000;343:674–681. doi: 10.1056/NEJM200009073431001. [DOI] [PubMed] [Google Scholar]
- 58.Clyman RI, Couto J, Murphy GM. Patent ductus arteriosus: Are current neonatal treatment options better or worse than no treatment at all? Semin Perinatol. 2012;36:123–129. doi: 10.1053/j.semperi.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katakam LI, Cotten CM, Goldberg RN, Dang CN, Smith PB. Safety and effectiveness of indomethacin versus ibuprofen for treatment of patent ductus arteriosus. Am J Perinatol. 2010;27:425–429. doi: 10.1055/s-0029-1243371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.