Abstract
Background
Despite antiretroviral therapy and trimethoprim-sulfamethoxazole (TMP-SMX) prophylaxis, Pneumocystis pneumonia (PCP) remains an important serious opportunistic infection in HIV-infected persons. Pneumocystis (Pc) colonization in HIV-infected individuals and in HIV-uninfected smokers is associated with chronic obstructive pulmonary disease (COPD). We previously developed a non-human primate (NHP) model of HIV infection and Pc colonization and demonstrated that Pc colonization correlated with COPD development. In the present study, we examined kinetics of COPD development in NHP and tested the effect of Pc burden reduction on pulmonary function by TMP-SMX treatment.
Methods
Cynomolgus macaques (n=16) were infected with simian/human immunodeficiency virus (SHIV89.6P) and natural Pc colonization was examined by nested polymerase chain reaction (PCR) of serial bronchoalveolar lavage (BAL) fluid and anti-Pc serology.
Results
Eleven of 16 monkeys became Pc-colonized by 16 weeks post-SHIV infection. Pc colonization of SHIV-infected monkeys led to progressive declines in pulmonary function as early as 4 weeks following Pc detection. SHIV-infected, Pc-negative monkeys maintained normal lung function. At 25 weeks post SHIV-infection, TMP-SMX treatment was initiated in 7 Pc-positive (Pc+) (20mg/kg TMP, 100mg/kg SMX, daily for 48 weeks) and 5 Pc-negative (Pc-) monkeys. Four SHIV+/Pc+ remained untreated for the duration of the experiment. Detection frequency of Pc in BAL fluid (p<0.001), as well as plasma Pc antibody titers (p=0.02), were significantly reduced in TMP-SMX-treated macaques compared to untreated.
Conclusion
Reduction of Pc colonization by TMP-SMX treatment did not improve pulmonary function, supporting the concept that Pc-colonization results in early, permanent obstructive changes in the lungs of immunosuppressed macaques.
Keywords: SIV/HIV, chronic obstructive pulmonary disease (COPD), Pneumocystis, trimethoprim-sulfamethoxazole
Introduction
Pulmonary disease remains a leading cause of morbidity and mortality in HIV-infected individuals, with Pneumocystis pneumonia (PCP) one of the most common AIDS-defining opportunistic infections in the United States 1-4. In addition, the number of HIV-uninfected individuals at risk for PCP has grown due to increased use of immunosuppressive therapies 5,6.
As there are no Pneumocystis vaccines available, current therapies and prophylaxis for PCP are restricted to chemotherapeutic agents. Trimethoprim-sulfamethoxazole (TMP-SMX) remains the most widely used antimicrobial agent for treatment of PCP and prophylaxis because of its safety, efficacy and low cost 7. TMP-SMX is recommended as first-line prophylaxis against PCP in HIV-infected individuals with CD4+ T cell counts less than 200 cells/μl, those with oral candidiasis, and those with PCP after completion of PCP treatment regimen 8-10. Pc prophylaxis is also recommended for HIV-uninfected persons receiving immunosuppressive medications or who have an underlying acquired or inherited immunodeficiency 11,12.
Recent studies have focused on the epidemiology and clinical consequences of Pc colonization, which is defined as detection of Pc in respiratory samples that may occur in subjects with or without symptoms of acute infection 13-15. Pc colonization is associated with low organism burden in respiratory samples and because Pc cannot be cultured in the laboratory, detection is accomplished using PCR-based assays of respiratory samples 16-18. The prevalence of Pc colonization is variable among HIV-infected individuals, with reported rates ranging from 20-69% 2,3,19-22, even among those receiving anti-Pc prophylaxis and those with high CD4+ T cell counts who are receiving anti-retroviral therapy (ART) 3,13. In the general population, Pc colonization rates may be higher than previously believed 23, and it is likely that Pc-colonized persons serve as a reservoir for transmission of Pc in PCP cases as well 24. Pc colonization has been reported in infants 25, persons receiving immunosuppressive therapies 26, healthcare workers 27, pregnant women 28 and persons with underlying pulmonary disease 26,29.
Colonization with Pc may have important clinical implications, in addition to its contribution to transmission or development of PCP. In particular, several recent studies have focused on the role of Pc colonization and the development of COPD 30-33. Pc colonization is associated with worse airway obstruction, increased risk of airway obstruction31 and COPD in HIV-infected individuals 31,32,34, independent of smoking history or corticosteroid use 32. Other studies have reported increased systemic inflammation, including higher levels of interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF)-α associated with Pc colonization in COPD 35. Furthermore, in experimental animal models, Pc colonization is associated with obstructive lung disease and emphysema36-38. In a study using an immunocompetent rat model, increased physiologic and anatomic emphysematous changes were reported in animals exposed to cigarette smoke in combination with Pc, compared with either alone 38. In a non-human primate (NHP) model of HIV infection, Pc colonization resulted in development of airway obstruction, radiographic emphysema and enlargement of lung airspaces 36.
To understand the relationship between Pc colonization and the development of HIV-associated COPD, our laboratory has developed a NHP model of naturally acquired Pneumocystis infection, in which macaques become persistently colonized with Pc following SIV or simian-human immunodeficiency virus (SHIV)-infection 36,39,40. Susceptibility to Pc colonization in this model is associated with low plasma anti-Pc antibody titer at baseline and CD4+ T cell levels below 500 cells/μl following virus infection 39,40. Pc colonization in SHIV-infected macaques correlated with declining pulmonary function and increased pulmonary inflammation, compared to monkeys infected with SHIV alone 36,37,40-42. As persistent Pc colonization has been noted in HIV-infected individuals despite Pc prophylaxis and colonization is associated with COPD, we sought to determine the effect of TMP-SMX treatment on established Pc colonization in SHIV-infected macaques. In addition, we tested whether reduction in Pc colonization improved pulmonary function in the macaque model of HIV-associated COPD.
Materials and Methods
Study design
Prior to the initiation of this study, all animal experiments were approved by the IACUC of the University of Pittsburgh. Animal husbandry and experimental procedures were conducted in accordance with standards set forth by the Guide for the Care and Use of Laboratory Animals 43 and the provisions of the Animal Welfare Act 44.
Adult cynomolgus macaques (Macacca fascicularis, n=16) were inoculated intravenously with 1×104.9 TCID50 of SHIV89.6P 45, which induces CD4+ T cell lymphopenia and AIDS-like disease, including wasting and opportunistic infections 45,46. To promote Pneumocystis transmission, SHIV-infected macaques were cohoused with SIV- or SHIV-immunosuppressed, Pc-colonized macaques, which served as a source of Pc 39,40. Determination of Pc colonization status was performed by detection of Pc DNA in BAL fluid samples by nested PCR of the mitochondrial large subunit rRNA gene (mtLSU)41,47, and by anti-Pc serology using recombinant Pc kexin protein as the target antigen 39,40. Pneumocystis colonization was defined as positive nested PCR of BAL fluid and/or at least a 3-fold increase in plasma anti-Pneumocystis kexin (KEX1) titers39,40. Study design is shown in Figure 1. For BAL fluid collection, a pediatric fiberoptic bronchoscope was directed into the right primary bronchus and wedged into a distal subsegmental bronchus that approximated the diameter of the bronchoscope. Four 10-mL aliquots of 0.9% saline were instilled, then aspirated; fluid from a single animal was pooled. BAL fluid and peripheral blood were processed as described previously36,39,41,42.
Figure 1. Study design schematic.
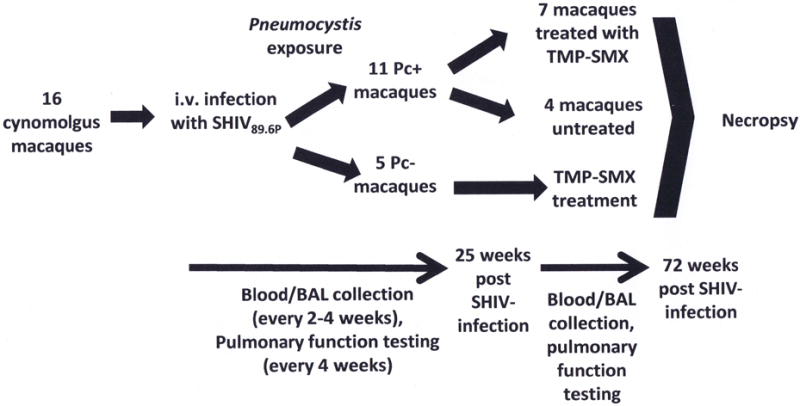
Sixteen cynomolgus macaques were intravenously infected with SHIV89.6P and exposed to Pneumocystis (Pc) via natural transmission and cohousing with other SIV+/Pc+ macaques. Eleven macaques became Pc-colonized, and 5 remained Pc-negative by 25 weeks post-SHIV infection and Pc-exposure, during which time serial blood and BAL fluid samples, as well as pulmonary function data, were collected. At 25 weeks post-SHIV infection, trimethoprim- sulfamethoxazole (TMP-SMX) treatment was initiated in 7 randomly-selected Pc-colonized animals and in all 5 Pc-negative monkeys. Four Pc-colonized animals were withheld from TMP-SMX treatment. Blood, BAL fluid and pulmonary function data collection was continued the remainder of the study duration, and at 72 weeks post-SHIV infection (47 weeks post TMP-SMX initiation), animals were sacrificed.
At twenty-five weeks post SHIV-infection, eleven macaques were Pc-colonized and five remained Pc-negative. At this time, 7 of the Pc-colonized macaques (randomly selected from Pc-colonized group of 11 animals), and all of the Pc-negative animals, were placed on TMP-SMX (20mg/kg TMP, 100mg/kg SMX, daily, administered orally, confirmed by direct observation) 48. Four Pc-colonized macaques remained untreated for the duration of the study (72 weeks post SHIV-infection).
Flow cytometry analysis of peripheral blood cells
Peripheral blood samples were collected from macaques as described in the supplemental digital content. Peripheral blood leukocytes (PBL) were counted, stained, and fixed for analysis by flow cytometry, as described 42. Antibodies used were: mouse anti-monkey CD3–FITC (clone SP34), mouse anti-monkey CD4–allophycocyanin (clone L200), mouse anti-monkey CD8-PacificBlue (RPA-T8) (BD Pharmingen, San Diego, CA). Acquisition was performed using BD FacsDiva software on BD LSRII flow cytometer (BD Biosciences, San Jose, CA). Forward-/side-scatter dot plots were used to gate the live lymphocyte population. All analyses were performed by using FlowJo flow cytometry analysis software (Tree Star, Ashland, OR). Antech Diagnostics (Lake Success, NY) performed differential cell counts, and lymphocyte counts were used to determine absolute numbers of CD4+ T cells.
Pulmonary function testing
Pulmonary function tests (PFT) were performed at baseline and monthly after SHIV infection using whole-body plethysmography 49 and forced deflation technique 36 to assess airflow obstruction. Briefly, animals were anesthetized with intravenous propofol (7.5 to 12.5 mg/kg [body weight]), and 2% lidocaine was given prior to intubation (3.5-mm endotracheal tube). Endotracheal tube placement was verified by a chest radiograph. Pulmonary function testing was performed using a Buxco whole-body plethysmograph (Buxco Electronics, Inc., Sharon, CT), and the BioSystems for Maneuvers Software (Buxco Electronics, Inc.) was used to collect data on flow rates and volumes. When three measurements for forced vital capacity were within 10% of each other, tests were considered valid.
Pneumocystis kexin antibody endpoint titer determination
Reciprocal endpoint titers to the Pneumocystis kexin-like protease (KEX1) were determined by ELISA as previously described 39,40. Serial dilutions were made to determine endpoint titers and goat anti-monkey horseradish peroxidase was used for detection. The reciprocal endpoint titer (RET) was calculated as the highest dilution at which the optical density values for the test sample were the same or less than an uninfected, Pc-negative control sample.
Statistical analyses
Statistical analyses were performed using Prism software or InStat software (GraphPad, La Jolla, CA). T-tests and one-way ANOVA were performed on ranked data50. For each group of animals, comparisons between baseline values and values at other time-points were made using a paired Student’s t-test. Comparisons between groups of animals at a single time-point were made using unpaired Student’s t-tests. When comparing Pc-colonized and Pc-negative monkeys over multiple time points, two-way repeated measures analysis of variance (ANOVA) was used for comparison. Fisher’s exact test was used to evaluate reduction of PCR+ samples following TMP-SMX treatment. A P value of <0.05 was considered significant.
Results
Pneumocystis colonization of SHIV89.6P -infected macaques
SHIV infection of macaques resulted in peak plasma viral load at 1-2 weeks post infection and rapid peripheral blood CD4+ T cell decline (Fig. 2). Monkeys were exposed to natural transmission of Pc by co-housing with SIV+/Pc+ macaques, as described 39,40. By 8 weeks post-SHIV infection and Pc exposure, 4 of 16 monkeys had detectable Pc in BAL fluid by nested PCR. By 16 weeks post-SHIV infection, 11 of 16 macaques became Pc-colonized and 5 macaques remained Pc-negative for the duration of the study (72 weeks).
Figure 2. CD4+ T cells and peak plasma viral loads were similar between Pneumocystis-colonized and Pneumocystis-negative macaques.
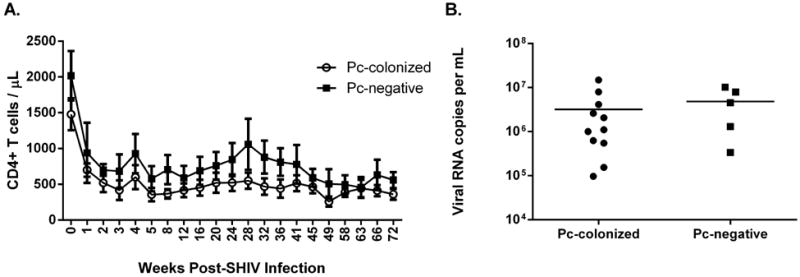
CD4+ T cell percentages were measured by flow cytometry of peripheral blood cells at baseline and at serial time-points following SHIV-infection. Counts were determined from absolute differential cell counts. SHIV RNA copies per mL of plasma were measured by an adapted quantitative PCR for detection of the SIV gag sequence. CD4+ T cell numbers (A, p=0.17, 2-way repeated measures ANOVA) and peak plasma viral loads (B, p=0.35, unpaired t-test).
Peripheral blood CD4+ T cell levels (cells/μL) were monitored monthly following infection, and no significant difference in absolute number was observed in Pc-colonized (n=11) and Pc-negative (n=5) animals (Fig 2A, p=0.17, repeated measures [RM] ANOVA). Additionally, peripheral blood peak viral loads were not significantly different between Pc-colonized and Pc-negative macaques (Fig 2B, p=0.35, unpaired t-test).
Pulmonary function in SHIV-infected macaques
Pulmonary function was measured at baseline and at monthly intervals following SHIV-infection. Pc-colonized macaques exhibited significant declines in peak expiratory flow (PEF, p=0.001) and FEV0.4 (forced expiratory volume in 0.4s, p=0.001) between baseline and 25 weeks post-SHIV infection (wpi) (Fig. 3A, C; paired t-test), indicating pulmonary obstruction. No significant changes in pulmonary function (baseline vs. 25 wpi) were observed in Pc-negative macaques (Fig 3, PEF, p=0.21 and FEV0.4, p=0.21, paired t-test). These results confirm previous studies that showed that Pc colonization is associated with the development of COPD in SHIV-infected macaques and that pulmonary function deficits occur early after detection of Pc colonization 36,40.
Figure 3. Pneumocystis-colonized macaques exhibited significant declines in pulmonary function, whereas pulmonary function was preserved in Pneumocystis-negative animals.
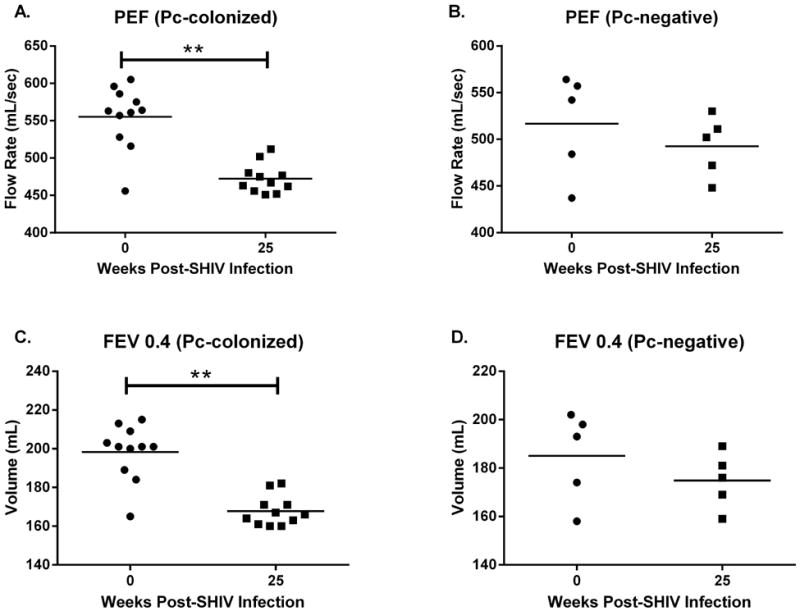
Significant declines in both peak expiratory flow (PEF, p=0.001, A) and forced expiratory volume in 0.4s (FEV 0.4, p=0.001, C) occurred in Pc-colonized macaques (n=11, paired t-test, week 0 vs week 25 post SHIV-infection). At 25 weeks post SHIV-infection, PEF (B, p=0.21) and FEV 0.4 (D, p=0.21) were similar to baseline values (n=5, paired t-test) in the Pc-negative animals.
TMP-SMX treatment of SHIV-infected macaques
We next tested whether treatment with TMP-SMX reduced Pc colonization in SHIV-infected macaques and whether reduction in Pc colonization restored pulmonary function or slowed decline in macaques with pulmonary obstruction. At 25 weeks following SHIV infection, macaques with persistent Pc colonization (n=11) were randomly assigned to TMP-SMX treated (n=7) or untreated groups (n=4) (Fig. 1). TMP-SMX treatment was initiated and bronchoscopy was performed monthly for detection of Pc by nested PCR. The percentage of positive nested PCR samples for all BAL fluid samples in each group (TMP-SMX-treated and untreated) was compared prior to (n=7 BAL fluid samples per animal) and during TMP-SMX treatment (n=9 BAL fluid samples per animal). TMP-SMX treatment significantly reduced the percentage of Pc-positive BAL fluid samples (1.6% of 63 total BAL fluid samples, p=0.0004, Fisher’s Exact Test) compared to untreated group (33.3% of 36 total BAL fluid samples) (Table S1). BAL samples from 6 of 7 TMP-SMX-treated monkeys were Pc-negative at all time-points for the duration of the treatment (47 weeks).
As a secondary indicator of Pc colonization, we determined anti-Pc KEX1 antibody titers in SHIV infected, Pc-exposed macaques pre- and post-TMP-SMX treatment. Plasma anti-KEX1 reciprocal endpoint (RET) antibody titers increased in SHIV-infected animals that became colonized with Pc (Fig. 4A). Prior to TMP-SMX treatment, there was no significant difference in the serial mean plasma KEX1 IgG titers in the group of Pc-colonized animals that were subsequently TMP-SMX treated (n=7) and the Pc-colonized animals that remained untreated (n=4) (p=0.51, RM ANOVA). Following 25 weeks post-SHIV infection, KEX1 IgG titers continued to increase in the untreated group. In contrast, KEX1 plasma IgG RET were significantly reduced in the Pc-colonized, TMP-SMX-treated macaques, compared with the Pc-colonized, untreated animals (p=0.021, RM ANOVA). These data indicate a treatment response to TMP-SMX, suggesting that when Pc burden is reduced, circulating antibody titers decline in response. TMP-SMX treatment did not significantly alter circulating IgG RET in the Pc-negative animals (Fig 4B, p=0.35, RM ANOVA). Additionally, IgG RET were not different between Pc-colonized, TMP-SMX-treated and Pc-negative animals (Fig 4C, p=0.34, RM ANOVA) or between Pc-colonized, untreated animals and Pc-negative monkeys (p=0.47, RM ANOVA) following TMP-SMX-treatment initiation.
Figure 4. Circulating antibody responses to Pneumocystis-kexin were reduced in Pc-colonized, TMP-SMX-treated macaques, compared to responses in untreated animals.
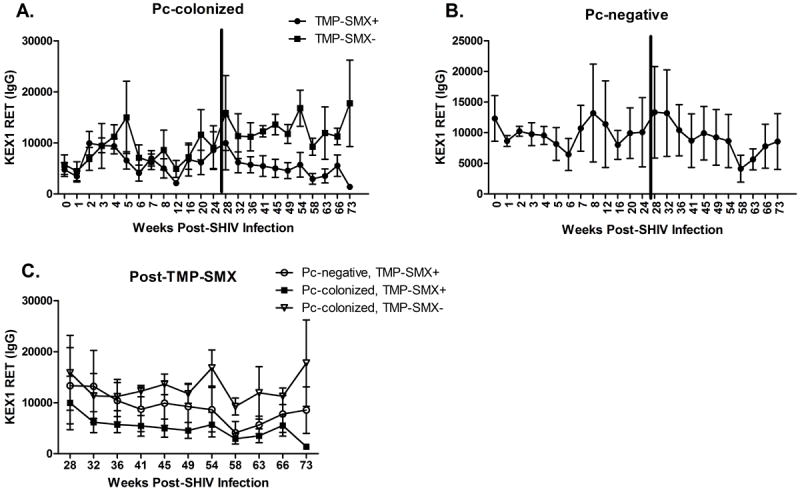
Prior to TMP-SMX treatment, there was no significant difference in the mean plasma Pc-kexin IgG reciprocal endpoint titers (RET) in the group of Pc-colonized animals that were subsequently TMP-SMX treated (n=7) and the Pc-colonized animals that remained untreated (n=4) (A, p=0.51, repeated measures [RM] ANOVA). During TMP-SMX treatment, Pc-kexin plasma IgG RET were significantly reduced in the Pc+, TMP-SMX-treated macaques, compared with the Pc+, untreated animals (A, p=0.021, RM ANOVA). Serial circulating IgG antibody titers to Pc-kexin in Pc-negative monkeys are shown in panel B. There was no significant difference from prior to TMP-SMX treatment (p=0.35, RM ANOVA). Following TMP-SMX treatment initiation (C), there was no difference between Pc-kexin titers in Pc-colonized, TMP-SMX+ and Pc-negative, TMP-SMX+ animals (C, p=0.34, RM ANOVA), or in Pc-colonized, TMP-SMX-untreated animals compared with Pc-negative, TMP-SMX+ animals (C, p=0.47, RM ANOVA).
Pulmonary function in TMP-SMX-treated and untreated Pneumocystis-colonized macaques
Pulmonary function was evaluated post-TMP-SMX treatment to determine whether the observed pulmonary function declines were the result of a transient response to Pc colonization and reversible with reduction in Pc burden, or whether pulmonary function decline was permanent. Pulmonary function was monitored at monthly intervals for the remainder of the study (25-72 weeks post-SHIV infection). Interestingly, while there was no significant improvement in pulmonary function more than 40 weeks post-treatment, pulmonary function did not continue to decline in either the TMP-SMX treated (PEF [p=0.29, Fig 5A] and FEV0.4 [p=0.46, Fig 5C]) or untreated group (PEF [Fig 5B, p=0.39] and FEV0.4 [Fig 5D, p=0.39]). PEF and FEV0.4 did not decline significantly from baseline values by 72 weeks post SHIV-infection in Pc-negative macaques (data not shown, p=0.70 [PEF], p=0.70 [FEV0.4]).
Figure 5. Pulmonary function remained steady in both TMP-SMX-treated and untreated Pneumocystis-colonized macaques for the remainder of the study (weeks 25-72 post SHIV-infection).
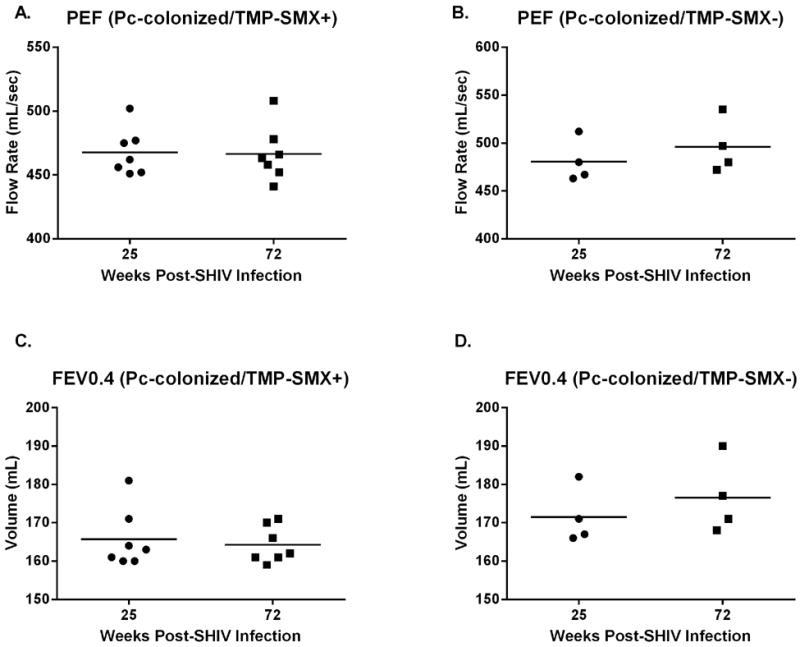
Peak expiratory flow (PEF, p=0.29, A) and forced expiratory volume in 0.4s (FEV 0.4, p=0.46, C) values were similar at experiment termination (72wpi), compared with pulmonary function measurements taken prior to TMP-SMX initiation (25wpi), in the TMP-SMX-treated macaques. PEF (B, p=0.39) and FEV 0.4 (D, p=0.39) values in the Pc+, untreated group were also not different compared to the time-point at which TMP-SMX was initiated in the treatment group (paired t-test). PEF and FEV 0.4 did not decline significantly by 72wpi in the Pc-negative group (data not shown, p=0.70).
To examine the reversibility of pulmonary obstruction in SHIV-infected, Pc-colonized macaques, animals were treated with the bronchodilator albuterol at 43 weeks post-SHIV infection (18 weeks of TMP-SMX treatment in the treated group), with no significant improvement in PEF or FEV0.4 following treatment (Fig S1).
Discussion
Increasing evidence suggests microbial colonization is associated with COPD exacerbations, perhaps through amplification of pulmonary inflammatory responses to noxious agents such as cigarette smoke 32,36,37,40,51-54. Several studies have shown that Pc colonization is associated with the development or progression of COPD in HIV-infected and non-HIV infected individuals 31,32,52, although a causal relationship is difficult to ascertain in clinical studies. In a SHIV-NHP model of HIV infection, we demonstrated that progressive declines in pulmonary function parameters followed Pc-colonization, and monkeys infected with virus alone maintained normal lung function 36. Here, we show that decline in pulmonary function occurs early after Pc colonization and that Pc-induced obstructive changes are not reversible following reduction of Pc colonization with TMP-SMX or albuterol treatment, indicating development of COPD-like disease in these animals.
In the SHIV model, Pc colonization occurs by natural transmission as early as 2-4 weeks post virus infection co-incident with CD4+ T cell decline. Pulmonary function decline was observed as early as four weeks following initial evidence of Pc colonization, with all Pc-colonized animals exhibiting significant pulmonary obstruction within 1-4 months of Pc colonization. Pulmonary function did not change significantly from baseline levels in SHIV-infected, Pc-negative animals, supporting previous findings that pulmonary function deficits in this model were not a consequence of virus infection alone36,40. We found no evidence of more profound SHIV infection in the Pc-colonized/COPD+ macaques based on peripheral blood CD4+ T cell levels or viral load, compared to Pc-negative monkeys with normal lung function, suggesting that decreased pulmonary function in the SHIV/Pc-colonized monkeys was not the direct result viral burden or more advanced AIDS. Previous studies showed that susceptibility of SHIV-immunosuppressed macaques to Pc colonization was associated with low baseline plasma anti-Pc antibody titers, suggesting a role for humoral immunity in control of Pc colonization and prevention of Pc-related COPD in this model40. SHIV-infected macaques withheld from TMP-SMX treatment remained persistently Pc-colonized although they did not develop PCP during the study period. This is likely due to the transmission of Pc from colonized macaques rather than macaques with PCP (K.A. Norris, unpublished data).
Pc colonization is common in HIV+ subjects 2,18,32. Reported incidence of Pc colonization varies, likely due to differences in patient populations examined, samples collected and detection methods employed. There may be substantial differences in colonization prevalence, for example, between samples collected from oropharyngeal washes versus BAL fluid. Additionally, the relationship between Pc colonization and CD4+ T cell counts is debated 3,55. It has been demonstrated that individuals with COPD are more likely to be Pc-colonized compared to healthy smokers, and the frequency of Pc colonization is associated with worse pulmonary obstruction in HIV-infected 3 and HIV-uninfected smokers 56. In HIV-uninfected persons, studies have demonstrated that Pc colonization is a risk factor for more severe COPD, independent of smoking history or corticosteroid use 32. The current study supports the concept that Pc colonization is associated with obstructive changes in a primate model of HIV infection, and demonstrates that obstructive changes occur within weeks of initial Pc colonization in the macaque model of HIV infection.
Although there is substantial evidence to indicate that TMP-SMX is effective in preventing and treating PCP 8,57,58, there have been limited studies on the effects of TMP-SMX prophylaxis on Pc colonization in HIV+ individuals 3. The present study demonstrates that it is possible to reduce Pc colonization by aggressive treatment with TMP-SMX, as indicated by reduced detection by PCR and decline in anti-Pc antibody titers. Nevertheless, continuous treatment did not improve lung function, suggesting that structural damage of the lung parenchyma, previously shown to be associated with Pc colonization 36, occurs as early as 6 months post-Pc exposure. Furthermore, no improvement in lung function was seen following bronchodilator treatment in the SHIV-infected/Pc-colonized macaques (supplemental data, Fig S1). We previously demonstrated that in addition to worse pulmonary function, SHIV-infected, Pc-colonized macaques had increased anatomic emphysema compared to macaques infected with SHIV alone 36. Taken together, these results support the conclusion that Pc colonization induces irreversible changes in pulmonary function rather than transient, inflammation-mediated airway hyper-responsiveness.
Interestingly, while TMP-SMX treatment and reduction in Pc burden did not improve pulmonary function, we did not see further decline in untreated, Pc-colonized monkeys. At 72 weeks post-SHIV infection, parameters of pulmonary function, PEF and FEV0.4, were similar to values recorded prior to TMP-SMX treatment initiation (25 wpi) in the treated, Pc-colonized group. These data suggest that the initial damage associated with Pc infection, which occurs early following Pc colonization, is not sustained at the same rate throughout infection, but is characterized by an initial sharp decline in pulmonary function, which is maintained, but does not decline further. As the kinetics of Pc colonization and development of COPD are difficult to assess in human populations, the present studies underscore the utility of the NHP model for examining the consequences of Pc colonization at its earliest measureable time-points.
It is interesting to note that Pc colonization results in early decline in pulmonary function in SHIV-infected macaques, but pulmonary function decline does not continue as typically occurs in human COPD. The development and progression of human COPD is multifactorial with genetic as well as extrinsic factors, such as cigarette smoke, contributing to pathogenesis59,60. The combination of Pc-colonization and smoking is associated with increased frequency of COPD and worse pulmonary function compared to that of non-Pc colonized individuals32. The role of Pc colonization, as well as other respiratory pathogens, in amplifying the host inflammatory response to cigarette smoke and other noxious agents has been proposed as a “vicious circle hypothesis”54. In the context of non-human primate SHIV-infection, Pc colonization is sufficient to induce COPD, but it may be that the absence of a “second hit” such as cigarette smoke precludes further decline in pulmonary function. Additionally, a number of studies in HIV-infected persons have found an association between respiratory symptoms, airway obstruction and ART34,61,62. The mechanism linking ART use with airway obstruction is not known, however, immune reconstitution inflammatory syndrome associated with ART initiation may result in a chronic inflammatory response, which may exacerbate COPD pathogenesis62. The non-human primate model of HIV-associated COPD is a valuable resource that should allow for direct assessment of the influence of extrinsic factors such as smoking, ART or illicit drugs on disease progression.
The relationship between Pc colonization and COPD development may be the result of chronic inflammatory changes that occur in the alveoli in response to Pc persistence. We have previously shown increased levels of pro-inflammatory mediators (IL-1b, IL-6, IL-8 and GM-CSF) as well as Th2-type cytokines (IL-4, IL-5 and IL-13) in bronchoalveolar lavage fluid of SHIV-infected macaques following Pc colonization compared to macaques infected with SHIV alone 36. Peak levels of these mediators occurred by 20 weeks post SHIV/Pc exposure, supporting a role for inflammation in the early response to Pc colonization and development of COPD. Many of the inflammatory changes reported in Pc infection, including influx of CD8+ T cells and neutrophils, and increased IL-8 production, are similar to inflammatory profiles associated with COPD63-66
The data presented here suggest TMP-SMX treatment may mitigate Pc colonization in a NHP model of HIV infection; however, damage to the host lung, likely resulting from host immune responses to Pc colonization, occurs early following colonization and cannot be reversed with chemotherapeutic treatment. While TMP-SMX has been shown to effectively prevent PCP in immunocompromised hosts, prolonged TMP-SMX therapy would likely have little effect in preventing or improving Pc-induced COPD. Several studies have explored the development of prophylactic Pc immunization to protect against PCP67-70. The relationship between Pc colonization and the development of permanent obstructive lung damage in at-risk populations, such as HIV+ individuals, and the lack of efficacy of TMP-SMX treatment in preventing Pc colonization or COPD, as demonstrated in this model, supports the rationale for expanding such vaccine development to include prevention of Pc colonization and obstructive lung disease.
Supplementary Material
Acknowledgments
The authors thank Dr. Chris Janssen for excellent veterinary care and Dr. Kurtis Moseley for consultation regarding statistical analyses. Funding for the work presented here was provided by NIH grants HL077095-01A1 and HL077914-01 (KAN) and NIH training grant T32 AI49820 (HMK).
Footnotes
Data included in this manuscript were presented in part at the American Thoracic Society International Conference, San Francisco, CA, USA, May 2012.
Conflicts of Interest: The authors declare no conflicts of interest.
Literature Cited
- 1.Louie JK, Hsu LC, Osmond DH, Katz MH, Schwarcz SK. Trends in causes of death among persons with acquired immunodeficiency syndrome in the era of highly active antiretroviral therapy, San Francisco, 1994-1998. The Journal of infectious diseases. 2002 Oct 1;186(7):1023–1027. doi: 10.1086/343862. [DOI] [PubMed] [Google Scholar]
- 2.Huang L, Crothers K, Morris A, et al. Pneumocystis colonization in HIV-infected patients. The Journal of eukaryotic microbiology. 2003;50(Suppl):616–617. doi: 10.1111/j.1550-7408.2003.tb00651.x. [DOI] [PubMed] [Google Scholar]
- 3.Morris A, Kingsley LA, Groner G, Lebedeva IP, Beard CB, Norris KA. Prevalence and clinical predictors of Pneumocystis colonization among HIV-infected men. AIDS (London, England) 2004 Mar 26;18(5):793–798. doi: 10.1097/00002030-200403260-00011. [DOI] [PubMed] [Google Scholar]
- 4.Morris A, Norris KA. Colonization by Pneumocystis jirovecii and its role in disease. Clinical microbiology reviews. 2012 Apr;25(2):297–317. doi: 10.1128/CMR.00013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Komano Y, Harigai M, Koike R, et al. Pneumocystis jiroveci pneumonia in patients with rheumatoid arthritis treated with infliximab: a retrospective review and case-control study of 21 patients. Arthritis and rheumatism. 2009 Mar 15;61(3):305–312. doi: 10.1002/art.24283. [DOI] [PubMed] [Google Scholar]
- 6.Yale SH, Limper AH. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illness and prior corticosteroid therapy. Mayo Clinic proceedings. Mayo Clinic. 1996 Jan;71(1):5–13. doi: 10.4065/71.1.5. [DOI] [PubMed] [Google Scholar]
- 7.Huang L, Morris A, Limper AH, Beck JM. An Official ATS Workshop Summary: Recent advances and future directions in pneumocystis pneumonia (PCP) Proceedings of the American Thoracic Society. 2006 Nov;3(8):655–664. doi: 10.1513/pats.200602-015MS. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan JE, Benson C, Holmes KH, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control. 2009 Apr 10;58(RR-4):1–207. quiz CE201-204. [PubMed] [Google Scholar]
- 9.Carmona EM, Limper AH. Update on the diagnosis and treatment of Pneumocystis pneumonia. Therapeutic advances in respiratory disease. 2011 Feb;5(1):41–59. doi: 10.1177/1753465810380102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas CF, Jr, Limper AH. Pneumocystis pneumonia. The New England journal of medicine. 2004 Jun 10;350(24):2487–2498. doi: 10.1056/NEJMra032588. [DOI] [PubMed] [Google Scholar]
- 11.Green H, Paul M, Vidal L, Leibovici L. Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochrane database of systematic reviews. 2007;(3) doi: 10.1002/14651858.CD005590.pub2. CD005590. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez M, Fishman JA. Prevention of infection due to Pneumocystis spp. in human immunodeficiency virus-negative immunocompromised patients. Clinical microbiology reviews. 2004 Oct;17(4):770–782. doi: 10.1128/CMR.17.4.770-782.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris A, Wei K, Afshar K, Huang L. Epidemiology and clinical significance of pneumocystis colonization. The Journal of infectious diseases. 2008 Jan 1;197(1):10–17. doi: 10.1086/523814. [DOI] [PubMed] [Google Scholar]
- 14.Calderon EJ. Pneumocystis infection: seeing beyond the tip of the iceberg. Clin Infect Dis. 2010 Feb 1;50(3):354–356. doi: 10.1086/649870. [DOI] [PubMed] [Google Scholar]
- 15.Cushion MT. Are members of the fungal genus pneumocystis (a) commensals; (b) opportunists; (c) pathogens; or (d) all of the above? PLoS pathogens. 2010;6(9):e1001009. doi: 10.1371/journal.ppat.1001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wakefield AE, Guiver L, Miller RF, Hopkin JM. DNA amplification on induced sputum samples for diagnosis of Pneumocystis carinii pneumonia. Lancet. 1991 Jun 8;337(8754):1378–1379. doi: 10.1016/0140-6736(91)93062-e. [DOI] [PubMed] [Google Scholar]
- 17.Wakefield AE, Pixley FJ, Banerji S, et al. Amplification of mitochondrial ribosomal RNA sequences from Pneumocystis carinii DNA of rat and human origin. Mol Biochem Parasitol. 1990 Nov;43(1):69–76. doi: 10.1016/0166-6851(90)90131-5. [DOI] [PubMed] [Google Scholar]
- 18.Wakefield AE, Pixley FJ, Banerji S, et al. Detection of Pneumocystis carinii with DNA amplification. Lancet. 1990 Aug 25;336(8713):451–453. doi: 10.1016/0140-6736(90)92008-6. [DOI] [PubMed] [Google Scholar]
- 19.Nevez G, Raccurt C, Jounieaux V, Dei-Cas E, Mazars E. Pneumocystosis versus pulmonary Pneumocystis carinii colonization in HIV-negative and HIV-positive patients. AIDS (London, England) 1999 Mar 11;13(4):535–536. doi: 10.1097/00002030-199903110-00020. [DOI] [PubMed] [Google Scholar]
- 20.Wakefield AE, Lindley AR, Ambrose HE, Denis CM, Miller RF. Limited asymptomatic carriage of Pneumocystis jiroveci in human immunodeficiency virus-infected patients. The Journal of infectious diseases. 2003 Mar 15;187(6):901–908. doi: 10.1086/368165. [DOI] [PubMed] [Google Scholar]
- 21.Gutierrez S, Morilla R, Leon JA, et al. High prevalence of Pneumocystis jiroveci colonization among young HIV-infected patients. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2011 Jan;48(1):103–105. doi: 10.1016/j.jadohealth.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi T, Goto M, Endo T, et al. Pneumocystis carinii carriage in immunocompromised patients with and without human immunodeficiency virus infection. J Med Microbiol. 2002 Jul;51(7):611–614. doi: 10.1099/0022-1317-51-7-611. [DOI] [PubMed] [Google Scholar]
- 23.Ponce CA, Gallo M, Bustamante R, Vargas SL. Pneumocystis colonization is highly prevalent in the autopsied lungs of the general population. Clin Infect Dis. 2010 Feb 1;50(3):347–353. doi: 10.1086/649868. [DOI] [PubMed] [Google Scholar]
- 24.Yazaki H, Goto N, Uchida K, Kobayashi T, Gatanaga H, Oka S. Outbreak of Pneumocystis jiroveci pneumonia in renal transplant recipients: P. jiroveci is contagious to the susceptible host. Transplantation. 2009 Aug 15;88(3):380–385. doi: 10.1097/TP.0b013e3181aed389. [DOI] [PubMed] [Google Scholar]
- 25.Vargas SL, Hughes WT, Santolaya ME, et al. Search for primary infection by Pneumocystis carinii in a cohort of normal, healthy infants. Clin Infect Dis. 2001 Mar 15;32(6):855–861. doi: 10.1086/319340. [DOI] [PubMed] [Google Scholar]
- 26.Khodadadi H, Mirhendi H, Mohebali M, Kordbacheh P, Zarrinfar H, Makimura K. Pneumocystis jirovecii Colonization in Non-HIV-Infected Patients Based on Nested-PCR Detection in Bronchoalveolar Lavage Samples. Iranian journal of public health. 2013;42(3):298–305. [PMC free article] [PubMed] [Google Scholar]
- 27.Durand-Joly I, Soula F, Chabe M, et al. Long-term colonization with Pneumocystis jirovecii in hospital staffs: a challenge to prevent nosocomial pneumocystosis. The Journal of eukaryotic microbiology. 2003;50(Suppl):614–615. doi: 10.1111/j.1550-7408.2003.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 28.Vargas SL, Ponce CA, Sanchez CA, Ulloa AV, Bustamante R, Juarez G. Pregnancy and asymptomatic carriage of Pneumocystis jiroveci. Emerging infectious diseases. 2003 May;9(5):605–606. doi: 10.3201/eid0905.020660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gutierrez S, Respaldiza N, Campano E, Martinez-Risquez MT, Calderon EJ, De La Horra C. Pneumocystis jirovecii colonization in chronic pulmonary disease. Parasite. 2011 May;18(2):121–126. doi: 10.1051/parasite/2011182121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Probst M, Ries H, Schmidt-Wieland T, Serr A. Detection of Pneumocystis carinii DNA in patients with chronic lung diseases. Eur J Clin Microbiol Infect Dis. 2000 Aug;19(8):644–645. doi: 10.1007/s100960000329. [DOI] [PubMed] [Google Scholar]
- 31.Morris A, Alexander T, Radhi S, et al. Airway obstruction is increased in pneumocystis-colonized human immunodeficiency virus-infected outpatients. Journal of clinical microbiology. 2009 Nov;47(11):3773–3776. doi: 10.1128/JCM.01712-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris A, Sciurba FC, Lebedeva IP, et al. Association of chronic obstructive pulmonary disease severity and Pneumocystis colonization. American journal of respiratory and critical care medicine. 2004 Aug 15;170(4):408–413. doi: 10.1164/rccm.200401-094OC. [DOI] [PubMed] [Google Scholar]
- 33.Morris AM, Huang L, Bacchetti P, et al. Permanent declines in pulmonary function following pneumonia in human immunodeficiency virus-infected persons. The Pulmonary Complications of HIV Infection Study Group. American journal of respiratory and critical care medicine. 2000 Aug;162(2 Pt 1):612–616. doi: 10.1164/ajrccm.162.2.9912058. [DOI] [PubMed] [Google Scholar]
- 34.George MP, Kannass M, Huang L, Sciurba FC, Morris A. Respiratory symptoms and airway obstruction in HIV-infected subjects in the HAART era. PloS one. 2009;4(7):e6328. doi: 10.1371/journal.pone.0006328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varela JM, Respaldiza N, Sanchez B, et al. Lymphocyte response in subjects with chronic pulmonary disease colonized by Pneumocystis jirovecii. The Journal of eukaryotic microbiology. 2003;50(Suppl):672–673. doi: 10.1111/j.1550-7408.2003.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 36.Shipley TW, Kling HM, Morris A, et al. Persistent Pneumocystis colonization leads to the development of chronic obstructive pulmonary disease (COPD) in a non-human primate model of AIDS. The Journal of infectious diseases. 2010;202(2):302–312. doi: 10.1086/653485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norris KA, Morris A, Patil S, Fernandes E. Pneumocystis colonization, airway inflammation, and pulmonary function decline in acquired immunodeficiency syndrome. Immunologic research. 2006;36(1-3):175–187. doi: 10.1385/IR:36:1:175. [DOI] [PubMed] [Google Scholar]
- 38.Christensen PJ, Preston AM, Ling T, et al. Pneumocystis murina infection and cigarette smoke exposure interact to cause increased organism burden, development of airspace enlargement, and pulmonary inflammation in mice. Infection and immunity. 2008 Aug;76(8):3481–3490. doi: 10.1128/IAI.00165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kling HM, Shipley TW, Patil S, Morris A, Norris KA. Pneumocystis colonization in immunocompetent and simian immunodeficiency virus-infected cynomolgus macaques. The Journal of infectious diseases. 2009 Jan 1;199(1):89–96. doi: 10.1086/595297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kling HM, Shipley TW, Patil SP, et al. Relationship of Pneumocystis jiroveci humoral immunity to prevention of colonization and chronic obstructive pulmonary disease in a primate model of HIV infection. Infection and immunity. 2010 Oct;78(10):4320–4330. doi: 10.1128/IAI.00507-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Board KF, Patil S, Lebedeva I, et al. Experimental Pneumocystis carinii pneumonia in simian immunodeficiency virus-infected rhesus macaques. The Journal of infectious diseases. 2003 Feb 15;187(4):576–588. doi: 10.1086/373997. [DOI] [PubMed] [Google Scholar]
- 42.Croix DA, Board K, Capuano S, 3rd, et al. Alterations in T lymphocyte profiles of bronchoalveolar lavage fluid from SIV- and Pneumocystis carinii-coinfected rhesus macaques. AIDS research and human retroviruses. 2002 Mar 20;18(5):391–401. doi: 10.1089/088922202753519179. [DOI] [PubMed] [Google Scholar]
- 43.Research IfLA, Sciences CoL, Council NR. 8. Washington DC: National Academies Press; 2010. Guide for the care and use of laboratory animals. [Google Scholar]
- 44.Animal Welfare Act, as Amended. US Government Printing Office; 2009. [Google Scholar]
- 45.Reimann KA, Li JT, Veazey R, et al. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. Journal of virology. 1996 Oct;70(10):6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pawar SN, Mattila JT, Sturgeon TJ, et al. Comparison of the effects of pathogenic simian human immunodeficiency virus strains SHIV-89.6P and SHIV-KU2 in cynomolgus macaques. AIDS research and human retroviruses. 2008 Apr;24(4):643–654. doi: 10.1089/aid.2007.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patil SP, Board KF, Lebedeva IP, Norris KA. Immune responses to Pneumocystis colonization and infection in a simian model of AIDS. The Journal of eukaryotic microbiology. 2003;50(Suppl):661–662. doi: 10.1111/j.1550-7408.2003.tb00675.x. [DOI] [PubMed] [Google Scholar]
- 48.Pickering LK, editor. Tables of Antibacterial Drug Dosages. Red Book: 2003 Report of the Committee on Infectious Diseases. 26. Vol. 2003. Elk Grove Village, IL: American Academy of Pediatrics; 2003. pp. 699–712. [Google Scholar]
- 49.Proskocil BJ, Sekhon HS, Clark JA, et al. Vitamin C prevents the effects of prenatal nicotine on pulmonary function in newborn monkeys. American journal of respiratory and critical care medicine. 2005 May 1;171(9):1032–1039. doi: 10.1164/rccm.200408-1029OC. [DOI] [PubMed] [Google Scholar]
- 50.Conover WJ, Iman RL. Rank Transformations as a Bridge between Parametric and Nonparametric Statistics. Am Stat. 1981;35(3):124–129. [Google Scholar]
- 51.Morris A, Netravali M, Kling HM, et al. Relationship of pneumocystis antibody response to severity of chronic obstructive pulmonary disease. Clin Infect Dis. 2008 Oct 1;47(7):e64–68. doi: 10.1086/591701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morris A, Sciurba FC, Norris KA. Pneumocystis: a novel pathogen in chronic obstructive pulmonary disease? Copd. 2008 Feb;5(1):43–51. doi: 10.1080/1541255070181756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sethi S. Bacterial infection and the pathogenesis of COPD. Chest. 2000 May;117(5 Suppl 1):286S–291S. doi: 10.1378/chest.117.5_suppl_1.286s. [DOI] [PubMed] [Google Scholar]
- 54.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. The New England journal of medicine. 2008 Nov 27;359(22):2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 55.Leigh TR, Kangro HO, Gazzard BG, Jeffries DJ, Collins JV. DNA amplification by the polymerase chain reaction to detect sub-clinical Pneumocystis carinii colonization in HIV-positive and HIV-negative male homosexuals with and without respiratory symptoms. Respiratory medicine. 1993 Oct;87(7):525–529. doi: 10.1016/0954-6111(93)90008-n. [DOI] [PubMed] [Google Scholar]
- 56.Vidal S, de la Horra C, Martin J, et al. Pneumocystis jirovecii colonisation in patients with interstitial lung disease. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2006 Mar;12(3):231–235. doi: 10.1111/j.1469-0691.2005.01337.x. [DOI] [PubMed] [Google Scholar]
- 57.Thomas M, Rupali P, Woodhouse A, Ellis-Pegler R. Good outcome with trimethoprim 10 mg/kg/day-sulfamethoxazole 50 mg/kg/day for Pneumocystis jirovecii pneumonia in HIV infected patients. Scandinavian journal of infectious diseases. 2009;41(11-12):862–868. doi: 10.3109/00365540903214256. [DOI] [PubMed] [Google Scholar]
- 58.Kaplan JE, Masur H, Holmes KK. Guidelines for preventing opportunistic infections among HIV-infected persons--2002. Recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 2002 Jun 14;51(RR-8):1–52. [PubMed] [Google Scholar]
- 59.Sandford AJ, Silverman EK. Chronic obstructive pulmonary disease. 1: Susceptibility factors for COPD the genotype-environment interaction. Thorax. 2002 Aug;57(8):736–741. doi: 10.1136/thorax.57.8.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martinez CH, Han MK. Contribution of the environment and comorbidities to chronic obstructive pulmonary disease phenotypes. Med Clin North Am. 2012 Jul;96(4):713–727. doi: 10.1016/j.mcna.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gingo MR, George MP, Kessinger CJ, et al. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. American journal of respiratory and critical care medicine. 2010 Sep 15;182(6):790–796. doi: 10.1164/rccm.200912-1858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morris A, George MP, Crothers K, et al. HIV and chronic obstructive pulmonary disease: is it worse and why? Proceedings of the American Thoracic Society. 2011 Jun;8(3):320–325. doi: 10.1513/pats.201006-045WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. 2011 Sep 10;378(9795):1015–1026. doi: 10.1016/S0140-6736(11)60988-4. [DOI] [PubMed] [Google Scholar]
- 64.Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. American journal of respiratory and critical care medicine. 1996 Feb;153(2):530–534. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- 65.Di Stefano A, Capelli A, Lusuardi M, et al. Severity of airflow limitation is associated with severity of airway inflammation in smokers. American journal of respiratory and critical care medicine. 1998 Oct;158(4):1277–1285. doi: 10.1164/ajrccm.158.4.9802078. [DOI] [PubMed] [Google Scholar]
- 66.Saetta M, Di Stefano A, Turato G, et al. CD8+ T-lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 1998 Mar;157(3 Pt 1):822–826. doi: 10.1164/ajrccm.157.3.9709027. [DOI] [PubMed] [Google Scholar]
- 67.Gigliotti F, Haidaris CG, Wright TW, Harmsen AG. Passive intranasal monoclonal antibody prophylaxis against murine Pneumocystis carinii pneumonia. Infection and immunity. 2002 Mar;70(3):1069–1074. doi: 10.1128/IAI.70.3.1069-1074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gigliotti F, Hughes WT. Passive immunoprophylaxis with specific monoclonal antibody confers partial protection against Pneumocystis carinii pneumonitis in animal models. The Journal of clinical investigation. 1988 Jun;81(6):1666–1668. doi: 10.1172/JCI113503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harmsen AG, Chen W, Gigliotti F. Active immunity to Pneumocystis carinii reinfection in T-cell-depleted mice. Infection and immunity. 1995 Jul;63(7):2391–2395. doi: 10.1128/iai.63.7.2391-2395.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wells J, Haidaris CG, Wright TW, Gigliotti F. Active immunization against Pneumocystis carinii with a recombinant P. carinii antigen. Infection and immunity. 2006 Apr;74(4):2446–2448. doi: 10.1128/IAI.74.4.2446-2448.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


