Abstract
Aged ovariectomized female monkeys, a model for menopause in humans, show declines in spine density in the dlPFC and diminished performance in cognitive tasks requiring this brain region. Previous studies in our laboratory have shown that long-term cyclic treatment with 17β-estradiol (E) produces an increase in spine density and in the proportion of thinner spines in layer III pyramidal neurons in the dorsolateral prefrontal cortex (dlPFC) of both young and aged ovariectomized rhesus monkeys. Here we used 3D reconstruction of Lucifer yellow-loaded neurons to investigate whether clinically relevant schedules of hormone therapy would produce similar changes in prefrontal cortical neuronal morphology as long-term cyclic E treatment in young female monkeys. We found that continuously delivered E, with or without a cyclic progesterone treatment, did not alter spine density or morphology in the dlPFC of young adult OVX rhesus monkeys. We also found that the increased density of thinner spines evident in the dlPFC 24 hours after E administration in the context of long-term cyclic E therapy is no longer detectable 20 days after E treatment. When compared with the results of our previously published investigations, our results suggest that cyclic fluctuations in serum E levels may cause corresponding fluctuations in the density of thin spines in the dlPFC. By contrast, continuous administration of E does not support sustained increases in thin spine density. Physiological fluctuations in E concentration may be necessary to maintain the morphological sensitivity of the dlPFC to E.
Keywords: Hormone replacement therapy, aging, primate, menopause, dendritic spine, dorsolateral prefrontal cortex, Prefrontal cortex, estrogen, progesterone
1. Introduction
Levels of estrogens drop off precipitously in women as they go through menopause, and age-related cognitive decline can begin in the decade following the typical age of menopause. This decline is particularly apparent on tasks that rely on the dorsolateral prefrontal cortex (dlPFC), such as those that emphasize working memory and cognitive flexibility (Drogos et al., 2013; Weber et al., 2013). The interaction between this loss of estrogens and the risk of decline in cognitive function is not well understood, and studies designed to explore the cognitive benefits of hormone therapy in women have yielded conflicting results. Although some laboratory studies and randomized clinical trials have found that initiation of hormone replacement therapy (HRT) during perimenopause or soon after the menopausal transition can improve cognitive function (Carlson et al., 2001; Keenan et al., 2001) and reduce a woman's risk of developing cognitive impairment or dementia later in life (Kimura, 1995; Matthews et al., 1999; Carlson et al., 2001; Zandi et al., 2002; Bagger et al., 2005; Henderson et al., 2005; Greendale et al., 2009), others have found that initiation of HRT more than a few years after menopause is associated with an unchanged or increased risk of dementia and age-associated cognitive decline (Matthews et al., 1999; Shumaker et al., 2003, 2004; Henderson et al., 2005; MacLennan et al., 2006), and several randomized clinical trials have found equivocal or negative effects of HRT on cognitive function, even when initiated soon after menopause (reviewed in Maki and Sundermann, 2009).
One factor that may contribute to these discrepancies is the fact that menopausal women are most commonly prescribed a continuous regimen of one or more estrogens with or without a progestin. There is evidence from rodent studies that treatments consisting of a continuous dose of 17β-estradiol (E), the predominant active estrogen in young women (Stricker et al., 2006), may be less effective in enhancing cognitive function than are treatments that provide E on a cyclical schedule, i.e., one dose of E per cycle length (Markowska and Savonenko, 2002). We have previously reported that cyclical E treatment with one dose of E every 21 days for 2-3 years with improves the performance of aged OVX female rhesus monkeys on dlPFC-dependent tasks (Rapp et al., 2003) and that the same schedule of cyclic E treatment given for 3 weeks or for 2-3 years increases the density of dendritic spines on dlPFC pyramidal neurons in both young and aged animals (Tang et al., 2004; Hao et al., 2006, 2007). Higher levels of spine and synapse density in the dlPFC have been found to correlate with preservation of dlPFC function in aging rhesus monkeys (Peters et al., 1998; Dumitriu et al., 2010).
In order to determine whether treatment schedule affects the ability of E to alter dlPFC neuronal morphology, the present study examined whether continuous E therapy, with or without progesterone, is effective at increasing thin spine density in the dlPFC of young OVX monkeys. We also examined whether thin spine density in the dlPFC falls during the interval between injections in OVX monkeys receiving cyclical E therapy. We found that continuous E treatment fails to trigger an increase in spine density, and that the presence or absence of a cyclic progesterone treatment component does not affect this result. Additionally, we found that spine density in cyclic E-treated animals does decrease between E treatments when circulating E levels are low, and is indistinguishable from that of vehicle-treated animals by 20 days post-E administration.
2. Experimental Procedures
2.1. Animals
Twenty young adult female Rhesus monkeys (Macaca mulatta; age range, 7.6-14.7 years old; mean age ± SEM, 10.1 years ± 6.8 months) were used in this study. Animals were singly housed in colonies of 40 individuals under conditions identical to those used in previous studies (Rapp et al., 2003; Hao et al., 2006, 2007), and water and monkey chow were provided in excess of nutritional needs. All monkeys received bilateral OVX prior to the initiation of hormone therapy as in (Rapp et al., 2003). Briefly, animals were sedated with ketamine (10 mg/kg) and atropine (0.04 mg/kg), intubated, and placed on isoflurane anesthesia. After the ovaries were removed, the abdominal wall was closed in layers, and animals were observed until responsive. Oxymorphone (1.5 mg/kg) was administered 3 times/day for 2 days for postoperative analgesia. The mean duration between OVX and the beginning of treatment was 51 days (range 42-57 days). All experiments were conducted in compliance with the National Institutes of Health Guidelines for the Care and Use of Experimental Animals approved by the Institutional Animal Care and Use Committee at the University of California, Davis and Mount Sinai School of Medicine.
2.2. Hormone treatment
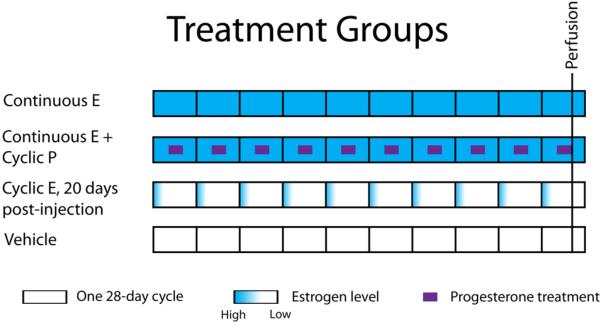
Five animals were randomly assigned to each of four treatment groups (Figure 1), which were as follows:
- Continuous E
- - Animals in this group received a continuous regimen of unopposed 17β-estradiol (E). Each animal was sedated with ketamine (10 mg/kg) and medetomidine (0.2-0.4 mg/kg) and subcutaneously implanted with two 6.5 cm lengths of Silastic tubing (inner diameter, 3.4 mm; outer diameter, 6.4 mm; Dow Corning, Midland, MI), each containing a 5 cm column of packed crystalline 17β-estradiol (Sigma-Aldritch, St. Louis, MO). These were designed to produce a target circulating estradiol level of approximately 80 pg/mL, corresponding to the mean serum E level in cycling rhesus monkeys during the luteal and mid-follicular phases of the menstrual cycle (Walker et al., 1983; Monfort et al., 1987).
- Continuous E + Cyclic P
- - Animals in this group received a continuous regimen of 17β-estradiol with a cyclical regimen of progesterone. Each animal was implanted with E-filled Silastic tubing in a manner identical to the animals in the Continuous E group. Each animal additionally received a regimen of one 100 mg capsule of progesterone (P) administered orally once per day on days 11-20 of a 28-day treatment cycle. This regimen was designed to mimic the broad progesterone peak in the luteal phase and near-absence of circulating P in the follicular phase of the natural rhesus menstrual cycle (Walker et al., 1983; Monfort et al., 1987).
- Cyclic E, cycle day 20
- - Each animal received a single injection of estradiol cypionate (100 µg in 1 ml of sterile peanut oil, IM; Andersham Pharmacia, Peapack, NJ) on day 1 of each 28-day cycle. This is the same dose of the same hormone used in previous studies, though on a 28-day rather than a 21-day cycle (Rapp et al., 2003; Hao et al., 2006, 2007), and has been shown to produce a peak in circulating E levels averaging nearly 300 pg/ml within 9 hours, which falls to zero over the next several days (Rapp et al., 2003). This regimen is designed to mimic the preovulatory surge in serum E levels present in cycling monkeys, which rise to a comparable level (266.3 ± 30.4 pg/ml per Monfort et al., 1987) before falling to baseline values over the course of approximately 2-4 days. Estradiol cypionate, which is cleaved i produce free 17β-estradiol, was used in place of 17β-estradiol for injection, as the cleavage step allows it to be absorbed more slowly, resulting in serum 17β-estradiol levels that more closely mimic the level and duration of the natural preovulatory E surge. Each animal was also implanted with two empty 6.5 cm lengths of Silastic tubing. In contrast to previous studies (Hao et al., 2007), in which animals were sacrificed 24 hours after their last E treatment, these animals were sacrificed 20 days after their final E injection.
- Vehicle
- - Animals in this group received a single injection of peanut oil vehicle on day 1 of each 28-day cycle and were implanted with two empty 6.5 cm lengths of Silastic tubing.
Figure 1. Hormone therapy treatment groups.
All hormone therapy was delivered using a calendar of ten 28-day treatment periods, each representing one menstrual “cycle.” The Continuous E group received a single, steady dose of estradiol through subcutaneously implanted Silastic capsules for the duration of the study. The Continuous E + Cyclic P group received the same continuous estradiol treatment, with the addition of an oral dose of progesterone taken daily on days 10-19 of each 28-day treatment cycle. The Cyclic E group received an injection of estradiol intramuscularly on the first day of each 28-day treatment cycle. The Vehicle group received an injection of oil vehicle on the first day of each 28-day treatment cycle. All animals were perfused on day 20 of the tenth treatment cycle.
Treatment continued for the equivalent of eight 28-day cycles. Silastic capsules were replaced under anesthesia on days 84 and 168 of treatment, with perfusion occurring on day 216. All animals were perfused on day 20 of the final treatment cycle, coinciding with the last day of P administration in the Continuous E + Cyclic P group, and 20 days after the last E injection in the Cyclic E group. All animals received an equivalent duration of E or vehicle treatment. The efficacy of OVX and hormone treatment was confirmed by measurement of serum levels of E and P using competitive chemiluminescent immunoassays as in (Lasley et al., 2012). Measurements were taken before OVX, after OVX before the start of treatment, at 18 time points spaced over the course of treatment, and at the time of perfusion.
2.3. Quantitative analysis of dendritic arborization, spine density, and spine morphology
Animals were perfused on the last day of treatment according to methods previously described (Hao et al., 2006). Briefly, each animal was deeply anesthetized with ketamine (25 mg/kg) and pentobarbital sodium (20-35 mg/kg), intubated, and mechanically ventilated. After 1.5 ml of 0.5% sodium nitrate was injected into the left ventricle of the heart, the descending aorta was clamped, and the animal was perfused transcardially with ice-cold paraformaldehyde in PBS (1% for 1 min followed by 4% for 12 min). Each brain was dissected into multiple standardized blocks, which were postfixed for 6 h in a solution of 4% paraformaldehyde and 0.125% glutaraldehyde in PBS. The frontal block, containing all of Brodmann's area 46, was cut on a Vibratome into 400 μm-thick sections, each spaced 2.6 mm apart. In this manner, a systematic random series of 8-12 sections throughout area 46 was obtained for intracellular injection of Lucifer yellow (Invitrogen).
Intracellular injection of layer III pyramidal cells with Lucifer yellow was performed according to methods previously described (Hao et al., 2006, 2007). Sections were stained with 4,6-diamidino-2-phenylindole (DAPI) to enable visualization of neuronal cell bodies and mounted on filter paper immersed in 0.1M PBS. Pyramidal cells in layer III of area 46 were identified using epifluorescence under a UV filter, and the cell bodies were impaled with sharp micropipettes and filled with 5% Lucifer yellow in dH2O for five to ten minutes under a 3-5 nA direct current. Approximately 4-6 neurons were so loaded per slice of tissue, sufficiently far apart to ensure no crossing of dendritic arbors. Sections containing loaded neurons were washed and mounted on slides with VectaShield mounting medium (Vector Laboratories, Burlingame, CA).
All filled neurons had somata located in layer III and within the boundaries of area 46, as determined by analysis after counterstaining selected slices with DAPI. Neurons used for analysis had completely filled dendritic trees, as demonstrated by well-defined endings, and were located sufficiently far away from other loaded cells to unambiguously determine the origin of every dendritic segment. Three animals, two from the vehicle group and one from the combined estradiol/progesterone group, lacked neurons meeting the above-specified criteria and were excluded from analysis.
In order to ensure systematic-random sampling of dendritic segments, well-filled neurons were first imaged at low magnification, and concentric circles 60 and 120 μm in diameter were drawn around the cell body. Only those dendritic segments intersecting these circles were used for high-magnification imaging and analysis. Six apical and six basal secondary or tertiary dendritic segments were imaged on each of five cells per animal at these points of intersection. Each dendritic segment was imaged in a sequential z-stack using a Zeiss LSM 510 confocal microscope (Carl Zeiss, Oberkochen, Germany) using a Plan-Apochromat 100x oil-immersion objective and a 488-nanometer Ar/Kr laser. Care was taken to completely image each dendritic segment, including a comfortable margin of whitespace on all sides. Image stacks were deconvolved with AutoDeblur (Media Cybernetics, Silver Spring, MD), and loaded into NeuronStudio software (Rodriguez et al., 2008) for automated measurement of spine length, head diameter, and head volume.
Spines were classified into “thin,” “mushroom,” and “other” types based on previously published criteria (Sorra and Harris, 2000; Dumitriu et al., 2010). All spines with a head diameter ≥ 0.6 μm were classified as mushroom spines. Spines with a head diameter < 0.6 μm were classified as thin spines if the maximum spine length was at least twice the head diameter. Spines meeting neither criterion were classified as “other.” To facilitate comparison, all analyses involving spine type were repeated using the criteria from Ohm et al., 2012 (spines with a ratio of head diameter to neck diameter of > 1.1 classified as thin if the head diameter was < 0.47 μm and as mushroom if the head diameter was > 0.47 μm, all other spines classified as “other”) with no change in results.
For analysis of dendritic arborization, five pyramidal neurons from each animal were visualized under a 40x objective and traced and reconstructed in 3D using NeuroLucida software (MicroBrightField, Williston, VT). Analyses of total dendritic length, number of branch points, and Sholl analyses (Sholl, 1953) were performed for each traced neuron using NeuroExplorer software (Nex Technologies).
2.4. Statistical analysis
As all variables tested were normally distributed according to Shapiro-Wilk tests (p > 0.1 for all variables), statistical analyses were performed using one-way ANOVA, followed by Bonferonni post hoc tests where appropriate, to assess possible differences in mean spine density, spine head diameter, dendritic length, and dendritic branch numbers between groups. Sholl analysis of the dendritic tree was performed using a two-way mixed-model repeated-measures ANOVA with treatment group as the between-subjects factor and distance from the soma (in 30 μm increments) as the within-subjects factor, followed by Bonferonni post hoc tests. All treatment groups were included in every analysis. The values are shown as means ± SEM, calculated based on one aggregate (average) value per cell. We elected to perform a cell-level rather an animal-level analysis because analysis of the variance of our core measures showed that the level of variation among cells within an animal was as great or greater than the level of variation among cells from two different animals within a treatment group. For example, the variances for spine density were as follows: (listed as measure, mean intra-animal variance vs. mean inter-animal within-group variance, ± SEM, in spines/um3) spine density, 0.220 ± 0.023 vs. 0.164 ± 0.058; thin spine density, 0.174 ± 0.019 vs. 0.151 ± 0.047; mushroom spine density, 0.049 ± 0.004 vs. 0.031 ± 0.009; stubby spine density, 0.041 ± 0.004 vs. 0.021 ± 0.003. Unless otherwise specified, all reported p values correspond to the main effect of treatment for each dependent measure. Observed power was calculated in all ANOVA to confirm that the sample size was sufficient to support the data. For changes in spine density, there was power of 0.8 to detect a change of 25%, approximately the effect size observed 24 hours after cyclic E administration in young animals in Hao et al., 2007. The statistical significance level was set at p < 0.05.
3. Results
3.1. Estradiol and progesterone levels
After ovariectomy, median serum E levels ranged from 0-35 pg/ml and P levels ranged from 0.5-1.9 ng/ml in all groups, consistent with cessation of ovarian activity. In the vehicle-treated group, median E levels remained undetectable and median P levels ranged from 1.0-1.3 ng/ml across the entire post-OVX study period. In the three groups not receiving progesterone, median P levels ranged between 0.6-1.4 ng/ml through the study. In the Continuous E + Cyclic P group, median P levels ranged from 3.7-7.8 ng/ml during P treatment, and from 0.7-0.9 ng/ml at all other times, mimicking the reported range of ~2.0-6.9 ng/ml during the luteal phase and ≤ 0.5 ng/ml during the follicular phase of the natural menstrual cycle (Monfort et al., 1987). Median serum E levels in the cyclic E-treated group ranged from 28 – 118 pg/ml when measured 48 hours post-injection and from 0 – 28 pg/ml when measured 9-28 days post-injection. In the two continuous E-treated groups, median serum E levels ranged between 75-160 pg/ml throughout the study. This is higher than the normal physiological average of ~70-80 pg/ml for baseline E levels present during the follicular and luteal phases of the menstrual cycle, but below the levels seen during the preovulatory surge (Walker et al., 1983; Monfort et al., 1987). Changes of the Silastic implants produced brief spikes in circulating E levels to between 205-820 pg/ml two days post-implant, with median circulating levels falling below 200 pg/ml by 9 days post-implant. Implants were changed twice during the experiment, and perfusion occurred 48 days after the last implant change. At the time of perfusion, median serum E and P levels were as follows: Vehicle group: 0 pg/ml E, 0.8 ng/ml P; Cyclic E, 20 days post-injection group: 5 pg/ml E, 1.3 ng/ml P; Continuous E group: 100 pg/ml E, 0.6 ng/ml P; Continuous E + Cyclic P group: 108 pg/ml E, 5.4 ng/ml P.
3.2. Effect of continuous 17β-estradiol treatment, with or without the addition of cyclic progesterone, on dendritic arbors in area 46
There was no significant effect of treatment on total apical (p = 0.06) or basal (p > 0.4) dendritic length, though a trend towards higher total apical dendritic length in the vehicle group resembles the higher dendritic length observed in the young vehicle-treated group compared to the cyclic E-treated group in Hao et al., 2007. Sholl analysis of the apical dendrites revealed a significant effect of distance from soma (p < 0.0005), no significant effect of treatment condition (p = 0.06), and a significant interaction (p = 0.04). Post hoc analysis revealed that the cyclic E group had lower dendritic length than the continuous E group at 30 μm from the soma (p = 0.04) and that the vehicle group had lower dendritic length than all three other groups at 420 μm from the soma (continuous E, p = 0.02; continuous E + cyclic P, p = 0.04; cyclic E, 20 days post-injection, p = 0.02). Analysis of the basal dendrites revealed a significant effect of distance from soma (p < 0.0005), with no effect of group (p > 0.4) or interaction (p > 0.4).
3.3. Continuous E, with or without cyclic P, does not alter dendritic spine density in area 46
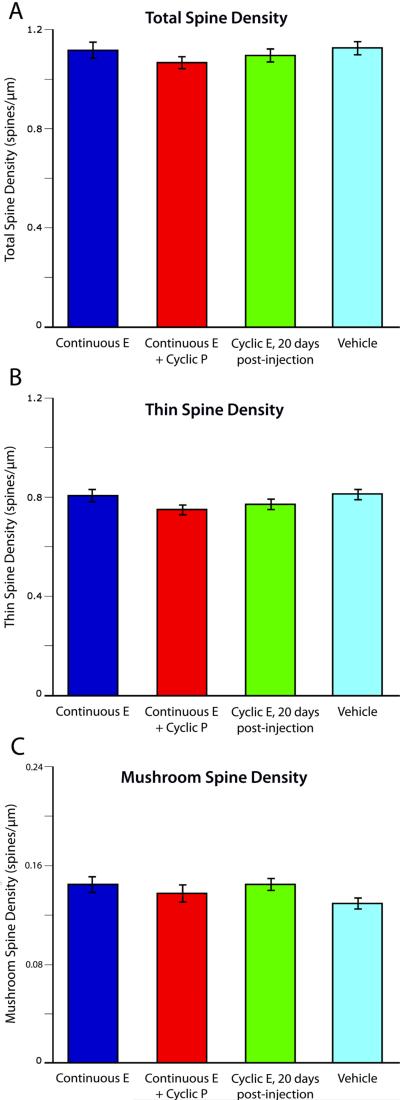
Previously, our laboratory reported a nearly 25% increase in apical and basal dendritic spine density 24 hours after E administration in the context of long-term cyclical E replacement in young OVX monkeys (Hao et al., 2007). However, despite effective hormone delivery and achievement of comparable circulating E levels at perfusion to those in Hao et al., we found that a continuous regimen of E treatment, with or without cyclic P, failed to alter spine density in the dlPFC (mean spines per micrometer: Continuous E, 1.12 +/- 0.07; Continuous E + Cyclic P, 1.07 +/- 0.05; vehicle alone, 1.13 +/- 0.06; p > 0.9). As expected, the effect of cyclic E was gone by day 20 post-injection (Figure 2A).
Figure 2. Dendritic spine density in area 46.
Though previous results have found that cyclic E treatment increases dendritic spine density and the proportion of thinner spines when measured 24 hours after injection (Hao et al., 2007), we found that continuous E treatment, with or without cyclic P, did not increase total (a), thin (b), or mushroom (c) spine density on layer III neurons in area 46. Twenty days after the final cyclic E treatment, total, thin, and mushroom spine density are indistinguishable from those in vehicle-treated animals.
3.4. Continuous E, with or without cyclic P, does not alter dendritic spine morphology in area 46
Our laboratory has also reported there is a shift in dendritic spine head diameter favoring smaller spines 24 hours after an E injection in young OVX monkeys receiving long-term cyclic E replacement (Hao et al., 2007). In contrast, we found that continuous E treatment, with or without cyclic P, had no effect on mean spine head diameter (p > 0.4), mean spine head volume (p > 0.3) or on the distribution of spine head diameters (p > 0.3) or volumes (p > 0.1).
As previous studies showed that that cyclic E treatment increases the proportion of spines with smaller head diameters (Hao et al., 2007), we tested whether there was a change in the density or morphology of any particular class of spines. We divided spines into thin, mushroom, or “other” types based on the shape of each individual spine (see Methods). We found that there was no change in thin spine density (p > 0.8) (Figure 2B), mushroom spine density (p > 0.8) (Figure 2C), thin spine head diameter (p > 0.3), mushroom spine head diameter (p > 0.5), thin spine head volume (p > 0.1) or mushroom spine head volume (p > 0.8) in animals receiving continuous E or continuous E + cyclic P treatment compared to vehicle-treated animals.
3.5. Fluctuation of E-induced morphological changes in area 46 over an E treatment cycle
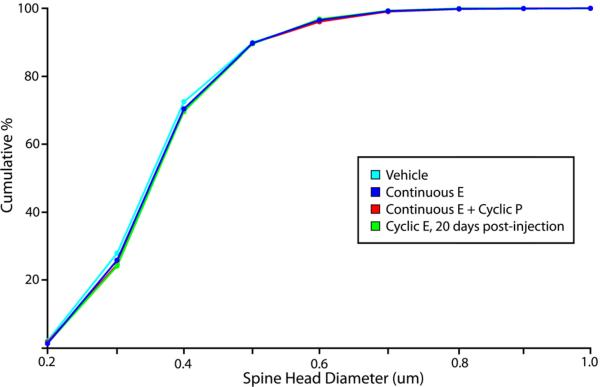
Our laboratory previously reported that cyclic E treatment produces a substantial increase in thin spine density in the dlPFC of young OVX rhesus monkeys when animals are perfused 24 hours after their last E injection (Hao et al., 2007). To test the effect of cyclical changes in E levels on dlPFC pyramidal neurons, we repeated this experiment with cyclically treated young OVX rhesus monkeys perfused 20 days after the final injection of E. We found that the morphological changes evident in the NHP dlPFC 24 hours after an injection of E are not present after 20 days, when E levels have returned to baseline. At this time point, both dendritic spine density (p > 0.9) (Figure 2) and the distribution of spine head diameters (p > 0.3) (Figure 3) and volumes (p > 0.3) are indistinguishable from those in vehicle-treated animals.
Figure 3. Dendritic spine morphology in area 46.
Previous results showed that, 24 hours after injection, cyclic E administration in both young OVX primates led to a left shift in the cumulative distribution curve for spine head diameter, indicating a greater proportion of thinner spines in the E-treated animals (Hao et al., 2007). We found that continuous E treatment, with or without cyclic P, does not increase the proportion of thinner spines in the dlPFC of young OVX animals, despite reaching similar levels of circulating E. We also found that, 20 days after the final E injection in the context of long-term cyclic E replacement, the distribution of spine head diameters in the dlPFC is not significantly different from that seen in vehicle-treated animals.
4. Discussion
This study was designed to examine the effects of cyclical and continuous E administration on dlPFC neurons in young NHPs. Previous results from our laboratory showed that dendritic spine density, and in particular, the density of thin dendritic spines, rises by over 20% 24 hours after an E injection (Hao et al., 2007). Our analysis yielded two interesting results. First, we found evidence that this previously noted increase in spine density in the dlPFC may not be maintained in cyclically treated animals over the course of the menstrual cycle. Rather, by 20 days post-injection, when circulating E levels are equivalent to those in vehicle-treated animals, dlPFC total and thin spine densities are also equivalent to those in vehicle-treated animals. It is worth noting, however, that the animals in the present study were treated with one E injection every 28 days, while the animals in the Hao 2007 study received an E injection once every 21 days. It is possible that the 28-day injection cycle, while more closely mimicking the natural cycle, fails either to produce or to maintain an increase in spine density which would be present in animals treated every 21 days.
Second, we found that long-term continuous E therapy fails to alter dendritic spine density or spine morphology in the dlPFC of young OVX monkeys. This second result is surprising given how, in other studies, spine density has been found to closely track circulating levels of estradiol. For example, in young, cycling female rats, spine density in area CA1 of the hippocampus rises and falls over the course of the estrous cycle, paralleling the rising and falling circulating E levels (Woolley et al., 1990). This effect is also seen in young OVX female rats treated with a cyclical course of E replacement: dendritic spine density in CA1 peaks when circulating E levels are high, and falls when circulating E levels are low (Woolley and McEwen, 1993). Indeed, the results of this study, in the context of previous findings, showed that monkeys treated with a dose of E show an increase in dlPFC spine density at 24 hours (Hao et al., 2007) that returns to baseline by 20 days post-injection. Given this sensitivity of dlPFC spine density to changes in serum E concentration, one might expect that consistent elevation of circulating E levels would produce a consistent elevation in spine density levels in the dlPFC.
However, our results show that, after approximately nine months of continuously elevated circulating E levels, spine density in the dlPFC of animals treated with continuous E is indistinguishable from that in animals treated with vehicle alone, in whom circulating E levels are nearly undetectable. This is especially striking in light of the fact that these continuously treated animals had circulating E levels at perfusion comparable to those in the cyclically treated animals in Hao et al., 2007 at their time of perfusion, 24 hours post-E administration. These results in young monkeys are consistent with recently published data in aged OVX female rhesus monkeys, which also failed to exhibit increased spinogenesis with chronic E treatment (Ohm et al., 2012). Our current thinking is that there may be some homeostatic mechanism that acts to decouple dlPFC spine density from serum E concentrations in response to a continuous, static elevation to high, but physiological, levels. Observations from rodent studies provide a possible mechanism for this effect, as prolonged continuous E treatment in OVX rats leads to a decrease in estrogen receptor mRNA and protein levels in several brain regions (Simerly and Young, 1991; Blaustein, 1993; DonCarlos et al., 1995; Brown et al., 1996; Bondar et al., 2009), though further research will be necessary to test the specific effects of continuous E exposure on estrogen receptors in primate dlPFC synapses. Our results support the idea that, at least from the perspective of dlPFC neuronal morphology, continuous E treatment may be no better than no E treatment at all. These results emphasize the importance of considering the schedule of hormone delivery in the attempt to create a hormone therapy regimen that will optimally protect cognitive function in aging women.
In conclusion, our study shows that continuous administration of E does not support a sustained increase in spine density or the proportion of thin spines in the dlPFC of young OVX rhesus monkeys. Rather, with continuous E administration, there is a return to baseline of these measures, possibly due to desensitization of the dlPFC to the effects of E. Therefore, cyclic, rather than continuous, administration of E may be required to support the periodic increases in thin spine density associated with improvements in cognitive function.
Continuous estradiol does not alter spine density or morphology in macaque dlPFC
Adding cyclic progesterone to continuous estradiol does not affect dlPFC spines
dlPFC spine density returns to baseline by 20 days post-estrogen injection
Acknowledgements
We would like to thank Dr. Dani Dumitriu for her assistance in loading neurons used in this study. We would also like to thank the staff at the University of California at Davis, National Primate Research Center for their hard work in caring for the animals used in this study. Finally, we would like to thank the National Institute of Aging for their funding of this work (P01AG016765).
Abbreviations
- NHP
nonhuman primate
- dlPFC
dorsolateral prefrontal cortex
- OVX
ovariectomized
- DR
delayed response
- DNMS
delayed nonmatching-to-sample
- WCST
Wisconsin card sorting task
- HRT
hormone replacement therapy
- E
estradiol
- P
progesterone
- DAPI
4,6-diamidino-2-phenylindole
- PBS
phosphate-buffered saline
- UV
ultraviolet
- ANOVA
analysis of variance
- SEM
standard error of the mean
- CA1
Cornu Ammonis area 1
- mRNA
messenger ribonucleic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnsten AF, Paspalas CD, Gamo NJ, Yang Y, Wang M. Dynamic Network Connectivity: A new form of neuroplasticity. Trends Cogn Sci. 2010;14:365–75. doi: 10.1016/j.tics.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagger YZ, Tankó LB, Alexandersen P, Qin G, Christiansen C. Early postmenopausal hormone therapy may prevent cognitive impairment later in life. Menopause. 2005;12:12–17. doi: 10.1097/00042192-200512010-00005. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. Estrogen receptor immunoreactivity in rat brain: rapid effects of estradiol injection. Endocrinology. 1993;132:1218–1224. doi: 10.1210/endo.132.3.7679973. [DOI] [PubMed] [Google Scholar]
- Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-alpha trafficking. J Neurosci. 2009;29:15323–30. doi: 10.1523/JNEUROSCI.2107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TJ, Scherz B, Hochberg RB, MacLusky NJ. Regulation of estrogen receptor concentrations in the rat brain: effects of sustained androgen and estrogen exposure. Neuroendocrinology. 1996;63:53–60. doi: 10.1159/000126935. [DOI] [PubMed] [Google Scholar]
- Carlson MC, Zandi PP, Plassman BL, Tschanz J, Welsh-Bohmer KA, Steffens DC, Bastian L, Mehta K, Breitner JC. Hormone replacement therapy and reduced cognitive decline in older women: The Cache County Study. Neurology. 2001;57:2210–2216. doi: 10.1212/wnl.57.12.2210. [DOI] [PubMed] [Google Scholar]
- DonCarlos LL, Malik K, Morrell JI. Region-specific effects of ovarian hormones on estrogen receptor immunoreactivity. Neuroreport. 1995;6:2054–2058. doi: 10.1097/00001756-199510010-00024. [DOI] [PubMed] [Google Scholar]
- Drogos LL, Rubin LH, Geller SE, Banuvar S, Shulman LP, Maki PM. Objective cognitive performance is related to subjective memory complaints in midlife women with moderate to severe vasomotor symptoms. Menopause. 2013 doi: 10.1097/GME.0b013e318291f5a6. [Epub ahead of print] doi:10.1097/GME.0b013e318291f5a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30:7507–15. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greendale GA, Huang M-H, Wight R, Seeman T, Luetters C, Avis NE, Johnston JM, Karlamangla A. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology. 2009;72:1850–7. doi: 10.1212/WNL.0b013e3181a71193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WG, Lou W, Lasley BL, Hof PR, Morrison JH. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Nat Acad Sci U S A. 2007;104:11465–70. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26:2571–8. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Benke K, Green R, Cupples L, Farrer L. Postmenopausal hormone therapy and Alzheimer's disease risk: interaction with age. J Neurol Neurosurg Psychiatry. 2005;76:103–5. doi: 10.1136/jnnp.2003.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon JG, Moss MB, Rosene DL, Killiany RJ. Patterns of cognitive decline in aged rhesus monkeys. Behav Brain Res. 1997;87:25–34. doi: 10.1016/s0166-4328(96)02256-5. [DOI] [PubMed] [Google Scholar]
- Keenan P, Ezzat W, Ginsburg K. Prefrontal cortex as the site of estrogen's effect on cognition. Psychoneuroendocrinology. 2001;26:577–590. doi: 10.1016/s0306-4530(01)00013-0. [DOI] [PubMed] [Google Scholar]
- Kimura D. Estrogen replacement therapy may protect against intellectual decline in postmenopausal women. Horm Behav. 1995;29:312–321. doi: 10.1006/hbeh.1995.1022. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Herndon JG, Moss MB. Cognitive function in aged ovariectomized female rhesus monkeys. Behav Neurosci. 2000;114:506–513. doi: 10.1037//0735-7044.114.3.506. [DOI] [PubMed] [Google Scholar]
- Lasley BL, Chen J, Stanczyk FZ, El Khoudary SR, Gee NA, Crawford S, McConnell DS. Androstenediol complements estrogenic bioactivity during the menopausal transition. Menopause. 2012;19:650–657. doi: 10.1097/gme.0b013e31823df577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan AH, Henderson VW, Paine BJ, Mathias J, Ramsay EN, Ryan P, Stocks NP, Taylor AW. Hormone therapy, timing of initiation, and cognition in women aged older than 60 years: the REMEMBER pilot study. Menopause. 2006;13:28–36. doi: 10.1097/01.gme.0000191204.38664.61. [DOI] [PubMed] [Google Scholar]
- Maki PM, Sundermann EE. Hormone therapy and cognitive function. Hum Reprod Update. 2009;15:667–681. doi: 10.1093/humupd/dmp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neurosci. 2002;22:10985–10995. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Cauley J, Yaffe K, Zmuda JM. Estrogen replacement therapy and cognitive decline in older community women. J Am Geriatr Soc. 1999;47:518–523. doi: 10.1111/j.1532-5415.1999.tb02563.x. [DOI] [PubMed] [Google Scholar]
- Monfort SL, Hess DL, Shideler SE, Samuels SJ, Hendrickx AG, Lasley BL. Comparison of serum estradiol to urinary estrone conjugates in the rhesus macaque (Macaca mulatta). Biol Reprod. 1987;37:832–837. doi: 10.1095/biolreprod37.4.832. [DOI] [PubMed] [Google Scholar]
- Ohm DT, Bloss EB, Janssen WG, Dietz KC, Wadsworth S, Lou W, Gee NA, Lasley BL, Rapp PR, Morrison JH. Clinically Relevant Hormone Treatments Fail to Induce Spinogenesis in Prefrontal Cortex of Aged Female Rhesus Monkeys. J Neurosci. 2012;32:11700–11705. doi: 10.1523/JNEUROSCI.1881-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Sethares C, Moss MB. The effects of aging on layer 1 in area 46 of prefrontal cortex in the rhesus monkey. Cereb Cortex. 1998;8:671–84. doi: 10.1093/cercor/8.8.671. [DOI] [PubMed] [Google Scholar]
- Presty SK, Bachevalier J, Walker LC, Struble RG, Price DL, Mishkin M, Cork LC. Age differences in recognition memory of the rhesus monkey (Macaca mulatta). Neurobiol Aging. 1987;8:435–40. doi: 10.1016/0197-4580(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Amaral D. Evidence for task-dependent memory dysfunction in the aged monkey. J Neurosci. 1989;9:3568–3576. doi: 10.1523/JNEUROSCI.09-10-03568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Amaral D. Recognition memory deficits in a subpopulation of aged monkeys resemble the effects of medial temporal lobe damage. Neurobiol Aging. 1991;12:481–6. doi: 10.1016/0197-4580(91)90077-w. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Kansky MT, Roberts JA. Impaired spatial information processing in aged monkeys with preserved recognition memory. Neuroreport. 1997;8:1923–8. doi: 10.1097/00001756-199705260-00026. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–14. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JA, Gilardi KV, Lasley BL, Rapp PR. Reproductive senescence predicts cognitive decline in aged female monkeys. Neuroreport. 1997;8:2047–51. doi: 10.1097/00001756-199705260-00048. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–58. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Young BJ. Regulation of estrogen receptor messenger ribonucleic acid in rat hypothalamus by sex steroid hormones. Mol Endocrinol. 1991;5:424–32. doi: 10.1210/mend-5-3-424. [DOI] [PubMed] [Google Scholar]
- Sorra KE, Harris KM. Overview on the structure, composition, function, development, and plasticity of hippocampal dendritic spines. Hippocampus. 2000;10:501–11. doi: 10.1002/1098-1063(2000)10:5<501::AID-HIPO1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Stricker R, Eberhart R, Chevailler MC, Quinn FA, Bischof P, et al. Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer. Clin Chem Lab Med. 2006;44:883–7. doi: 10.1515/CCLM.2006.160. [DOI] [PubMed] [Google Scholar]
- Tang Y, Janssen WG, Hao J, Roberts JA, McKay H, Lasley BL, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen Replacement Increases Spinophilin-immunoreactive Spine Number in the Prefrontal Cortex of Female Rhesus Monkeys. Cereb Cortex. 2004;14:215–223. doi: 10.1093/cercor/bhg121. [DOI] [PubMed] [Google Scholar]
- Tinkler GP, Voytko ML. Estrogen modulates cognitive and cholinergic processes in surgically menopausal monkeys. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:423–31. doi: 10.1016/j.pnpbp.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Walker ML, Gordon TP, Wilson ME. Menstrual cycle characteristics of seasonally breeding rhesus monkeys. Biol Reprod. 1983;29:841–848. doi: 10.1095/biolreprod29.4.841. [DOI] [PubMed] [Google Scholar]
- Weber MT, Rubin LH, Maki PM. Cognition in perimenopause: the effect of transition stage. Menopause. 2013;20:511–517. doi: 10.1097/GME.0b013e31827655e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytko ML, Murray R, Higgs CJ. Executive function and attention are preserved in older surgically menopausal monkeys receiving estrogen or estrogen plus progesterone. J Neurosci. 2009;29:10362–70. doi: 10.1523/JNEUROSCI.1591-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JC, Investigators CCMS. Hormone replacement therapy and incidence of Alzheimer disease in older women: The Cache County Study. JAMA. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]