Abstract
High rates of comorbidity between alcohol use disorder (AUD) and major depressive disorder (MDD) are reported. Preclinical models examining effects of primary depression on secondary AUD are currently absent, preventing adequate testing of drug treatment. Here, we combined social defeat-induced persistent stress (SDPS) and operant alcohol self-administration (SA) paradigms to assess causality between these two neuropsychiatric disorders. We then exploited guanfacine, an FDA-approved adrenergic agent reported to reduce drug craving in humans, against SDPS-induced modulation of operant alcohol SA. Wistar rats were socially defeated and isolated for a period of ⩾9 weeks, during which depression-like symptomatology (cognitive and social behavioral symptoms) was assessed. Subsequently, animals were subjected to a 5-month operant alcohol SA paradigm, examining acquisition, motivation, extinction, and cue-induced reinstatement of alcohol seeking. The effects of guanfacine on motivation and relapse were measured at >6 months following defeat. SDPS rats exhibited significant disruption of social and cognitive behavior, including short-term spatial and long-term social memory, several months following defeat. Notably, SDPS increased motivation to obtain alcohol, and cue-induced relapse vulnerability. Guanfacine reversed the SDPS-induced effects on motivation and relapse. Together, our model mimics core symptomatology of a sustained depressive-like state and a subsequent vulnerability to alcohol abuse. We show that SDPS is strongly associated with an enhanced motivation for alcohol intake and relapse. Finally, we show that the clinically employed drug guanfacine has potential as a novel treatment option in comorbid patients, as it effectively reduced the enhanced sensitivity to alcohol and alcohol-associated stimuli.
Keywords: comorbidity, alcohol use disorder, prolonged social defeat stress, motivation, cue-induced reinstatement, adrenergic agonist
INTRODUCTION
Major depressive disorder (MDD) and alcohol use disorder (AUD) are among the most prevalent psychiatric disorders that commonly co-occur (Pettinati, 2004). Core symptoms of MDD are persistent depressed mood (American Psychiatric Association, 2000), decreased interest for social- and other type of interactions, a general inability to experience reward or impaired responsivity to reward-relevant information (anhedonia) (Der-Avakian and Markou, 2012), as well as cognitive decline (Baune et al, 2010). AUD is characterized by chronic alcohol abuse, extreme preoccupation with alcohol-related activities, and frequent episodes of relapse into alcohol use (American Psychiatric Association, 2000). The two disorders reciprocally affect each other by severity of illness, their onset and temporal persistence (Davis et al, 2008). Comorbidity of MDD and AUD also impacts on the therapeutic outcomes (pharmacological and psychotherapeutic), as evident by low remission rates and increased disability (Kelly et al, 2012). Finally, experiencing one disorder is a risk factor for relapse to the other, further supporting a reciprocal relationship between the two (Petrakis et al, 2002).
Despite prevailing comorbidity between depression and AUD, direct evidence of causality of co-occurrence in clinical and preclinical data of the two pathologies is still scarce. Likewise, potentially shared molecular substrates underlying such co-existence are yet to be found. As a result, targeted pharmacotherapies against the comorbid phenotype are practically absent. To explore a possible causative role for primary depression on subsequent susceptibility to alcohol abuse, and to enable studies into the molecular interaction mechanisms of these disorders, a novel experimental animal model is required.
In terms of a preclinical model for depression-like behavior, social defeat has been explored extensively, reliably representing a wide range of manifestations of the disorder (Nestler and Hyman, 2012). In this respect, acute defeat-induced social avoidance has been repeatedly reported both in mice (Golden et al, 2011) and rats (Fanous et al, 2011), offering a consistent assessment of depressive-like symptomatology. However, the outcome of these studies relies on the presence and/or threat of acute stress, without taking into account the perpetuating nature of the depressive state. In order to model this lasting character of stressful life events that are typically associated with increased risk for depression in humans, we and others have used the social defeat-induced persistent stress (SDPS) paradigm. In this model, acute stress of social defeat, an etiologically relevant type of stressor for animals living in a social setting as rats, is perpetuated by protracted social isolation (de Jong et al, 2005). The SDPS paradigm has been successfully used to model core symptoms of depression, such as anhedonia, as well as severe and persistent cognitive decline (Von Frijtag et al, 2000), as commonly seen in MDD-diagnosed patients (Austin et al, 2001). In addition, the SDPS model emulates accompanying neurobiological hallmarks of depression, such as reduced neurogenesis and synaptic plasticity in the hippocampus (Von Frijtag et al, 2001; Van Bokhoven et al, 2011). Importantly, antidepressant treatment, as well as behavioral therapy, resulted in amelioration of these sustained depression-like effects (Artola et al, 2006), confirming the predictive validity of the SDPS model.
Social defeat was previously employed to model acute effects of social stress on alcohol home-cage consumption (Caldwell and Riccio, 2010), or operant responding for alcohol in rats (Funk et al, 2005). However, these approaches did not address long-term consequences of repeated stressful events and persistent negative mood, typical of depressive symptomatology, on the development of AUD (Briand and Blendy, 2010). We, therefore, developed a new paradigm, linking the SDPS model to an AUD model of operant alcohol self-administration (SA).
MATERIALS AND METHODS
Animals and SDPS
Paired-housed male Wistar rats (Harlan CPB, Horst, the Netherlands) 6–7 weeks old, weighing <200 g upon arrival were habituated (2 weeks) and exposed to SDPS (see Supplementary Methods, (Von Frijtag et al, 2000; Van Bokhoven et al, 2011)) and operant alcohol SA paradigms (Figure 1a). All experiments were approved by the VU University Amsterdam Animal Users Care Committee.
Figure 1.
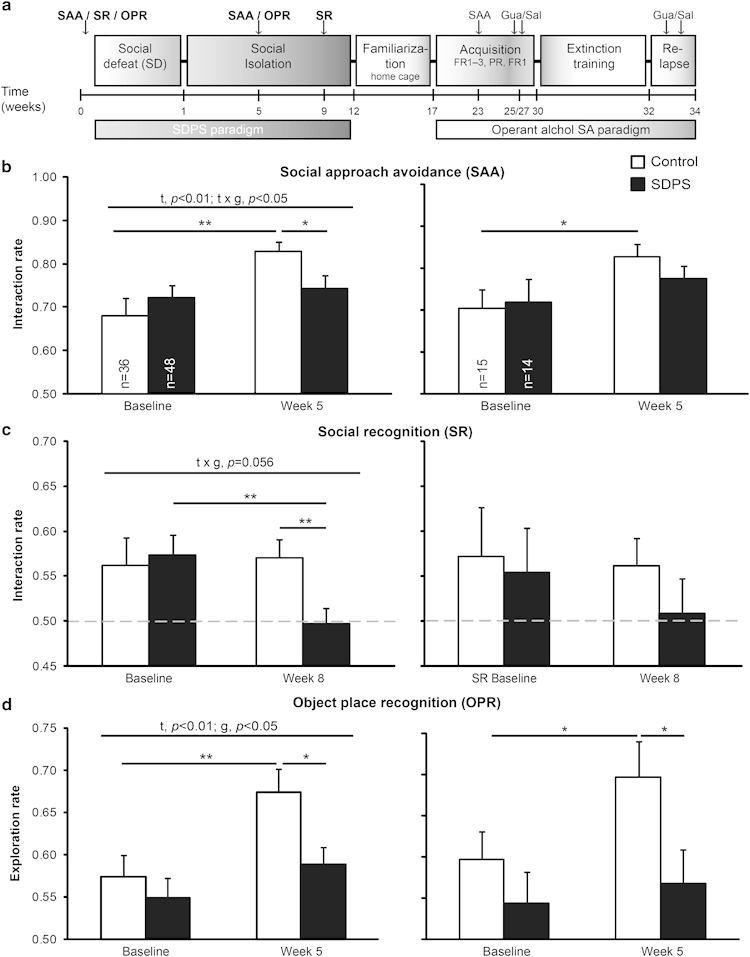
Long-term effects of social defeat-induced persistent stress (SDPS) on social behavior and cognition. (a) Experimental schedule: individually housed animals were used (controls; n=36), or they were exposed to the prolonged social defeat stress paradigm (SDPS; n=48), after which animals were subjected to operant alcohol self-administration (SA). All animals were tested in social approach-avoidance (SAA; b), social recognition (SR; c), and object place recognition (OPR; d) before (baseline) and after defeat (week 5 or 9). After ⩾9 weeks, representatives of each group (n=16) were selected for familiarization to alcohol in the home cage and subsequent operant alcohol SA. Animals were trained in fixed (fixed ratio1( FR1)–3) and progressive ratio (PR) schedules, followed by extinction training and an alcohol-coupled cue-induced reinstatement test. The effects of guanfacine (Gua) on motivation and reinstatement of alcohol seeking were assessed in a cross-over design. (b–d) Results from the total group of animals (left) or from the selected group for subsequent alcohol SA studies (right). (b) SAA: the SDPS group exhibited a significant reduction in interaction rates in week 5 and showed no improvement in the SAA performance over time as seen in controls. (c) Control and SDPS animals displayed significant between-group differences in week 9 following defeat, and SDPS animals exhibited a reduction in interaction rate over time. (d) The SDPS group displayed a significant reduction in exploration rate (week 5) and failed to improve the OPR performance. ANOVA/t-test results are indicated (t: time effect; g: group effect; t × g: time × group interaction) *p<0.05; **p<0.01. Dashed gray lines indicate 50% preference index.
Assessment of Depressive Symptomatology
Social approach-avoidance test (SAA)
Approach-avoidance behavior (interaction rates (Golden et al, 2011)), using an unfamiliar Long-Evans adult male rat (aggressor), was calculated as time spent in active zone (aggressor zone)/total exploration time (aggressor+neutral zone), in a 5-min test.
Social recognition
Long-term social discrimination memory (Reijmers et al, 2001) was tested using a social recognition (SR) test 24 h following first exposure to a juvenile Wistar rat (4–6 weeks old). Discrimination between familiar (inactive zone) and novel (active zone) social targets was used as measurement of SR (interaction rate=time spent in active zone/total interaction time) in a 5-min test.
Object place recognition
Hippocampal-dependent short-term memory (Dere et al, 2007) was determined using a 15-min retention interval. Discrimination between spatial locations of objects was used as measurement for spatial memory (exploration rate=time spent in active zone (novel location)/total exploration time (novel+familiar location) in a 4-min test.
All video recordings were analyzed with Viewer2 software (BiObserve GmbH, Bonn, Germany).
Selection procedure
Animals were selected to continue with operant alcohol SA paradigm based on the performance on SAA, SR, and OPR tests, as assessed at three different time points: (1) 1 week before defeat, baseline; (2) 4–6; and (3) 8–10 weeks following defeat, post-individual housing. From 48 defeated animals, 16 SDPS rats that displayed average performance were selected (Supplementary Figure S1), to assess the validity of our model in a moderately affected group, which most faithfully represents defeat-induced behavioral deficits in the general population. In accordance, 16 control rats were randomly assigned to the alcohol SA paradigm.
Alcohol Exposure
Home-cage consumption
All animals were habituated to alcohol consumption using the two-bottle free/limited-access paradigm (Wouda et al, 2011). Rats were exposed to gradually elevating alcohol concentrations (2–12% v/v) in the home cage (5 weeks; Figure 1a). During the last 2 weeks, alcohol availability was limited to 1 h/day.
Cue-coupled alcohol SA—Fixed Ratio
Rats were trained to nose-poke for a 0.20 ml 12% alcohol reward in 1-h sessions every other day. Alcohol delivery (US) was accompanied by discrete audiovisual stimuli (CS, 4-s active hole illumination and tone presentation). Different reinforcement schedules (fixed ratio, FR) were used (FR1–3).
Cue-coupled alcohol SA—Progressive Ratio
Animals were subjected to six 2-h progressive ratio (PR) sessions, during which the effort (number of nose-pokes) to obtain a reward was progressively increased according to: response ratio=(5e(0.2 * reward number))–5, rounded to the nearest integer.
Cue-coupled alcohol SA—Extinction and Relapse
After PR sessions, all animals were retrained (FR1) to minimize between-group differences that could affect subsequent analysis of extinction performance. Extinction training consisted of 1-h exposure to the training context in absence of alcohol and alcohol-associated cues. Following 15 daily sessions, operant responding was successfully extinguished (<5 active responses/session) and all animals participated in a 30-minute cue-induced reinstatement session, at the start of which a single 0.20 ml alcohol reward was delivered.
Treatment administration
Guanfacine-HCl (N-amidino-2-(2,6-dichlorophenyl) acetamide hydrochloride) was tested in PR sessions 3 and 6, and on cue-induced relapse in two separate tests, given at a 7-day interval without additional extinction training. Saline (1 ml/kg) or guanfacine (0.5 mg/kg dissolved in saline) were systemically (i.p.) administered 1 h before the session/test in a cross-over design.
Statistical Analyses
All statistics were performed using SPSS (version 15.0, IBM). Data are presented as mean±SEM, and (repeated) ANOVA or the t-test results are indicated. Interaction or memory indices were calculated based on a fictive group showing no discrimination, while retaining the variation of the tested sample (Akkerman et al, 2012).
RESULTS
Effects of SDPS on Depression-Like Symptomatology
We examined emotional state (SAA, SR) and cognitive performance (SR, object place recognition (OPR)) (Figure 1) indicative of a disrupted behavioral state resembling core symptoms of depression (Von Frijtag et al, 2000).
Social approach-avoidance test
All rats showed a significant interaction rate with the (potential) aggressor at baseline (p<0.001) and at week 5 (Con: p<0.001; SDPS, p<0.001) of the SDPS paradigm. Repeated measures ANOVA revealed a time effect (F(1,82)=8.62, p<0.01), and a significant time-group interaction (F(1,82)=4.66, p<0.05), indicating that social defeat differentially affects SAA performance over time. Whereas both groups showed similar performance at baseline (F(1,83)=0.77, ns), SDPS rats exhibited a significant reduction in interaction rate at the 5 weeks test when compared with controls (F(1,83)=4.49, p<0.05). In addition, SDPS animals failed to improve SAA performance-like controls (Con, t(35)=−3.37, p<0.01; SDPS, t(47)=−0.59, ns; Figure 1b). Likewise, in groups selected for alcohol SA, control animals showed a similar time-dependent increase in interaction rate (t(14)=−2.31, p<0.05), whereas SDPS animals failed to improve (t(13)=−0.96, ns) (Figure 1b). Notably, SAA performance at ∼6 months following the last defeat session confirmed the stability of the depression-like status (F(1,28)=7.37, p<0.05; Figures 2a and b). Our data indicate that the SDPS paradigm caused long-lasting impairment of social behavior.
Figure 2.
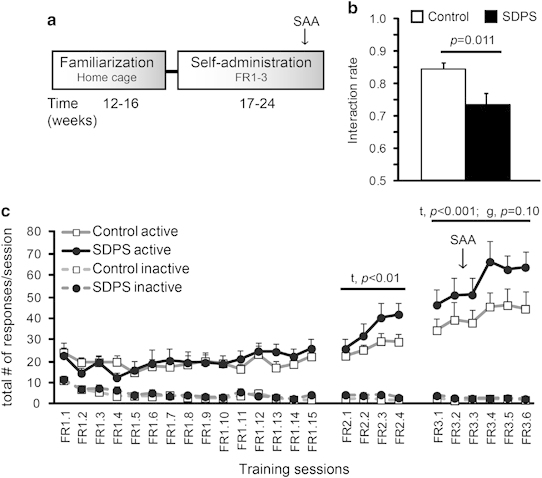
Effects of social defeat on acquisition of operant alcohol self-administration. (a) Experimental schedule: 2 months following social defeat, all animals got familiarized with 12% alcohol solution in the home-cage (5 weeks; cf. Figure 1a). Rats were then trained to self-administer alcohol according to different training schedules (fixed ratio1 (FR1)–FR3; c). Before the end of the acquisition phase both groups were subjected to an additional social approach-avoidance (SAA) test (b). (b) The social defeat-induced persistent stress (SDPS) group displayed a significant reduction in interaction on an alcohol-free day, at ∼6 months after the last defeat exposure. (c) Acquisition of cue-paired alcohol self-administration (SA): time effects were observed at FR2 and FR3. No between-group differences in number of active responses/session were observed at FR1 and FR2, with a trend for between-group differences at the FR3 schedule. No between-group differences in total number of inactive responses were found (FR1: F(1,27)=1.71, ns; FR2: F(1,27)=2.56, ns; FR3: F(1,27)=0.89, ns). ANOVA/t-test results are indicated (t: time effect; g: group effect).
Social recognition
All rats showed a significant interaction rate with the novel social target at baseline (p<0.01), but only controls maintained this at 9 weeks (Con, p<0.05; SDPS, ns). Indeed, repeated measures ANOVA revealed a trend for time-group interaction (F(1,82)=3.76, p=0.056), indicating that SDPS differentially affects SR performance over time. Although control and SDPS animals demonstrated equivalent baseline performance (F(1,83)=0.11, ns), at week 9 of the SDPS paradigm, significant between-group differences were observed (F(1,83)=7.38, p<0.01). Post hoc analysis showed that SDPS rats displayed a significant reduction in interaction rate when compared with their baseline performance (t(47)=2.92, p<0.01), an effect absent in controls (t(35)=−0.24, ns; Figure 1c). Similarly, in selected alcohol SA groups, control rats showed normal SR (p<0.08), whereas SDPS rats were significantly impaired (ns), indicating that SDPS triggers enduring deficits in affective cognition.
Object place recognition
All rats showed a significant exploration rate for the object at its new location at baseline (p<0.001) and at week 5 (Con, p<0.001; SDPS, p<0.01). Importantly, repeated measures ANOVA revealed a significant time (F(1,76)=8.39, p<0.01) and group ((F(1,76)=4.33, p<0.05) effect. Post hoc analysis showed that, whereas both groups performed similar at the baseline (F(1,81)=0.51, ns), at week 5, SDPS animals displayed a significant reduction in exploration rate when compared with controls (F(1,79)=6.42), p<0.05). Notably, SDPS rats displayed no increases in exploration rate, as opposed to controls (Con, t(33)=−2.73, p<0.05; SDPS, t(43)=−1.28, ns; Figure 1d). Likewise, selected alcohol SA animals showed no between-group difference at baseline (F(1,28)=1.09, ns), whereas a significant group effect (F(1,25)=4.47, p<0.05) was present at week 5, with the SDPS group showing no improvement of OPR performance (Con, t(13)=−2.35, p<0.05; SDPS, t(11)=−0.49, ns; Figure 1d). In an independent group, OPR memory at the 8-week time point was not present in SDPS animals (Supplementary Figure S2b). Together, we observed a persistent defeat-driven impairment in short-term spatial memory, indicative of cognitive rigidity observed in depressive patients (Austin et al, 2001).
Social isolation alone did not affect OPR, SR, and SAA behaviors, as tested in independent batches of animals (Supplementary Figures S2 and S3). Thus, the effects measured here originate specifically from coupling social defeat with prolonged social isolation, and cannot be attributed solely to adulthood isolation.
Effects of SDPS on Alcohol Taking and Seeking
Acquisition of operant alcohol SA
Alcohol habituation in the home-cage showed no significant between-group difference in alcohol or water consumption (Supplementary Figure S4). Control and SDPS animals showed similar performance on an FR1 schedule (F(1,27)=0.04, ns). FR2 increased responding (session effect: F(2.44,65.95)=7.48, p<0.01) in both groups, without a clear significant between-group difference (F(1,27)=2.43, p=0.13). FR3 showed a significant session effect (F(4.37,118.13)=5.55, p<0.001), and a trend for between-group differences (F(1,27)=2.90, p=0.10), reflecting a stronger escalation of responding in SDPS animals. Similar results were obtained for alcohol consumption (Supplementary Figure S5). SDPS and control rats performed identical for inactive responses (Figure 2b).
PR and guanfacine treatment
To detect differences in motivational drive in SDPS animals, PR schedules were introduced. SDPS rats significantly elevated responding for an alcohol reward (PR1–2: (F(1,27)=6.38, p<0.05; PR4–5: (F(1,27)=11.69, p<0.01; Figures 3a and b) and displayed significantly increased breaking points compared with controls (F(1,28)=8.96, p<0.01; Figure 3c). Thus, SDPS increases the motivational drive to obtain an alcohol reward.
Figure 3.
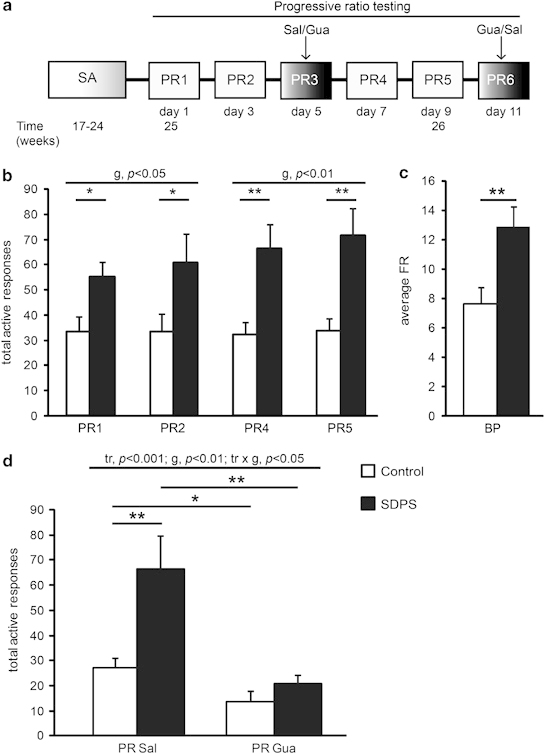
Social defeat affects motivation for alcohol intake: rescue by guanfacine administration. (a) Experimental schedule: following fixed ratio (FR), all rats were trained under a progressive ratio (PR) schedule for a period of 2 weeks every other day (cf. Figure 1a). Defeat effects on motivation to obtain alcohol were examined in 4 × 2-h sessions, whereas the effect of guanfacine (Gua; 0.5 mg/kg, i.p.) was tested at intermediate training days 5 and 11 using a within-subject design. (b) Social defeat-induced persistent stress (SDPS) animals displayed a robust increase in number of total active responses. (c) SDPS animals exhibited significantly augmented breaking points (BP). (d) Pretreatment with Gua significantly reduced the number of active responses in both groups. Importantly, Gua reversed defeat-induced increases in responding, eliminating between-group differences (F(1,28)=1.64, ns) that were present following SAL administration. ANOVA/t-test results are indicated (tr: treatment effect; g: group effect; tr × g: treatment × group interaction) *p<0.05; **p<0.01.
Guanfacine, an α2 adrenergic agonist, has been shown to attenuate stress-induced relapse to alcohol seeking in rats (Lê et al, 2011). To analyze possible effects of guanfacine on motivation to take alcohol, both groups were treated with guanfacine in a cross-over design. This revealed a significant treatment effect (F(1.00,27.00)=22.88, p<0.001), indicating that guanfacine treatment greatly reduced active responding in both groups. A treatment-group interaction was observed (F(1.00,27.00)=6.98, p<0.05). Indeed, guanfacine affected SDPS animals to a greater extent (F(1,27)=8.10, p<0.01), abolishing between-group differences (Figure 3d). At this low-dose of guanfacine (Lê et al, 2011), we confirmed the absence of gross effects on motor activity (Supplementary Figure S6, Supplementary Table S1).
Cue-induced reinstatement and guanfacine treatment
After retraining to FR1, all animals showed similar performance (Supplementary Figure S7a). During extinction training, similar extinction rates and no between-group differences were observed (Supplementary Figure S7b), indicating that SDPS did not affect extinction of previously learned operant responding for an alcohol reward.
Next, we examined the effect of SDPS on cue-induced alcohol relapse and its interaction with guanfacine treatment in two cue-induced reinstatement tests, using a cross-over design (Figure 4). First, presentation of alcohol-associated cues and the ability to respond for these cues significantly reinstated alcohol seeking in SDPS and control groups as compared with the last three extinction sessions (F(1.00,27.00)=134.28, p<0.001; control: t(14)=−6.86, p<0.001; SDPS: t(13)=−9.48, p<0.001)). The significant group effect (F(1,27)=6.63, p<0.05) and a relapse-group interaction (F(1.00,27.00)=4.28, p<0.05) indicated that SDPS animals exhibited more robust reinstatement. Treatment with guanfacine 1 h before relapse completely abolished reinstatement in both groups, as compared with the last three extinction sessions (F(1.00,27.00)=1.46, ns) with a trend for group (F(1,27)=3.40, p=0.076). Notably, after guanfacine administration, significant group differences were no longer observed (F(1,28)=2.35, ns), indicating that guanfacine suppressed SDPS-induced facilitation of reinstatement of alcohol seeking.
Figure 4.
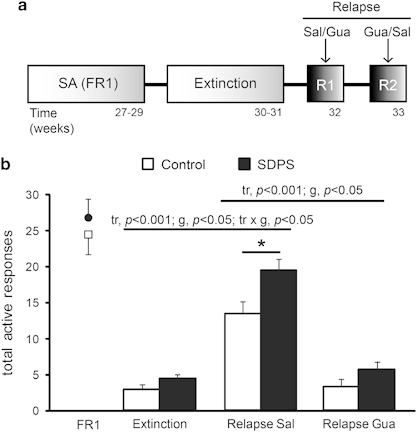
Social defeat affects cue-induced relapse: effects of guanfacine. (a), Experimental schedule: all rats were trained on an fixed ratio1 (FR1) schedule before extinction (cf. Figure 1a). Operant responding was then extinguished in 15 × 1-h extinction sessions (<5 responses/session). Social defeat-induced persistent stress (SDPS) effect on cue-induced reinstatement of alcohol seeking was examined in two consecutive tests (R1 and R2) and the effect of guanfacine (Gua; 0.5 mg/kg, i.p.) was tested using a within-subject design. (b) After similar performance at FR1 and extinction training, both SDPS animals and controls showed a significant cue-induced relapse, with the SDPS group exhibiting more robust relapse. Guanfacine significantly suppressed responding in both groups (control: t(14)=7.77, p<0.001; SDPS: t(13)=−9.48, P<0.001). Importantly, guanfacine abolished group differences. ANOVA/t-test results are indicated (tr: treatment effect; g: group effect; tr × g: treatment × group interaction). *p<0.05.
DISCUSSION
In the current study, we addressed whether a sustained depressive-like state has subsequent effects on alcohol intake and/or relapse, to enable future studies into the molecular mechanisms of these comorbid psychiatric disorders. By combining two behavioral paradigms, namely SDPS and operant alcohol SA in rats, we could reliably evoke an enduring depressive-like state that dramatically affected subsequent alcohol taking and seeking, leading to a co-occurrence of depression-like and alcohol dependence-like phenotypes, similar to the comorbid phenotype of MDD and AUD. Using this combined model, we provide first evidence for the efficacy of guanfacine, an FDA-approved adrenergic agent, on the depression-induced enhancement of the motivational drive to seek alcohol.
SDPS Evokes a Maintenance State of Depression-Like Symptomatology
Our data indicate that SDPS in rats, ie, social defeat coupled with prolonged social isolation in absence of living in close proximity to the dominant male (a paradigm often used in mice (Golden et al, 2011)), affects social and cognitive performance (Von Frijtag et al, 2000), as assessed in SAA, SR, and OPR tests, effects that cannot be attributed to depression-associated motor retardation (Supplementary Figure S6).
Our results are in accordance with the vast majority of current clinical research describing the multifaceted consequences of depression on cognitive and executive functions (Millan et al, 2012). Although social defeat has been explored in great extent, the majority of studies focused on acute effects of defeat failing to address the sustained character of the depressive state. Using the SDPS model, we describe long-lasting effects of social defeat, in particular social withdrawal, that persists up to 6 months following the last defeat episode, and that is independent of acute stress-triggered increases in serum corticosterone (Van Bokhoven et al, 2011). Apart from social withdrawal, we show that SDPS impairs long-term SR, as previously shown (Reijmers et al, 2001), as well as hippocampus-dependent spatial memory for up to 2 months following the last defeat episode. The latter is in agreement with the well-characterized depression-associated reduction of hippocampal volume (Bremner et al, 2000), and its consequent cognitive deficiencies (Femenía et al, 2012). Our current data confirm that the SDPS model reflects the magnitude and temporal persistence of the depressive-like state in terms of etiological-, face-, construct- and predictive validity (Nestler and Hyman, 2012) and forms a firm basis to subsequently investigate comorbidity of alcohol abuse. Depression-associated behavioral impairments were moderate in the group selected for alcohol SA, in line with our aim to assess the validity of our model in a moderately affected population.
SDPS Induces an AUD Phenotype and Models Depression-AUD Comorbidity
A major finding is that the persistent depressive-like state of the SDPS paradigm led to profound alcohol reward-related changes, exaggerating the incentive salience of alcohol, and facilitating cue-induced relapse to alcohol seeking. Social defeat was previously employed to model acute effects of social stress on alcohol consumption in rodents. However, in these studies, the outcome, to a large extent, depended on the behavioral read-out at use (eg, alcohol home-cage consumption or operant alcohol SA). Indeed, whereas acute social defeat stress yielded mixed results for alcohol home-cage consumption (Caldwell and Riccio, 2010), acute defeat during operant alcohol SA reduced responding for alcohol (van Erp and Miczek, 2001; Funk et al, 2005), with presentation of defeat-coupled cues being sufficient to reinstate alcohol seeking (Funk et al, 2005). In addition, the majority of studies examined acute stress effects on alcohol taking, without addressing the impact of long-lasting negative effect on the incentive motivation for alcohol use (Koob, 2009), as observed during depression. Here, we examined alcohol taking and seeking during a persistent depressive-like state in the absence of the actual stressor or stress-signifying cues and demonstrated a persistent enhanced motivation to consume and seek alcohol.
The operant nature of the alcohol SA paradigm allowed us to model several phases of alcohol taking and seeking. We demonstrate that SDPS affected motivation to consume alcohol, with the magnitude of SDPS-induced depressive-like state correlating with increased breaking points (Supplementary Figure S8). These results suggest defeat-induced dysregulation of the motivational response, a characteristic of the human depressive phenotype (Nestler and Carlezon, 2006; Der-Avakian and Markou, 2012). Similarly, we demonstrate that SDPS robustly enhanced reinstatement of alcohol seeking. These results indicate a depressive-like state-driven amplification of the incentive value attributed to alcohol-associated cues and/or the presence of dysfunctional inhibitory control, as observed in drug-dependent human subjects (Kalivas and Volkow, 2005). Thus, we could reliably mimic the primary depression-secondary AUD diagnosis often seen in comorbid patients. We here provide first preclinical evidence for a direct causality between depressive-like symptomatology and subsequent increased vulnerability to alcohol seeking.
Isolation Facilitates Incubation and Preservation of Depressive-Like Symptoms
Cognitive and social decline might be caused solely by isolation from conspecifics, ie, single-housed rats display significant deficits in spatial memory (Quan et al, 2010), impaired social interest and high anxiety levels (Hermes et al, 2011). However, these isolation-induced behavioral manifestations are to a large extent limited to animals reared in isolation, thereby disturbing important developmental periods in the brain maturation, such as adolescence (Fone and Porkess, 2008). In our paradigm, all animals were single-housed after adulthood (ie, ⩾postnatal day 70). Furthermore, we specifically showed, given there is no difference between single-housed and paired-housed controls, that depression-like impairments in social and cognitive behavior were not due to an isolation-driven increase in exploration/interaction rates. Moreover, both control groups showed intact social and cognitive behavior and differed from the SDPS group (Figure 1, Supplementary Figures S1 and S2). Therefore, we conclude that isolation facilitated incubation and preservation of depressive-like symptoms, as social housing directly after defeat ameliorates both physiological and behavioral defeat-induced impairments (de Jong et al, 2005). Thus, the synergistic effect of defeat- and isolation-induced social stress poses a model of the maintenance of the depressive-like state. In our paradigm, all animals, including controls, were single-housed for ∼2 months before initial exposure to alcohol, ruling out acute isolation effects on alcohol intake, as well as isolation being a confounding factor in the increased motivation and relapse to alcohol.
Adrenergic Receptor as Possible Target for Depression-Induced Increased Motivation and Relapse
The α2 adrenoreceptor agonist guanfacine is an FDA-approved drug known for its cognitive enhancing effects, improving working memory and attention (Arnsten and Jin, 2012). It is proposed that guanfacine can directly modulate prefrontal cortex (PFC) functioning, thereby strengthening signal-to-noise ratio of relevant information and boosting cognitive control (Gamo and Arnsten, 2011). In theories of addiction, it is well-accepted that disruptions of inhibitory or executive control could explain hallmarks of addictive behavior, such as continued substance use despite adverse consequences, impaired control over behavior and repeated unsuccessful attempts to reduce or cut down use (Goldstein and Volkow, 2011). Taken together with the well-documented detrimental effects of chronic stress on PFC functioning (Arnsten, 2009) and preclinical work, in which adrenoreceptor α2 agonists were able to reduce stress-induced drug-seeking in rodents and monkeys (Lee et al, 2004; Lê et al, 2011; Smith and Aston-Jones, 2011), cognitive enhancers have been proposed as plausible treatments for addictive disorders (Sofuoglu et al, 2013). Indeed, guanfacine lowered stress and cue-induced cocaine craving in a clinical setting (Fox et al, 2012). Here, we show that the treatment effects of guanfacine can be extended to cue-induced alcohol seeking, both in controls and SDPS rats. Furthermore, guanfacine can be used to reduce the motivational overdrive observed in SDPS rats. Guanfacine effects on other behavioral readouts (SAA, OPR, and SR) were not addressed in this study; however, it is interesting to assess its efficacy against a larger spectrum of depressive-like manifestations.
Norepinephrine (NE) receptors of the α2A type, for which guanfacine shows highest affinity, are mainly expressed in locus coeruleus (LC) and PFC and have stress-protective roles (Schramm et al, 2001). At the LC, α2A acts as autoreceptor to inhibit NE transmission. Reduced LC inhibition and subsequent increased modulation of PFC-mediated cognitive control are implicated in hyper-arousal, poor stress coping, chronic anxiety, and disturbances of higher cognitive functions typically met in depressive disorders, justifying the use of noradrenergic therapeutic agents (Goddard et al, 2010). Thus, the beneficial effects of guanfacine might be mediated by reducing LC-originated NE transmission to output regions and the subsequent stress response (Gamo and Arnsten, 2011). In addition, direct effects of postsynaptic α2A receptor activation in the PFC, which has been shown to enhance the area's functional connectivity and to improve cognitive performance (Wang et al, 2007), might account for the observed results. In a recent model, α2A postsynaptic receptors modulate the balance between facilitation and inhibition of prefrontal cortical glutamatergic signaling (Ji et al, 2008), which could be disturbed in SDPS animals, leading to decreased PFC output. In accordance, guanfacine-precipitated reduction in drug craving is accompanied by reversal of stress- and cue-induced hypofrontality in human addicts (Sinha et al, 2011).
Noradrenergic transmission in the PFC is essential for the attribution of incentive value, engaging dopamine in the nucleus accumbens (NAc) to process motivationally relevant stimuli, thereby guiding goal-directed conduct (Ventura et al, 2007). As frontal NE transmission has a role in the expression of depression, stress coping, and drug vulnerability (Arnsten, 2009; Lê et al, 2009; Goddard et al, 2010; Perry et al, 2011), it is plausible that the observed SDPS-induced reward-related behavioral deficits originate from a dysregulated NE-mediated (LC-)PFC-NAc reciprocal communication. This hypothesis is supported by the ability of guanfacine to reduce both enhanced motivation and susceptibility to relapse. As such, guanfacine could weaken the LC-to-PFC NE response and/or directly enhance PFC top-down control, thereby allowing for more optimal cognitive performance, which in turn can reduce behavioral sensitivity to reward-coupled cues and the accompanied maladaptive drug-seeking response. Future studies are required to further explore this hypothetical construct.
In conclusion, we have established a putative inductive role of the depressive state on subsequent vulnerability to alcohol abuse, and contributed to the imperative need to introduce preclinical models that mimic the comorbid phenotype of depressive disorders and AUD, including the enduring nature of depression. Only after characteristics of the comorbid phenotype are better understood, more targeted and successful treatments can be implemented. Novel animal models, as described here, might help to uncover possible neuronal mechanisms relevant for depression-AUD comorbidity and elucidate novel potential therapeutic targets. Our study indicates that guanfacine could be beneficial in the treatment of alcohol dependence in comorbid depression and AUD.
FUNDING AND DISCLOSURE
ABS and SS received funding by the Center for Medical Systems Biology (CMSB). ABS and LJMS received funding from Neurobsik. JEvH is employed at Deltaphenomics BV, in which the social defeat paradigm is offered for contract-based research. The authors declare no conflict of interest.
Acknowledgments
We thank Jon-Ruben van Rhijn and Dustin Schetters for their assistance in the behavioral experiments, as well as Michel van de Oever for his valuable advice on the manuscript.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Akkerman S, Prickaerts J, Steinbusch H, Blokland A. Object recognition testing: statistical considerations. Behav Brain Res. 2012;232:317–322. doi: 10.1016/j.bbr.2012.03.024. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association 2000Diagnostic and statistical manual of mental disorders4th edn.text rev. American Psychiatric Press: Washington, DC, USA [Google Scholar]
- Arnsten A, Jin L. Guanfacine for the treatment of cognitive disorders: a century of discoveries at Yale. Yale J Biol Med. 2012;85:45–58. [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artola A, von Frijtag J, Fermont P, Gispen W, Schrama L, Kamal A, et al. Long-lasting modulation of the induction of LTD and LTP in rat hippocampal CA1 by behavioural stress and environmental enrichment. Eur J Neurosci. 2006;23:261–272. doi: 10.1111/j.1460-9568.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- Austin M, Mitchell P, Goodwin G. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001;178:200–206. doi: 10.1192/bjp.178.3.200. [DOI] [PubMed] [Google Scholar]
- Baune BT, Miller R, McAfoose J, Johnson M, Quirk F, Mitchell D. The role of cognitive impairment in general functioning in major depression. Psychiatry Res. 2010;176:183–189. doi: 10.1016/j.psychres.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Bremner J, Narayan M, Anderson E, Staib L, Miller H, Charney D. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Briand L, Blendy J. Molecular and genetic substrates linking stress and addiction. Brain Res. 2010;1314:219–234. doi: 10.1016/j.brainres.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell E, Riccio D. Alcohol self-administration in rats: modulation by temporal parameters related to repeated mild social defeat stress. Alcohol. 2010;44:265–274. doi: 10.1016/j.alcohol.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Davis L, Uezato A, Newell J, Frazier E. Major depression and comorbid substance use disorders. Curr Opin Psychiatry. 2008;21:14–18. doi: 10.1097/YCO.0b013e3282f32408. [DOI] [PubMed] [Google Scholar]
- de Jong J, van der Vegt B, Buwalda B, Koolhaas J. Social environment determines the long-term effects of social defeat. Physiol Behav. 2005;84:87–95. doi: 10.1016/j.physbeh.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E, Huston J, De Souza Silva M. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Fanous S, Terwilliger E, Hammer RJ, Nikulina E. Viral depletion of VTA BDNF in rats modulates social behavior, consequences of intermittent social defeat stress, and long-term weight regulation. Neurosci Lett. 2011;502:192–196. doi: 10.1016/j.neulet.2011.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femenía T, Gómez-Galán M, Lindskog M, Magara S. Dysfunctional hippocampal activity affects emotion and cognition in mood disorders. Brain Res. 2012;1476:58–70. doi: 10.1016/j.brainres.2012.03.053. [DOI] [PubMed] [Google Scholar]
- Fone K, Porkess M. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Fox H, Seo D, Tuit K, Hansen J, Kimmerling A, Morgan P, et al. Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. J Psychopharmacol. 2012;26:958–972. doi: 10.1177/0269881111430746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Harding S, Juzytsch W, Lê A. Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2005;183:341–349. doi: 10.1007/s00213-005-0194-1. [DOI] [PubMed] [Google Scholar]
- Gamo N, Arnsten A. Molecular modulation of prefrontal cortex: rational development of treatments for psychiatric disorders. Behav Neurosci. 2011;125:282–296. doi: 10.1037/a0023165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard A, Ball S, Martinez J, Robinson M, Yang C, Russell J, et al. Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress Anxiety. 2010;27:339–350. doi: 10.1002/da.20642. [DOI] [PubMed] [Google Scholar]
- Golden S, Covington HI, Berton O, Russo S. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R, Volkow N. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes G, Li N, Duman C, Duman R. Post-weaning chronic social isolation produces profound behavioral dysregulation with decreases in prefrontal cortex synaptic-associated protein expression in female rats. Physiol Behav. 2011;104:354–359. doi: 10.1016/j.physbeh.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Ji J, Zhang H, Li B. Stimulation of alpha2-adrenoceptors suppresses excitatory synaptic transmission in the medial prefrontal cortex of rat. Neuropsychopharmacology. 2008;33:2263–2271. doi: 10.1038/sj.npp.1301603. [DOI] [PubMed] [Google Scholar]
- Kalivas P, Volkow N. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kelly T, Daley D, Douaihy A. Treatment of substance abusing patients with comorbid psychiatric disorders. Addict Behav. 2012;37:11–24. doi: 10.1016/j.addbeh.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G. Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry. 2009;42:S32–S41. doi: 10.1055/s-0029-1216356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê A, Funk D, Harding S, Juzytsch W, Fletcher P. The role of noradrenaline and 5-hydroxytryptamine in yohimbine-induced increases in alcohol-seeking in rats. Psychopharmacology (Berl) 2009;204:477–488. doi: 10.1007/s00213-009-1481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê A, Funk D, Juzytsch W, Coen K, Navarre B, Cifani C, et al. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology (Berl) 2011;218:89–99. doi: 10.1007/s00213-011-2178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt D, Spealman R. Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Millan M, Agid Y, Brüne M, Bullmore E, Carter C, Clayton N, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11:141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- Nestler E, Carlezon WJ. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nestler E, Hyman S. Animal models of neuropsychiatric disorders. Nat Neurosci. 2012;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J, Joseph J, Jiang Y, Zimmerman R, Kelly T, Darna M, et al. Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions. Brain Res Rev. 2011;65:124–149. doi: 10.1016/j.brainresrev.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis I, Gonzalez G, Rosenheck R, Krystal J. Comorbidity of alcoholism and psychiatric disorders: an overview. Alcohol Res Health. 2002;26:81–89. [Google Scholar]
- Pettinati H. Antidepressant treatment of co-occurring depression and alcohol dependence. Biol Psychiatry. 2004;56:785–792. doi: 10.1016/j.biopsych.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Quan M, Tian Y, Xu K, Zhang T, Yang Z. Post weaning social isolation influences spatial cognition, prefrontal cortical synaptic plasticity and hippocampal potassium ion channels in Wistar rats. Neuroscience. 2010;169:214–222. doi: 10.1016/j.neuroscience.2010.04.048. [DOI] [PubMed] [Google Scholar]
- Reijmers L, Hoekstra K, Burbach J, van Ree J, Spruijt B. Long-term impairment of social memory in the rat after social defeat is not restored by desglycinamide–vasopressin. Neurosci Lett. 2001;305:145–148. doi: 10.1016/s0304-3940(01)01834-1. [DOI] [PubMed] [Google Scholar]
- Schramm N, McDonald M, Limbird L. The alpha(2a)-adrenergic receptor plays a protective role in mouse behavioral models of depression and anxiety. J Neurosci. 2001;21:4875–4882. doi: 10.1523/JNEUROSCI.21-13-04875.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Shaham Y, Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology (Berl) 2011;218:69–82. doi: 10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Aston-Jones G. α(2) Adrenergic and imidazoline receptor agonists prevent cue-induced cocaine seeking. Biol Psychiatry. 2011;70:712–719. doi: 10.1016/j.biopsych.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Devito E, Waters A, Carroll K. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–463. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bokhoven P, Oomen C, Hoogendijk W, Smit A, Lucassen P, Spijker S. Reduction in hippocampal neurogenesis after social defeat is long-lasting and responsive to late antidepressant treatment. Eur J Neurosci. 2011;33:1833–1840. doi: 10.1111/j.1460-9568.2011.07668.x. [DOI] [PubMed] [Google Scholar]
- van Erp A, Miczek K. Persistent suppression of ethanol self-administration by brief social stress in rats and increased startle response as index of withdrawal. Physiol Behav. 2001;73:301–311. doi: 10.1016/s0031-9384(01)00458-9. [DOI] [PubMed] [Google Scholar]
- Ventura R, Morrone C, Puglisi-Allegra S. Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. Proc Natl Acad Sci USA. 2007;104:5181–5186. doi: 10.1073/pnas.0610178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Frijtag J, Kamal A, Reijmers L, Schrama L, van den Bos R, Spruijt B. Chronic imipramine treatment partially reverses the long-term changes of hippocampal synaptic plasticity in socially stressed rats. Neurosci Lett. 2001;309:153–156. doi: 10.1016/s0304-3940(01)02062-6. [DOI] [PubMed] [Google Scholar]
- Von Frijtag J, Reijmers L, Van der Harst J, Leus I, Van den Bos R, Spruijt B. Defeat followed by individual housing results in long-term impaired reward- and cognition-related behaviours in rats. Behav Brain Res. 2000;117:137–146. doi: 10.1016/s0166-4328(00)00300-4. [DOI] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu YS, Simen A, Duque A, et al. alpha 2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Wouda J, Riga D, De Vries W, Stegeman M, Van Mourik Y, Schetters D, et al. Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology. 2011;216:267–277. doi: 10.1007/s00213-011-2213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


