Abstract
Polymorphisms in the gene encoding the serotonin synthesis enzyme Tph2 have been identified in mental illnesses, including bipolar disorder, major depression, autism, schizophrenia, and ADHD. Deficits in cognitive flexibility and perseverative behaviors are shared common symptoms in these disorders. However, little is known about the impact of Tph2 gene variants on cognition. Mice expressing a human TPH2 variant (Tph2-KI) were used to investigate cognitive consequences of TPH2 loss of function and pharmacological treatments. We applied a recently developed behavioral assay, the automated H-maze, to study cognitive functions in Tph2-KI mice. This assay involves the consecutive discovery of three different rules: a delayed alternation task, a non-alternation task, and a delayed reversal task. Possible contribution of locomotion, reward, and sensory perception were also investigated. The expression of loss-of-function mutant Tph2 in mice was associated with impairments in reversal learning and cognitive flexibility, accompanied by perseverative behaviors similar to those observed in human clinical studies. Pharmacological restoration of 5-HT synthesis with 5-hydroxytryptophan or treatment with the 5-HT2C receptor agonist CP809.101 reduced cognitive deficits in Tph2-KI mice and abolished perseveration. In contrast, treatment with the psychostimulant methylphenidate exacerbated cognitive deficits in mutant mice. Results from this study suggest a contribution of TPH2 in the regulation of cognition. Furthermore, identification of a role for a 5-HT2 receptor agonist as a cognition-enhancing agent in mutant mice suggests a potential avenue to explore for the personalized treatment of cognitive symptoms in humans with reduced 5-HT synthesis and TPH2 polymorphisms.
Keywords: cognitive flexibility, H-maze, perseveration, R439H-Tph2-KI mouse, reversal learning, 5-HT2C receptor
INTRODUCTION
Tryptophan hydroxylase 2 (Tph2) is the rate-limiting enzyme for brain serotonin (5-HT) synthesis (Walther et al, 2003; Zhang et al, 2004). This member of the amino-acid hydroxylase family catalyzes the formation of the direct 5-HT precursor, 5-hydroxy-tryptophan (5-HTP) from tryptophan (Walther et al, 2003). Multiple noncoding or loss-of-function polymorphisms in the TPH2 gene have been identified as potential genetic risk factors for several psychiatric illnesses, including bipolar disorder, major depression, attention deficit hyperactivity disorder (ADHD), and schizophrenia (Popova and Kulikov, 2010; Russo et al, 2009; Waider et al, 2011).
Cognitive dysfunctions, including impaired reversal learning, deficits in cognitive flexibility, and perseverative behaviors are shared symptoms in these illnesses (Iosifescu, 2012). Interestingly, studies have shown that acute 5-HT depletion, in humans and animals, may contribute to the development of these cognitive symptoms (Clarke et al, 2004; Izquierdo et al, 2012). Noncoding TPH2 single nucleotide polymorphisms (SNP) have also been identified in humans exhibiting such cognitive deficits (Strobel et al, 2007). However, the consequences on cognition of a chronic alteration in Tph2 enzymatic activity resulting from loss of function mutations have remained unexplored.
The R439H Tph2-KI mice carry a rare coding single point mutation (SNP) identified in a cohort of humans with major depression as well as in one individual with low 5-HT synthesis (Zhang et al, 2005). This mutation results in a reduction of brain 5-HT synthesis by ∼40% in R/H heterozygous (HET) and ∼80% in H/H homozygous (HO) animals (Beaulieu et al, 2008). Tph2-KI mice replicate alterations in mood disorder and serotonin-related biomarkers observed in human pathologies (Jacobsen et al, 2012b; Lavoie et al, 2013), which provide the opportunity to use R439H Tph2-KI mice as a model with construct, face, and predictive validity to investigate consequences of 5-HT depletions (Dzirasa et al, 2013; Jacobsen et al, 2012a).
Here we investigate the consequences of chronic 5-HT deficiency and pharmacological treatments on cognitive functions in R439H Tph2-KI mice with the automated H-maze (Del'Guidice et al, 2009; Girard et al, 2012). This behavioral test is a recently developed cognitive assay that involves a delayed reaction paradigm (Belhaoues et al, 2005), consisting in the consecutive discovery of three different rules: a delayed alternation task, a non-alternation task, and a delayed reversal task requiring a stronger mobilization of cognitive resources (Del'Guidice et al, 2009). The inclusion of this task was used as an analog to observations made in humans for whom the successive challenges of inhibiting a rule and finding a new one become more and more difficult with repetitions (Baddeley, 2012). These tasks, respectively, evaluate rule learning, cognitive flexibility/reversal learning and adaptability to increasingly complex problem-solving conditions. Our results showed that long-lasting 5-HT synthesis reduction in Tph2-KI mice is associated with altered cognitive flexibility, deficits in reversal learning and the development of perseverative behaviors. Acute rescue of 5-HT synthesis in these mutant mice using the 5-HT precursor 5-HTP or the stimulation of 5HT2C receptors, with the agonist CP809.101, improved cognitive functions and abolished perseverative behaviors.
MATERIAL AND METHODS
Animals
R439H Tph2-KI mice have been described previously (Beaulieu et al, 2008). Respective wild-type R/R (WT) littermates were used as controls for mutant mice and all mice were 3–4 months old. Experiments were carried out using cohorts composed of 50% male and 50% female mice. No behavioral differences were observed between sexes in any of the tests. Different groups of animals were used for each behavioral test. All mice were housed 3–4 per cage and maintained on a 12-h light/dark cycle (lights on at 0700 hours) in a humidity-controlled room at 23 °C. Mice were kept with food and water available ad libitum throughout the experiments, except for habituation and training periods. All procedures were conducted in accordance with guidelines from the Canadian Council on Animal Care and approved by Laval University Animal Care Committee.
Drug Administration
All drugs were administered intraperitoneally (i.p.) in a volume of 10 ml/kg saline. Methylphenidate (MPH; Tocris Cookson, Ballwin, MI) was injected at 30 mg/kg as described (Beaulieu et al, 2006). 5-carboxamidotryptamine (5-CT; Sigma-Aldrich, Oakville, Canada) and CP809.101 (Tocris Cookson, Ballwin, MI) were administered at 0.1 and 1 mg/kg, respectively as described (Beaulieu et al, 2006; Siuciak et al, 2007). 5-hydroxy-L-tryptophan (5-HTP; Sigma-Aldrich, Oakville, Canada) was administered at a 20 mg/kg dose (Jacobsen et al, 2012b). Drugs were administered 30 min before the different tests.
Automated H-Maze
The automated H-maze was performed as described previously in (Del'Guidice et al, 2009), see Supplementary Information for Material And Methods. Briefly, mice were assessed in three consecutive delayed response tasks (Belhaoues et al, 2005)—Alternance (ALT), Nonalternance (N-ALT), and Reversal (REV). In the first task (ALT), mice had to learn to alternately move between both chambers of the H-maze to induce the automatic delivery of a hydric reward (turn left, then turn right, in alternation) (Supplementary Figure S1A). In the second task, mice had to accomplish a response inhibition of the first task in order to learn the second rule (N-ALT) that consisted in always turning to the same side to enter each chamber (Supplementary Figure S1A). In the third task (REV), mice had to execute the opposite strategy of the one learned in the N-ALT task and reward could only be obtained by learning the new strategy (Supplementary Figure S1A). For each task, the overall completion or ‘rate of survival' as well as the number of trials taken to complete the task were measured. Occurrence of specific behavioral strategies was also quantified. Shifting deficits, and indication of a deficit in reversal learning, were defined as maintenance of the ALT strategy at the beginning of the N-ALT task (Supplementary Figure S1B). Perseveration errors were defined as episodes of more than six repetitive failed attempts indicating that the mice display a deficit in the test by repetitively applying an invalid strategy (Del'Guidice et al, 2009).
Cross Maze Test
The cross-maze procedure was based on previous behavioral investigations (Marquis et al, 2006, 2008), see Supplementary Information for material and methods.
Olfactory Perception in a Novelty-Related Test
The olfactory perception test was performed as described (Breton-Provencher et al, 2009), see Supplementary Information for material and methods.
Sucrose Preference and Quinine Aversion Tests
Sucrose preference and quinine avoidance tests were performed as described (Ninomiya et al, 1995), see Supplementary Information for material and methods.
Locomotion in a Novel Environment
Locomotion was assessed under illuminated conditions in an automated Omnitech Digiscan apparatus (AccuScan Instruments, Columbus, OH) as described (Beaulieu et al, 2004). Mice were injected 30 min before the test and then placed into the activity monitor for 30 min. Locomotor activity was expressed as the total distance covered.
Motor Coordination in the Rotarod Test
Motor abilities of mice were assessed on a rotarod (Med-Associates, St Albans, VT) using the 2 to 20 RPM acceleration program as described (Xi et al, 2005).
Statistical Analysis
To compare performances between mice from the three genotypes in the H-maze, data were processed in analysis of variance (ANOVA) followed, when necessary, by Newman–Keuls post hoc comparisons, using the Prism statistic software 2007 (GraphPad Software, La Jolla, CA). To compare the effects of drugs on cognition in the H-maze, olfactory perception, preference/avoidance tests, and motor tests, we proceeded with two-way ANOVAs followed by Bonferroni post hoc tests to compare replicate means by genotype. Nonparametric Mann–Whitney U test has been used to compare percentage of cumulative successes. Two-tailed t-tests were also used to compare the performances between vehicle WT and vehicle HO mice used as control for drug treatments. The p-levels (p⩽0.05, p⩽0.01, p⩽0.001) in the figures are denoted with asterisks (*, **, and ***, respectively). Only significant statistical analyses are presented.
RESULTS
Tph2-KI Mice Show Reversal Learning Deficits and Perseverative Behaviors in the H-Maze
Performances of Tph2-KI mice were evaluated in a traditional, non-automated cross-maze paradigm involving the sequential learning of two rules (Marquis et al, 2006, 2008). All WT mice learned the tasks in this test, whereas only 82% of HO mice completed both tasks. No significant difference was found between groups in the number of trial taken to complete each task (two-way ANOVA: F(1,58)=0.83, NS; Bonferroni post hoc test: NS) (Supplementary Figure S2A). However, HO mice showed a trend toward perseverative strategies (Supplementary Figure S2B). Nevertheless, this trend remained nonsignificant (two-way ANOVA: F(1,58)=0.83, NS; Bonferroni post hoc test: NS), possibly due to elevated variability between experimental groups. This suggested that application of a more stringent behavioral test might uncover cognitive differences between WT and Tph2-KI mice.
Mice were then tested using the automated H-maze (Supplementary Figure S1). Individual performances of each mouse were analyzed, for each consecutive task (Figure 1). The performances of representative untreated WT and HET mice that completed the three tasks were similar (Figure 1a and b, Supplementary Figure S1A). In contrast, the few HO R439H Tph2-KI mice that completed the tasks did it within more trials than mice from other genotypes (Figure 1c). Analysis of HO mice strategies showed two types of perseverative behaviors that were first manifested at the transition between the ALT and N-ALT task. Immediately after learning the first task, HO mice continued with the same alternation strategy for several trials (Figure 1c and d, Supplementary Figure S1B), although this pattern was only reinforced half of the time (ANOVA: F(2,27)=4.84, p⩽0.05). The second behavior was observed in both the N-ALT and REV tasks only in HO mice and consisted in perseveration errors characterized by repetitive, consecutive, and nonrewarded trials (Figure 1c).
Figure 1.
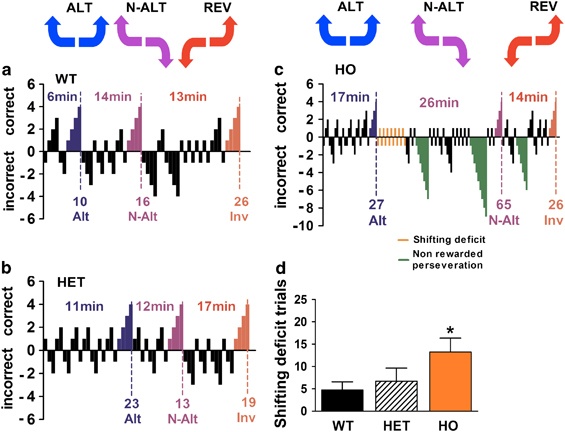
Tph2-KI mice show perseveration and strong impairments in reversal learning in the H-maze. (a–c) Representative examples of the performance of WT (a), HET (b) and HO (c) mice that have completed the three tasks. Each positive (top) and negative (bottom) bar corresponds to a rewarded or a failed trial, respectively. Dotted lines indicate that the mouse has reached the criterion of four consecutive successful trials for the task as defined (30). Expected correct behaviors are indicated above each task (ALT: blue; N-ALT: purple; REV: red, see Supplementary Figure S1A for details). Numbers on top of each task indicate the time required for the mouse to complete the task. Numbers on the bottom part indicate the number of trials required to complete each task. In c, the shifting deficits (orange bars) and perseveration errors (green bars) are indicated. Shifting deficits were defined as maintenance of the ALT strategy at the beginning of the N-ALT task (see Supplementary Figure S1B for details). Perseveration errors were defined as episodes of more than six repetitive failed attempts as defined (Del'Guidice et al, 2009). (d) Quantification of shifting deficit from ALT to N-ALT task for each Tph2-KI mouse genotype. It should be noted here that the term perseveration errors is an operational definition that can only be applied to describe a strategy used by a mouse in the test. The occurrence or nonoccurrence of such errors should not be construed as a measurement of all perseverative or repetitive behaviors (Del'guidice et al, 2013). Data are means±SEM of number of trials corresponding to a correct ALT strategy at the beginning of the N-ALT task. n=10 mice per genotype. (Detailed gender repartition of mice used for the different automated H-Maze experiments are listed in Supplementary Table S2) , *p⩽0.05, one-way ANOVA with Newman–Keuls post hoc test.
Impact of tph2 Genotype on Performances for each of the Three Different Tasks
Mice from all genotypes were able to reach the criterion of four consecutive successful trials in the ALT task (Figure 2a and b). However, there were statistically significant differences between the three groups (Figure 2a: ANOVA: F(2,27)=5.51, p⩽0.01). Compared with the performances of WT mice, HET, and HO mice required more trials to learn the first rule (Newman–Keuls test: p⩽0.05 and p⩽0.01, respectively).
Figure 2.
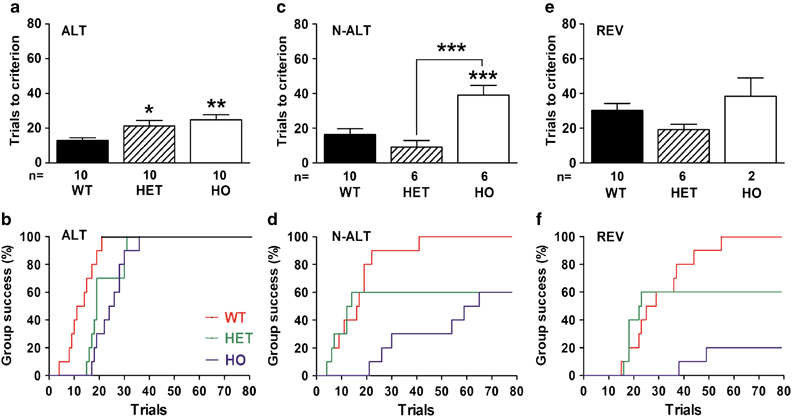
Performances per genotype of Tph2-KI mice in each task of the automated H-Maze. (a, c, and e) Average number of trials (±SEM) needed to reach the criterion of four consecutive successful trials for mice of the WT, HET, and HO genotype in (a) the ALT task, (c) the N-ALT task and (e) the REV task. Average number of trials was calculated only from the performances of mice that completed each task. (b, d, and f) Survival curves representing the number of trials required to reach maximum group success for genotype in each task (b) the ALT, (d) the N-ALT and (f) the REV tasks. Initial n=10 mice per genotype, number of remaining animals used to calculate average number of trials are indicated below each condition. *p⩽0.05, **p⩽0.01, and ***p⩽0.001, one-way ANOVA test with Newman–Keuls post hoc test.
A significant difference was found between the three genotypes in the N-ALT task (ANOVA: F(2,219)=13.47, p⩽0.001) (Figure 2c). HO mice required approximately three times more trials than mice from the WT and HET groups to learn the second task (Newman–Keuls test: p⩽0.001). Furthermore, only 60% of HET and HO mice completed the N-ALT task (Figure 2d).
In the REV task (Figure 2e and f), no significant difference was found between the WT and the remaining mice of the HET group (Student's t-test, NS). No statistical analysis was ran on HO mice, as only two of them completed the REV task.
Olfactory Perception is not Affected in Tph2-KI Mice
As the H-maze relies on odor-assisted learning (Del'Guidice et al, 2009), an olfactory perception test (Breton-Provencher et al, 2009) was carried out to establish whether reduced performances in mutant mice could be attributed to altered olfactory sensitivity. Mice from all genotypes showed a similar increase in odor detection between 10−6 and 10−5 μl/l for both odors (Figure 3a and b). Therefore, no difference in odor detection was observed between the three groups across two different odors. Furthermore, no significant difference in exploration was found between genotypes during the habituation sessions.
Figure 3.
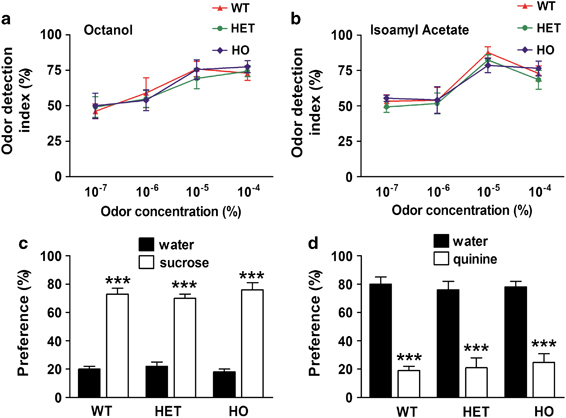
5-HT deficiency does not affect olfactory perception, sucrose preference, or quinine avoidance in Tph2-KI mice. (a and b) Odor detection threshold of WT, HET, and HO Tph2-KI mice for the two odors isoamyl acetate and octanol. Normalized values are expressed as the mean ratio between time spent investigating the odor and total sniffing time (ie, odor plus mineral oil). Data are presented as mean±SEM (n=8 mice per group). (c and d) Preference/avoidance test in WT, HET, and HO Tph2-KI mice for two different tastes (C-sucrose and D-quinine). Normalized values are expressed as the mean ratio between the quantity of sucrose or quinine drank and total volume of liquid absorbed (ie, water plus sucrose or water plus quinine). Data are presented as mean±SEM (n=17 mice per group). Two-way ANOVA with Bonferroni post hoc tests. ***p⩽0.001.
Normal Sucrose Preference and Quinine Avoidance in Tph2-KI Mice
Sucrose preference and a quinine avoidance test were also carried out to establish if differences in the performances observed in the automated H-maze could be attributed to deficits in reward or avoidance (Figure 3c and d). No difference was observed between the three genotypes in the two tests (two-way ANOVA: F(2,54)=1.11 and 0.3, NS, respectively). Mice from all genotypes showed a significant preference for sucrose (two-way ANOVA: CF(2,54)=377.3, p⩽0.001, Figure 3c) and an aversion to quinine (two-way ANOVA: CF(254)=167, p⩽0.001, Figure 3d) as compared with water.
Deterioration of Cognitive Performances Following Methylphenidate Treatment
As the neurotransmitter dopamine (DA) modulates the 5-HT system (Alex and Pehek, 2007), we first attempted to improve cognitive functions of Tph2-KI mice using the dopaminergic psychostimulant MPH. This drug is used for the treatment of attention deficit hyperactivity disorder (ADHD) and is known to increase some cognitive abilities in humans (Arnsten and Dudley, 2005). Furthermore, recent data from our group have shown that acute treatment with methylphenidate restore the cognitive performance of dopamine transporter knockout (DAT-KO) mice in the automated H-maze (Del'guidice et al, 2013).
Figure 4a and b show representative examples of vehicle-treated WT and HO mice. Most MPH-treated WT mice failed to complete the three tasks (Figure 4c, Figure 5a) (Mann–Whitney test: U=8, p⩽0.01). This treatment also reduced the success rate of Tph2-KI HO animals in the N-ALT and REV tasks (Figure 5b). One contributing factor to this further deterioration of performances may come from a dramatic increase in the occurrence of perseveration errors in Tph2-KI mice treated with MPH (Figure 4d, Figure 5c) (ANOVA: F(4,39)=14.40, p⩽0.001; Newman–Keuls test: p⩽0.001). Interestingly, while showing reduced performances, WT mice treated with MPH did not display the perseverative behaviors observed in vehicle-treated HO-mutant mice (Figure 4b and c), suggesting that enhanced DA tones and reduction of 5-HT synthesis have different effects on cognitive abilities, at least in the H-maze.
Figure 4.
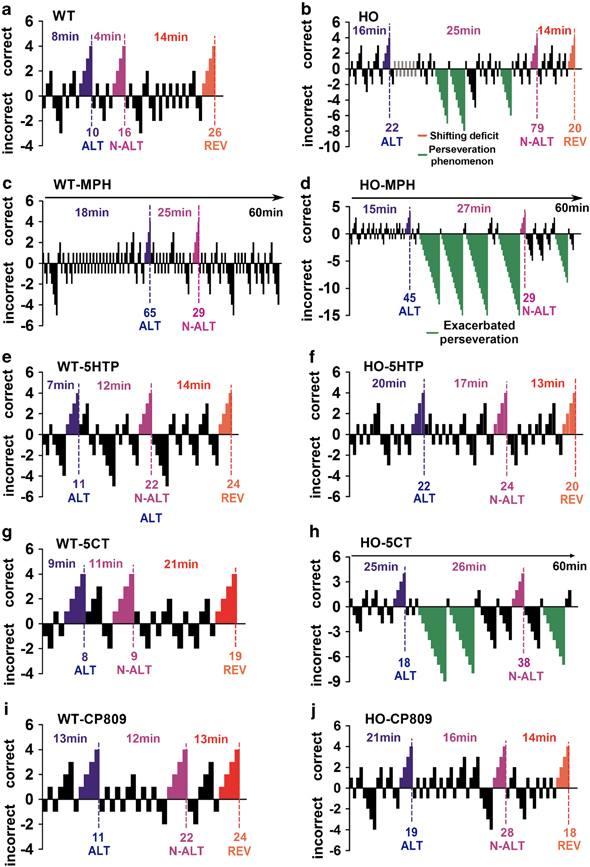
Representative example of the effects of monoaminergic drugs on cognitive performances of Tph2-KI mice. (a and b) Vehicle-treated WT (a) and HO (b) mice. (c and d) MPH-treated WT (c) and HO (d) mice. (e and f) 5-HTP-treated WT (e) and HO (f) mice. (g and h) 5-CT-treated WT (g) and HO (h) mice. (i and j) CP809.101-treated WT (i) and HO (j) mice. Each positive (top) and negative (bottom) bar corresponds to a rewarded or a failed trial, respectively. Dotted lines indicate that the mouse has reached the criterion of four consecutive successful trials for the task as defined (Del'Guidice et al, 2009). Expected correct behaviors are indicated above each task (ALT: blue; N-ALT: purple; REV: red, see Supplementary Figure S1A for details). Numbers on top of each task indicate the time required for the mouse to complete the task. Numbers on the bottom part indicate the number of trials required to complete each task. Shifting deficits (orange bars) and perseveration errors (green bars) are indicated. Shifting deficits were defined as maintenance of the ALT strategy at the beginning of the N-ALT task (see Supplementary Figure S1B for details). Perseveration errors were defined as episodes of more than six repetitive failed attempts as described (Del'Guidice et al, 2009). See method section for a description of drug administration conditions and doses. n=8 mice per conditions, detailed gender repartition and effectives of mice used for the different automated H-Maze experiments are listed in Supplementary Table S2. Relative affinity of 5-CT and CP809.101 for different 5-HT receptors is listed in Supplementary Table S3.
Figure 5.
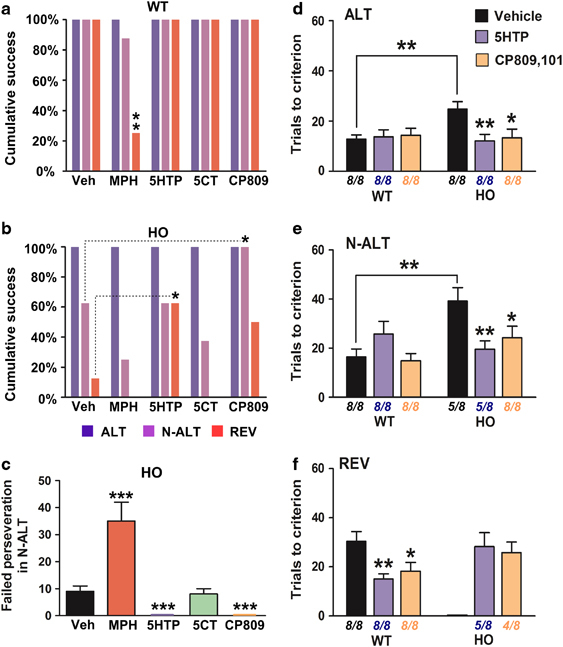
Effects of monoaminergic drug treatment on cumulative success rates, perseveration, and task performances in the automated H-maze. (a and b) Cumulative success rates (%) for WT (a) and HO (b) Tph2-KI mice in all three tasks of the automated H-maze following treatment with: vehicle, MPH, 5-HTP, 5-CT, or CP809.101. See method section for a description of drug administration conditions and doses. Data are presented as % of animals from each initial group that succeeded in completing each tasks. Initial n=8 mice per group. (c) Average number (±SEM) of perseveration errors for HO Tph2-KI mice during the N-ALT task. Perseveration errors were defined as episodes of more than six repetitive failed attempts as defined (Del'Guidice et al, 2009). Calculation of perseveration errors included all animals independently of their ability to complete the N-ALT task. n=8 animals per group. (d–f) Average number of trials (±SEM) required for reaching the criterion of 4 consecutive successful trials for mice of the WT and HO genotype after treatment with vehicle, 5-HTP or CP809.101. See method section for a description of drug administration conditions and doses. (d) the ALT task, (e) the N-ALT task, and (f) the REV task. Average number of trials was calculated only from the performances of mice that completed each task. Initial n=8 mice per genotype, number of remaining animals used to calculate average numbers of trials are indicated below each condition. *p⩽0.05, **p⩽0.01, and ***p⩽0.001, a and b, Mann–Whitney test; c, one-way ANOVA test with Newman–Keuls post hoc test, d–f, two-way ANOVA with Bonferroni post hoc tests; f, one-way ANOVA test with Newman–Keuls post hoc test.
Acute 5-HT Restoration Therapy Improves Cognitive Performances in Tph2-KI Mice
We then explored the effect of an acute restoration of 5-HT synthesis on cognitive performances in Tph2-KI mice. To achieve this, mice were treated with a dose of the 5-HT precursor 5-HTP that has been shown to restore normal 5-HT levels in R439H Tph2-KI mice (Siesser et al, 2013). Administration of 5-HTP did neither affect the performances nor success rates of WT mice in the automated H-Maze (Figure 4e, Figure 5a). In contrast, this treatment completely abolished the perseverative behaviors (Figure 4f, Figure 5c) (Newman–Keuls test: p⩽0.001) and improved overall completion of the three tasks in HO mice (Figure 5b) (Mann–Whitney test: U=16, p⩽0.05). As visual observation of 5-HTP-treated mice suggested an effect on locomotion, the effect of treatment on spontaneous and forced locomotion was evaluated using a locomotor activity monitor and an accelerating rotarod test (Supplementary Figure S3A-B). These tests confirmed that 5-HTP reduced spontaneous locomotor activity only in HO mice (two-way ANOVA: F(1,28)=4.75, p⩽0.05; Bonferroni post hoc test: p⩽0.01) without affecting motor functions as evaluated with the accelerating rotarod (Supplementary Figure S3B).
5-HT2C Agonist Improves Cognitive Performances in Tph2-KI Mice
Different agonists were then used to identify the 5-HT receptor types involved in the regulation of cognitive performances in Tph2-KI mice. Co-stimulation of the 5-HT1, 5-HT5, and 5-HT7 receptors with 5-CT did not improve overall completion of the test by WT or HO mice (Figure 4g and h, Figure 5a and b). Furthermore, this drug had no effect on the perseverative behaviors of HO mice (Figure 5c) while reducing spontaneous locomotor activity in both WT and HO mice (Supplementary Figure S3C) (two-way ANOVA: F(1,28)=33.64, p⩽0.001). In contrast, the 5-HT2C preferring agonist CP809.101 had no effect on WT mice performances (Figure 4i, Figure 5a) but reinstated the reversal learning and shifting abilities of HO mice (Figure 4j), increased overall completion of the three tasks (Figure 5b) (Mann–Whitney test: U=20, p⩽0.05) and completely abolished perseveration (Figure 5c) (Newman–Keuls test: p⩽0.001). This compound also reduced locomotor activity in mice from both genotypes (Supplementary Figure S3E) (two-way ANOVA: F(1,28)=19.16, p⩽0.001). None of these two serotonergic drugs impaired motor coordination or skills of WT and HO mice as measured by an accelerating rotarod test (Supplementary Figure S3D, F).
Impact of 5-HTP and CP809.101 Treatments on Tph2-KI Mice Performances in the Three Different Tasks
Beyond their effects on perseveration and overall completion of the different tasks, we then verified the impact of 5-HTP and CP809.101 treatments on learning performances. In the first task (ALT) (Figure 5d), a significant difference in the number of trials required to complete the task was found between the different treatments (two-way ANOVA: F(2,42)=3.88, p⩽0.05). Vehicle HO mice required significantly more trials to complete the ALT task as compared with vehicle WT mice (Two-tailed t-test: p⩽0.01). 5-HTP and CP809.101 significantly improved performances of HO mice (Bonferroni post hoc test: p⩽0.01 and p⩽0.05, respectively).
In the N-ALT task (Figure 5e), the interaction between genotypes and treatments was significant for the number of trials required to complete the task (two-way ANOVA: F(2,39)=5.85, p⩽0.01). Only 60% of vehicle HO mice completed the task within more trials than animals from the WT group (Two-tailed t-test: p⩽0.01). Treatment with 5-HTP or CP809.101 reduced the number of trials required by Tph2-KI HO mice to complete the task as compared with vehicle-treated mice (Bonferroni post hoc test: p⩽0.01 and p⩽0.05, respectively).
In REV task (Figure 5f), a significant reduction was found between the number of trials required by WT mice to complete the task after 5-HTP and CP809.101 treatments as compared with vehicle (ANOVA: F(2,23)=6.04, p⩽0.01 followed by Newman–Keuls multiple comparison test: p⩽0.01 and p⩽0.05, respectively). In HO mice, while only one vehicle-treated mouse found the rule, 60% of the 5-HTP-treated HO mice and 50% of CP809.101-treated HO mice learned the third rule within a number of trials that was equivalent to those required by vehicle- treated WT mice, therefore confirming the efficacy of these two treatments on cognitive performances across the three tasks of the automated H-Maze.
DISCUSSION
The results presented here show that a single nucleotide loss of function polymorphism in tph2 is sufficient to induce gene-dose-dependent cognitive deficits in mice. Furthermore, acute restoration of 5-HT levels through administration of 5-HTP or the 5-HT2c receptor agonist CP809.101 can improve cognitive flexibility and reversal learning abilities independently from their effects on locomotion.
Mice with tph2 loss of function mutation displayed shifting deficit accompanied by unrewarded perseveration strategies. Perseveration is reminiscent of broad cognitive symptoms observed in several neurological and psychiatric illnesses (Hauser, 1999; Joseph, 1999). Similar patterns of cognitive deficits have previously been associated with prefrontal cortex (PFC) dysfunctions in humans and mice with PFC-lesions (Del'Guidice et al, 2009; Verin et al, 1993). Recent characterization of brain circuit dysfunctions in Tph2-KI mice (Dzirasa et al, 2013) revealed increased synchrony between the amygdala and the PFC. Furthermore, amygdala lesions have been shown to facilitate reversal learning in rats (Izquierdo et al, 2012). Taken together with our observations, this suggests that cognitive deficits associated with tph2 loss of function mutation in mice may arise, at least in part, from increased PFC amygdala connectivity resulting from 5-HT deficiency.
In addition to the possible contribution of the PFC, Tph2-KI mice have been shown to display exaggerated emotional responses in several behavioral tests relevant to the assessment of ‘depressive-like', ‘anxiety-like', and aggressive behaviors (Beaulieu et al, 2008). In line with our observations, humans carrying TPH2 non-coding SNPs (Strobel et al, 2007), patients with affective disorders and healthy subject submitted to chronic stress and anxiety all tend to present deficits of memory, attention, and/or executive functions (Castaneda et al, 2008; Gass and Curiel, 2011; O'Toole and Pedersen, 2011). High levels of anxiety have also been associated with perseverative behaviors in a number of cognitive tasks in rodents (Gill et al, 2012; Jiao et al, 2011). Consequently, anxiety might also represent a mediating factor contributing to cognitive deficits in mice with tph2 loss of function mutation.
Recent investigations have shown that an increase of 5-HT transmission may improve performances in different behavioral tests assessing cognitive flexibility in rodents (Bari et al, 2010; Charles et al, 2011). Furthermore, impaired reversal learning and perseveration have also been reported following acute tryptophan and 5-HT depletion in humans and other primates (Clarke et al, 2004; Evers et al, 2010). In line with this, the 5-HT precursor 5HTP or the 5HT2C preferring receptor agonist CP809.101 improved cognitive flexibility and reversal learning in Tph2-KI mice (Supplementary Table S1). These two drugs also exerted a positive effect on WT mice performances, albeit only in the more complicated last task of the test. This indicates that cognitive deficits induced by a tph2 loss of function variant are not fully explained by long-term adaptation to 5-HT depletion and can therefore be alleviated, at least in part, by an acute stimulation of 5-HT neurotransmission. Importantly, the effects of 5HTP and CP809.101 cannot be solely explained by a reduction of locomotor activity, as 5-CT had a comparable effect on locomotion without alleviating cognitive deficits in Tph2-KI mice.
Pharmacological and genetic studies have suggested that several 5-HT receptors may contribute to the regulation of PFC-mediated reversal learning and cognitive flexibility (Homberg, 2012; Jensen et al, 2010). The different effects of 5-CT and CP809.101 on cognitive deficits in Tph2-KI mice suggest that targeting 5HT2C receptors may be a viable avenue to improve cognition in humans with TPH2 mutations. CP809.101 is a recently developed 5HT2C receptor agonist (Siuciak et al, 2007) (Supplementary Table S3). However, we cannot fully exclude potential effects of this drug on others 5-HT receptors as CP809.01 can also bind to the 5HT2A receptors, albeit with approximately ninefold lesser affinity (Jensen et al, 2013). Furthermore, while CP809.101 was reported to improve cognitive functions in rodents without affecting emotional behavior (Siuciak et al, 2007), results obtained with several other 5HT2C receptor agonists are variable (Dougherty and Oristaglio, 2013; Schmidt et al, 1995). Given the complex phenotype of Tph2-KI mice and the anxiolytic effects of several serotonergic drugs (Bandelow et al, 2012; Witkin, 2008), it could thus also be premature to conclude that the effect of CP809.101 on Tph2-KI mice in the H-maze is entirely independent from an effect on anxiety.
In contrast to CP809.101 and 5HTP, MPH worsened the cognitive deficits of Tph2-KI mice and exacerbated their perseverative behaviors (Supplementary Table S1). This result is in contrast with beneficial effect of the same drug at the same dose on the cognitive functions of DAT-KO mice in the H-maze (Del'guidice et al, 2013). Furthermore, we have also shown that CP809.101 does not rescue the cognitive deficits of DAT-KO mice in the same test (Del'guidice et al, 2013). We elected to test the effects of MPH because TPH2 polymorphisms have been implicated in reversal learning deficits observed in ADHD (Baehne et al, 2009). Furthermore, this drug is often the first line of pharmacological treatment for children with learning disabilities. If a drug can induce opposite cognitive effects at the same dose and in the same tests in mice carrying different mutations, it is also possible that similar divergent effects may also occur in humans. This underscores the need for studies of drug–gene interaction to develop better cognition enhancing agents and personalized treatments for cognitive symptoms in people with gene polymorphisms affecting monoamine-mediated neurotransmission (Baehne et al, 2009; Pasini et al, 2013; Serretti et al, 2011). Furthermore, the distinct cognitive profiles of DAT-KO and Tph2-KI mice observed in the H-maze denotes the needs to develop strategies to further study the specific impact of such polymorphisms on behavior by coupling the use of animals carrying specific human variants with new technical approaches like the H-maze.
Various TPH2 polymorphisms are potential genetic risk factors for major depression, ADHD, bipolar disorder and schizophrenia (Baehne et al, 2009; Serretti et al, 2011). However, the specific impact of such polymorphisms on behavior needs to be further studied using animals carrying specific human variants in order to understand their contribution to psychiatric symptomatology and their potential usefulness as diagnostic tools (Millan et al, 2012). While limited to a single rare human TPH2 gene variant, the results obtained using the automated H-Maze and R439H Tph2-KI mice indicate that TPH2 loss of function polymorphisms might contribute to cognitive deficits. Furthermore, our observations on the relative efficacy of MPH and CP809.101 in Tph2-KI mice suggest that information about TPH2 loss of function polymorphisms can potentially instruct treatment strategies. This may provide an opportunity to develop better cognition-enhancing agents and personalized treatments for cognitive symptoms in people with reduced 5-HT synthesis and TPH2 polymorphisms.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
We thank N Bouchard and K Aubé for assistance maintaining mice colonies, and Hugues Dufour for assembling the H-Maze. TD is recipient of fellowships from the CRCN. FL is supported by a scholarship from the Natural Sciences and Engineering Research Council of Canada (NSERC). JMB is NARSAD Vital Projects Fund. investigator and Canada research Chair in Molecular Psychiatry. This work was supported by a Canadian Institute of Health Research (CIHR) operating grant (NSA 93798) to JMB, a NSERC discovery grant to FYD and a FRSQ project for innovative strategic development.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: Relevance to therapeutic effects in attention deficit hyperactivity disorder. Behav Brain Funct. 2005;1:2. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. Working memory: theories, models, and controversies. Annu Rev Psychol. 2012;63:1–29. doi: 10.1146/annurev-psych-120710-100422. [DOI] [PubMed] [Google Scholar]
- Baehne CG, Ehlis AC, Plichta MM, Conzelmann A, Pauli P, Jacob C, et al. Tph2 gene variants modulate response control processes in adult ADHD patients and healthy individuals. Mol Psychiatry. 2009;14:1032–1039. doi: 10.1038/mp.2008.39. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Sher L, Bunevicius R, Hollander E, Kasper S, Zohan J, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77–84. doi: 10.3109/13651501.2012.667114. [DOI] [PubMed] [Google Scholar]
- Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW, et al. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology. 2010;35:1290–1301. doi: 10.1038/npp.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Paradoxical striatal cellular signaling responses to psychostimulants in hyperactive mice. J Biol Chem. 2006;281:32072–32080. doi: 10.1074/jbc.M606062200. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci USA. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, et al. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci USA. 2008;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhaoues R, Soumireu-Mourat B, Caverni JP, Roman FS. A novel experimental paradigm for studying cognitive functions related to delayed response tasks in mice. Brain Res Cogn Brain Res. 2005;23:199–206. doi: 10.1016/j.cogbrainres.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Breton-Provencher V, Lemasson M, Peralta MR, 3rd, Saghatelyan A. Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. J Neurosci. 2009;29:15245–15257. doi: 10.1523/JNEUROSCI.3606-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda AE, Tuulio-Henriksson A, Marttunen M, Suvisaari J, Lonnqvist J. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord. 2008;106:1–27. doi: 10.1016/j.jad.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Charles PD, Ambigapathy G, Geraldine P, Akbarsha MA, Rajan KE. Bacopa monniera leaf extract upregulates tryptophan hydroxylase (TPH2) and serotonin transporter (SERT) expression: implications in memory formation. J Ethnopharmacol. 2011;134:55–61. doi: 10.1016/j.jep.2010.11.045. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Del'guidice T, Lemasson M, Etievant A, Manta S, Magno LA, Escoffier G, et al. 2013Dissociations between cognitive and motor effects of psychostimulants and atomoxetine in hyperactive DAT-KO mice Psychopharmacology (Berl)(e-pub ahead of print). [DOI] [PubMed]
- Del'Guidice T, Nivet E, Escoffier G, Baril N, Caverni JP, Roman FS. Perseveration related to frontal lesion in mice using the olfactory H-maze. Behav Brain Res. 2009;205:226–233. doi: 10.1016/j.bbr.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Dougherty JP, Oristaglio J. Chronic treatment with the serotonin 2A/2C receptor antagonist SR 46349B enhances the retention and efficiency of rule-guided behavior in mice. Neurobiol Learn Mem. 2013;103:50–63. doi: 10.1016/j.nlm.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Dzirasa K, Kumar S, Sachs BD, Caron MG, Nicolelis MA. Cortical-amygdalar circuit dysfunction in a genetic mouse model of serotonin deficiency. Jneuroscience: the official journal of the Society for Neuroscience. 2013;33:4505–4513. doi: 10.1523/JNEUROSCI.4891-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers EA, Sambeth A, Ramaekers JG, Riedel WJ, van der Veen FM. The effects of acute tryptophan depletion on brain activation during cognition and emotional processing in healthy volunteers. Curr Pharm Des. 2010;16:1998–2011. doi: 10.2174/138161210791293060. [DOI] [PubMed] [Google Scholar]
- Gass CS, Curiel RE. Test anxiety in relation to measures of cognitive and intellectual functioning. Arch Clin Neuropsychol. 2011;26:396–404. doi: 10.1093/arclin/acr034. [DOI] [PubMed] [Google Scholar]
- Gill DA, Perry MA, McGuire EP, Perez-Gomez A, Tasker RA. Low-dose neonatal domoic acid causes persistent changes in behavioural and molecular indicators of stress response in rats. Behav Brain Res. 2012;230:409–417. doi: 10.1016/j.bbr.2012.02.036. [DOI] [PubMed] [Google Scholar]
- Girard SD, Baranger K, Gauthier C, Jacquet M, Bernard A, Escoffier G, et al. Evidence for early cognitive impairment related to frontal cortex in the 5XFAD Mouse Model of Alzheimer's Disease. J Alzheimers Dis. 2012;33:781–796. doi: 10.3233/JAD-2012-120982. [DOI] [PubMed] [Google Scholar]
- Hauser MD. Perseveration, inhibition and the prefrontal cortex: a new look. Curr Opin Neurobiol. 1999;9:214–222. doi: 10.1016/s0959-4388(99)80030-0. [DOI] [PubMed] [Google Scholar]
- Homberg JR. Serotonin and decision making processes. Neurosci Biobehav Rev. 2012;36:218–236. doi: 10.1016/j.neubiorev.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Iosifescu DV. The relation between mood, cognition and psychosocial functioning in psychiatric disorders. Eur Neuropsychopharmacol. 2012;22 (Suppl 3):S499–S504. doi: 10.1016/j.euroneuro.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Carlos K, Ostrander S, Rodriguez D, McCall-Craddolph A, Yagnik G, et al. Impaired reward learning and intact motivation after serotonin depletion in rats. Behav Brain Res. 2012;233:494–499. doi: 10.1016/j.bbr.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JP, Medvedev IO, Caron MG. The 5-HT deficiency theory of depression: perspectives from a naturalistic 5-HT deficiency model, the tryptophan hydroxylase 2Arg439His knockin mouse. Philosoph Transact Royal Soc Lond Series B, Biol Sci. 2012;367:2444–2459. doi: 10.1098/rstb.2012.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JP, Siesser WB, Sachs BD, Peterson S, Cools MJ, Setola V, et al. Deficient serotonin neurotransmission and depression-like serotonin biomarker alterations in tryptophan hydroxylase 2 (Tph2) loss-of-function mice. Mol Psychiatry. 2012;17:694–704. doi: 10.1038/mp.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AA, Plath N, Pedersen MH, Isberg V, Krall J, Wellendorph P, et al. Design, synthesis, and pharmacological characterization of N- and O-substituted 5,6,7,8-tetrahydro-4H-isoxazolo[4,5-d]azepin-3-ol analogues: novel 5-HT(2A)/5-HT(2C) receptor agonists with pro-cognitive properties. J Med Chem. 2013;56:1211–1227. doi: 10.1021/jm301656h. [DOI] [PubMed] [Google Scholar]
- Jensen NH, Cremers TI, Sotty F. Therapeutic potential of 5-HT2C receptor ligands. ScientificWorldJournal. 2010;10:1870–1885. doi: 10.1100/tsw.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao X, Pang KC, Beck KD, Minor TR, Servatius RJ. Avoidance perseveration during extinction training in Wistar-Kyoto rats: an interaction of innate vulnerability and stressor intensity. Behav Brain Res. 2011;221:98–107. doi: 10.1016/j.bbr.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R. Frontal lobe psychopathology: mania, depression, confabulation, catatonia, perseveration, obsessive compulsions, and schizophrenia. Psychiatry. 1999;62:138–172. doi: 10.1080/00332747.1999.11024862. [DOI] [PubMed] [Google Scholar]
- Lavoie J, Illiano P, Sotnikova TD, Gainetdinov RR, Beaulieu JM, Hebert M. The electroretinogram as a biomarker of central dopamine and serotonin: potential relevance to psychiatric disorders. BiolPsychiatry. 2013;S0006-3223:01032–01033. doi: 10.1016/j.biopsych.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Marquis JP, Goulet S, Dore FY. Neonatal lesions of the ventral hippocampus in rats lead to prefrontal cognitive deficits at two maturational stages. Neuroscience. 2006;140:759–767. doi: 10.1016/j.neuroscience.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Marquis JP, Goulet S, Dore FY. Dissociable onset of cognitive and motivational dysfunctions following neonatal lesions of the ventral hippocampus in rats. Behav Neurosci. 2008;122:629–642. doi: 10.1037/0735-7044.122.3.629. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Agid Y, Brune M, Bullmore ET, Carter CS, Clayton NS, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11:141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Sako N, Imai Y. Enhanced gustatory neural responses to sugars in the diabetic db/db mouse. Am J Physiol. 1995;269 (4 Pt 2):R930–R937. doi: 10.1152/ajpregu.1995.269.4.R930. [DOI] [PubMed] [Google Scholar]
- O'Toole MS, Pedersen AD. A systematic review of neuropsychological performance in social anxiety disorder. Nord J Psychiatry. 2011;65:147–161. doi: 10.3109/08039488.2011.565801. [DOI] [PubMed] [Google Scholar]
- Pasini A, Sinibaldi L, Paloscia C, Douzgou S, Pitzianti MB, Romeo E, et al. Neurocognitive effects of methylphenidate on ADHD children with different DAT genotypes: a longitudinal open label trial. Eur J Paediatr Neurol. 2013;17:407–414. doi: 10.1016/j.ejpn.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Popova NK, Kulikov AV. Targeting tryptophan hydroxylase 2 in affective disorder. Expert Opin Ther Targets. 2010;14:1259–1271. doi: 10.1517/14728222.2010.524208. [DOI] [PubMed] [Google Scholar]
- Russo S, Kema IP, Bosker F, Haavik J, Korf J. Tryptophan as an evolutionarily conserved signal to brain serotonin: molecular evidence and psychiatric implications. World J Biol Psychiatry. 2009;10:258–268. doi: 10.1080/15622970701513764. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Sorensen SM, Kehne JH, Carr AA, Palfreyman MG. The role of 5-HT2A receptors in antipsychotic activity. Life Sci. 1995;56:2209–2222. doi: 10.1016/0024-3205(95)00210-w. [DOI] [PubMed] [Google Scholar]
- Serretti A, Chiesa A, Porcelli S, Han C, Patkar AA, Lee SJ, et al. Influence of TPH2 variants on diagnosis and response to treatment in patients with major depression, bipolar disorder and schizophrenia. Psychiatry Res. 2011;189:26–32. doi: 10.1016/j.psychres.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Siesser WB, Sachs BD, Ramsey AJ, Sotnikova TD, Beaulieu JM, Zhang X, et al. Chronic SSRI treatment exacerbates serotonin deficiency in humanized Tph2 mutant mice. ACS Chem Neurosci. 2013;4:84–88. doi: 10.1021/cn300127h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, Chapin DS, McCarthy SA, Guanowsky V, Brown J, Chiang P, et al. CP-809,101, a selective 5-HT2C agonist, shows activity in animal models of antipsychotic activity. Neuropharmacology. 2007;52:279–290. doi: 10.1016/j.neuropharm.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Strobel A, Dreisbach G, Muller J, Goschke T, Brocke B, Lesch KP. Genetic variation of serotonin function and cognitive control. J Cogn Neurosci. 2007;19:1923–1931. doi: 10.1162/jocn.2007.19.12.1923. [DOI] [PubMed] [Google Scholar]
- Verin M, Partiot A, Pillon B, Malapani C, Agid Y, Dubois B. Delayed response tasks and prefrontal lesions in man—evidence for self generated patterns of behaviour with poor environmental modulation. Neuropsychologia. 1993;31:1379–1396. doi: 10.1016/0028-3932(93)90105-9. [DOI] [PubMed] [Google Scholar]
- Waider J, Araragi N, Gutknecht L, Lesch KP. Tryptophan hydroxylase-2 (TPH2) in disorders of cognitive control and emotion regulation: a perspective. Psychoneuroendocrinology. 2011;36:393–405. doi: 10.1016/j.psyneuen.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- Witkin JM.2008Animal models of obsessive compulsive-disorder Curr Protoc NeurosciChapter 9: Unit 9.30. [DOI] [PubMed]
- Xi ZX, Gilbert JG, Pak AC, Ashby CR, Jr, Heidbreder CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. Eur J Neurosci. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gainetdinov RR, Beaulieu JM, Sotnikova TD, Burch LH, Williams RB, et al. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005;45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


